Abstract
Two kinds of modified macro porous silicon (maPsi) layer with trimetallic nanoparticles surface alloying and core–shell forms CO2 gas sensors were fabricated and tested successfully. Trimetallic Au–Ag–Pd nanoparticles were prepared by an ion reduction process of different metallic salts on maPsi layer by a simple immersion process. As-formed maPsi layer was fabricated by a laser assisted etching (LAE) process with laser power density illumination, current density and etching time of about: 25 mW/cm2, 20 mA/cm2 and 20 min, respectively. Immersion process with different immersion times were carried out at a fixed concentration 1 mM of HAuCl4, AgNO3 and PdCl2 to synthesize Au–Ag–PdNPs/maPsi hybrid structures. Trimetallic core–shell nanoparticles form was prepared at (6, 8) min immersion times, while for surface alloying nanoparticles form was prepared at (2, 4) min immersion times in a mixed solution with a fixed ratio 1:1:1 at room temperature. The structural aspects of the as-formed maPsi layer and trimetallic Au–Ag–Pd nanoparticles were demonstrated by field emission-scanning electron microscopy (FE-SEM), X-ray diffraction (XRD) and energy dispersive X-ray analysis (EDS). The electrical properties of the modified maPsi layers with trimetallic nanoparticles with and without CO2 gas sensor were found at room temperature. A specific enhancement in gas sensitivity for trimetallic surface alloying nanoparticles form compared with that of the core–shell nanoparticles form was obtained due to the higher specific area to volume ratio.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Advance in nanotechnology is very important for chemical sensors with a high efficiency, fast sensing and little power consumption [1]. Monitor and control of air pollution in the environment are needed todays, in laboratories, hospitals or generic technical installations, it pushes the development of extremely sensitive gas detectors to avoid the accidents produced by gas leakages [2]. Porous silicon (Psi) sensor is inexpensive with a high surface area to volume ratio and permits the multiplexing of sensor arrays and remote sensing [3,4,5]. The sensitivity of Psi sensors depend on the morphological features of pores, including the pores size and homogeneity, regularity of the surface and the layer thickness [4]. Hydrogen-terminated (Si-Hx) of Psi can allow a reduction of the metal ion at the Psi surface to generate small metal nanoparticles distributed inside the pore walls of the Psi interface and hence modified the morphological properties [6]. In general, the trimetallic Au–Ag–Pd nanoparticle catalysis produced better results than that of monometallic and bimetallic catalysts. The high catalytic activity of trimetallic nanoparticles is possibly due to the sequential electronic effect between elements in a particle [7]. The control of the metallic nanoparticles such as: the shape, particle size, density, surface morphology, and elemental composition of alloy NPs has been identified as the main part to tune the sensitivity of the device and chemical reactivity [8]. Alwan and Dheyab have reported a study on the modified porous silicon with monometallic nanoparticles on the CO2 gas sensing properties, they found that the morphological properties of the nanoparticle intensely influence on the sensitivity and temporal response of the gas sensor [9]. Litovchenko et al. incorporated the porous silicon with the nanoparticles of transition metals (Pd, W, Cu), and they found that an increase of adsorption sensitivity for various gases (H2, H2S, H2O) in comparison with an ordinary porous silicon [10]. Potemkin et al. prepared Psi supported with bimetallic nanoparticles Au–Pd and PdAu2/Psi for H2O2 gas sensing, the performance of catalyst was related to the amount of Pd in the catalyst and also due to the high surface area to volume ratio of the bimetallic nanoparticles [11].
In this work, the preparation of two kinds of maPsi layer modified with trimetallic nanoparticles for gas sensing applications has been reported. The morphological properties of as-formed maPsi layer and the modified layer, involving nano size, distribution, and surface area were inspected based on the FE-SEM, X-ray diffraction and EDS analysis. The electrical behavior of Au–Ag–PdNPs/maPsi hybrid structures with and without CO2 was investigated extensively.
2 Experimental section
2.1 Chemicals materials
High purity chemical salts of PdCl2, AgNO3 and HAuCl4:3H2O were used to prepare the required electrolyte solution for ion reduction process. A 1 mM PdCl2 salt (high purity 99%) was dissolved in the 0.15 M HF and few drops from HCL with concentration 37% at the 60 °C under magnetic stirring for about 30 min to prepare an aqueous solution. The AgNO3 salt with a purity of 99.99% was dissolved by triply distilled water to prepare an aqueous solution with a concentration of 1 mM. The HAuCl4·3H2O at concentration (1 mM) was prepared with the assistant of 2.9 M from HF and distilled water. Hydrofluoric acid 40% was employed as received and diluted with absolute ethanol 99.8%, to create etching solutions 20% HF, all the chemicals were supplied from (SIGMA-ALDRICH, Germany). 2M aqueous KOH was used to remove the porous layer to determine the porosity and layer thickness. The concentration of these salt solutions was calculated according to the following equation [12]:
where, W (g) is the weight of the salts, M.Wt (g/Mol) is the molecular weight, and V is the volume of the dissolved solution.
2.2 Preparation process of MaPsi layer
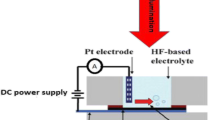
As-formed maPsi samples were prepared by a laser assisted etching (LAE) process from n-type silicon wafer (100) oriented with resistivity 10 Ω cm. The sample was etched in the etching solution consisted of HF/C2H5OH with ratio 1:1 under a current density of about 20 mA/cm2, etching time 20 min and illumination conditions 25 mW/cm2 and 630 nm laser power density and wavelength, respectively. The maPsi samples were washed in water and permitted to dry at ambient air. The setup of etching process is illustrated in Fig. 1.
2.3 Modification of as-formed macro Psi layers with trimetallic nanoparticles
As-formed maPsi layers were modified by trimetallic nanoparticles Au–Ag–Pd using an immersing process of the maPsi layer in aqueous solution of 1 mM HAuCl4, AgNO3 and PdCl2 with an equal mixing ratio of 1:1:1 for different immersion times (2, 4, 6 and 8) min to prepare hybrid structures with two forms (surface alloying and core–shell) for CO2 gas sensors. The ion reduction process of Au3+, Ag1+ and Pd2+ occurs according to the following reactions, respectively [12,13,14,15,16]:
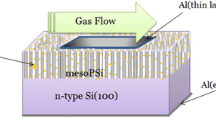
The schematic diagram of the proposed procedures for the formation of the trimetallic nanoparticles: surface alloy form and core–shell form is shown in Fig. 2. An aluminum wire was connected by suitable conductive paste on the top layer and bottom of the modified maPsi structure to carry out the electrical properties. These sensors were placed in a small closed dark cavity with inlet and outlet to evaluate the CO2 gas detection process. Finally, the sensor’s performance at room temperature in vacuum and with two gas pressures 0.5 and 1 mbar was investigated by measuring the current–voltage characteristics at a forward case using an electrometer Kiethly 6485 and D.C power supply Furnall LT30-2.
3 Results and discussions
3.1 Morphological properties of as-formed MaPsi layer
Figure 3 shows the FE-SEM image of the reference as-formed maPsi layer, from this image, one can note that the surface contains a dark uniform semi- circular shape pores (pores-like structure). The pores sizes range from 0.25 to 6.25 µm, and the peak of the pore size distribution is around 0.75 µm. The gravimetrical method was used to calculate the porosity and the layer thickness of maPsi, the obtained result is about 67% and 36.146 µm, respectively.
3.2 Morphological properties of modified as-formed MaPsi layers with trimetallic nanoparticles
The surface morphology of the macroporous layer plays an important feature in the growing of trimetallic nanoparticles Au–Ag–Pd, where the density of the growth sites (Si–H dangling bonds) is various according to it [17]. The morphological and structural features of trimetallic nanoparticles were investigated and classified as a function of immersion time, the surface alloying nanoparticles form behavior can be recognized at low immersion time 2 and 4 min, while the core–shell nanoparticles form behavior at a high immersion time 6 and 8 min. Figure 4 shows the typical FE-SEM images of modified maPsi layers with trimetallic nanoparticles. Figure 4a, b clarifies the surface morphology of the surface alloying nanoparticles form, while Fig. 4c, d clarifies the surface morphology of core–shell nanoparticles form. In general, for both types of modification, the deposited nanoparticles are mainly located in the pore walls, especially when their integrated size is larger than the pore size. The main reason behind the formation of the surface alloying process is related to the electron affinity of the metallic ions, gold, silver and palladium which is about (2.308, 1.304 and 0.562) eV [18]. High immersion time will exhaust all the dangling bonds on the porous surface, so that the remained metallic ions will attack each other to form its nanoparticle and hence it will take electrons from the metal of lower electron affinity. Circular shielding is the approximate resulting form of core–shell form [19, 20]. The statistical distributions of the trimetallic nanoparticles of surface alloying form and core–shell form modified structures are shown in Fig. 5a–d, respectively. For the surface alloying form, the nanoparticles grain sizes range from 35 to 125 nm to 37.5–187 nm as the immersion time increases from (2 to 4) min. For the core–shell form, the nanoparticles grain sizes range from 50 to 450 nm to 90–510 nm as the immersion time increases from (6 to 8) min. These fluctuations in trimetallic nanoparticles grain sizes really depend on the ion reduction rates, where this rate increases with the immersion time [21].
The EDS analysis, which confirms the existence of trimetallic Au–Ag–Pd nanoparticles, is shown in Fig. 6. From these figures, it can be easily noted that the density of gold nanoparticles is less than that of silver and palladium nanoparticles. Higher amount of deposited nanoparticles was shown in silver nanoparticles rather than the other deposited metals. This is due to the fact that the silver ions just need only one electron in the reduction process, so the silver nanoparticles have a higher growth rate than the other nanoparticles. The growth of silver nanoparticles is confirmed by the EDS analysis, from this analysis, the density of the silver nanoparticles increases with the immersion time increasing [21].
3.3 X-ray diffraction patterns of modified as-formed MaPsi layers with trimetallic nanoparticles
Figure 7a, b elucidates the pattern of diffraction peaks for Au–Ag–PdNPs/maPsi hybrid structure for surface alloying form. The alloy Au–Ag–Pd nanoparticles have similar lattice parameters (4.065, 4.079 and 3.859) Å, for gold, silver and palladium, respectively. The interface between the peaks of these metals confirms its being alloyed nanoparticles [18, 22]. While, the pattern of diffraction peaks for Au–Ag–PdNPs/maPsi hybrid structure for core–shell form is shown in Fig. 7c, d. The XRD peaks of the Au–Ag–PdNPs/maPsi alloy reflect that the plane (100) at 39° with FWHM is broader than that of monometallic and bimetallic nanoparticles due to the overlapping process of each type of individual nanoparticles.
The nanoparticles grain size was calculated by the Sherres equation [23].
where, L is the nanoparticles grain size in nm, k is the constant shape factor 0.09, λ is the wavelength of the X-ray (1.5406 Å), β is the full width half- maximum in radian, and P is the diffraction angle in degree. The specific surface area (S.S.A.) is one of the figures of merit of material nanostructured, this one is known as [24]:
The density of the trimetallic Au–Ag–Pd nanoparticles is about 12.093, 12, 11.868 and 12.293 g/cm3, these values can be determined using equation [25]:
where, D is the density of trimetallic Au–Ag–Pd nanoparticles (g/cm3), \(\rho\) is the density of the monometallic Au, Ag and Pd for about 19.3, 10.5 and 12.023 g/cm3, correspondingly and a = wt% Au, b = wt% Ag and c = wt% Pd (precentages of metals depending on the EDS analysis). Table 1 demonstrates the trimetallic nanoparticles grain size and S.S.A.
From Table 1, it is observed that the Au–Ag–Pd NPs/maPsi hybrid structure, surface alloying nanoparticles form has a high specific surface area compared with the core–shell nanoparticles form, these results are in agreements with [23].
4 Electrical properties
4.1 Electrical properties of as-formed MaPsi layer
Figure 8 displays the I–V characteristics of as-formed maPsi layer in vacuum and with two gas pressures 1 and 0.5 mbar. I–V characteristics are displayed as an ohmic contact behavior due to the high density of the dangling bonds in the porous structures [26, 27]. In the presence of CO2 gas molecules, the forward current increases with the increasing gas pressure due to the passivation of the dangling bonds with the gas molecules [9]. The CO2 desorption will change the relative permittivity of the maPsi layer. The dependence of relative permittivity εrPsi on the porosity of the maPsi layer and the gas (εrgas) is set by the Eq. 9 [28].
where, P % is the porosity of the maPsi layer, and εrgas is the relative permittivity of the embedding medium (CO2 molecule).
4.2 Electrical properties of modified as-formed MaPsi layers with trimetallic nanoparticles
The I–V characteristics of trimetallic Au–Ag–Pd NPs/maPsi hybrid structure for surface alloy and core–shell forms are shown in Fig. 9a-c, it is observed that the forward current for surface alloying nanoparticles form is higher than that for the core–shell nanoparticles form the as a function of gas pressure. There is a number of attitudes to clarify the increasing of current in the existence of CO2 gas. For stimulation of the electrical charge carriers trapping (initiating from the adsorbate interaction with maPsi layer (dangling bonds)), the Si–H states improve the conductance variations; due to the trap/release of the charge carriers [29]. This difference is mainly related to desorption of the CO2 molecules on the maPsi layer as a result of Vander Waals interaction [30]. Trimetallic nanoparticles deposited on the walls in maPsi layer act as a supplementary reason to increase the surface area and therefore improve the gas adsorption rate. The sensitivity (S) of the sensor was calculated via the following formula [31]:
where, Igas and I without gas are the current in the existence and nonexistence of CO2 gas, correspondingly.
Figure 10a–d elucidates the sensitivity–voltage characteristics of the as-formed maPsi layer and the modified layer with trimetallic nanoparticles Au–Ag–PdNPs under two CO2 gas pressures at room temperature. As shown from this figure, the relative response of the modified structures is higher than that of as-formed layer, and the relative response grows with the increased concentrations of gas molecules. The maximum response obtained for sensors having Au–Ag–PdNPs/maPsi hybrid structure for surface alloying form is higher than that of Au–Ag–PdNPs/maPsi hybrid structure for core–shell form. In general, higher sensitivity is observed at a low bias voltage of surface alloying nanoparticles form compared with both the core–shell nanoparticles form and as-formed maPsi layer hybrid structure sensors.
These results indicate the presence of Schottky barrier height between the trimetallic Au–Ag–Pd nanoparticles and the maPsi layer; the forward current is strongly controlled by the changes in barrier height, which formed between the nanoparticles and maPsi layer [16]. The value of the barrier height (ΦBn) for n-type silicon can be calculated experimentally, according to the thermionic emission; J0 is given by the expression [32]:
where, A* is the effective Richardson, which is equal to 120 (A/K2 cm2) for n-type silicon, from this equation. The barrier height is given by [33]:
From Eq. (12), and the obtained experimental results, one can estimate the value of the barrier height as shown in Table 2. Also, the value of the barrier height can be evaluated theoretically based on Eq. 13 by taking into account that the electron affinity (χ) of porous silicon is same the electron affinity of silicon for about 4.05 eV with considering the metal percentages in the EDS analysis. The theoretical value of the barrier height (ØBn) for n-type silicon is connected to the work function of metal (Φm) and the electron affinity of the silicon (χ) by the following relation [32].
The theoretical calculated value of barrier height (ØBn) is in good agreement with that calculated experimentally, especially at high density of silver nanoparticles within the trimetallic regime. From the current voltage characteristics in the presence of gas molecules and the resulting barrier height, it’s clear that the optimized response was achieved at structures with a higher value of the barrier height.
From Table 2, it can be observed that the Schottky barrier height Φbn is depending on the density of the nanoparticles. So that with increased density of silver NPs as a function of immersion times, the Schottky barrier height Φbn decreases from 0.75 to 0.69 eV [29].
The temporal response features of as-formed maPsi layer and the modified layer with trimetallic nanoparticles Au–Ag–Pd NPs hybrid structure for CO2 gas sensing were measured for two gas pressures 0.5 and 1 mbar at room temperature, as shown in Fig. 11. The results proved that the response increases with the pressures of the gas for all sensors. The sensing process with trimetallic surface alloying nanoparticles form is higher than that of trimetallic core–shell nanoparticles form. The main reason behind this experimental behavior is related to the S.S.A. Table 3 summarizes the temporal response results for all gas sensors (as-formed maPsi layer, and hybrid structure). It can be noticed that the surface alloying nanoparticles form compared with the core–shell nanoparticles form has the considerable short time of response and recovery of the sample, this may be owing to the high rate of CO2 gas adsorption and desorption, respectively.
5 Conclusion
In this work, an efficient CO2 gas sensors were prepared by modifying as-formed maPsi layers with trimetallic nanoparticles to synthesize Au–Ag–PdNPs/maPsi hybrid structures: surface alloying form and core–shell form. The modification process of as-formed maPsi layers was carried out by incorporating trimetallic nanoparticles via an immersion process in aqueous solution of HAuCl4, AgNO3 and PdCl2 with equal mixing ratio at different immersion times. The maPsi layer modified by surface alloying nanoparticles form has a higher specific area to volume ratio compared with maPsi layer modified with core–shell nanoparticles form. Maximum sensitivity with the lowest response and recovery times was attained with surface alloying form rather than core–shell form, especially for forms with higher value of barrier height.
References
C. Wei, L. Dai, A. Roy, T.B. Tolle, Multifunctional chemical vapor sensors of aligned carbon nanotube and polymer composite. J. Am. Chem. Soc. 128(5), 1412–1413 (2006)
G. Barillaro, A. Nannini, F. Pieri, A. New, Porous silicon-based gas sensing device. Sens. Actuators B 93, 263–270 (2003)
N.A. Yebo, P. Lommens, Z. Hens, R. Baets, An integrated optic ethanol vapor sensor based on silicon on insulator microing resonatorcoated with porous ZnO film. Opt. Express 18, 11859–11866 (2010)
B. Timmer, W. Othuis, A. Berg, Ammonia sensor and their application. Sens. Actuators B 107, 666–677 (2005)
A.M. Alwan, R.A. Abbas, A.B. Dheyab, Study the characteristic of planer and sandwich psi gas sensor (comparative study). Silicon 10, 2527–2534 (2018)
S. Polisski, B. Goller, A. Lapkin, S. Fairclough, D. Kovalev, Synthesis and catalytic activity of hybrid metal/silicon nanocomposites. Phys. Stat. Sol. 2, 132–134 (2008)
P. Venkatesan, J. Santhanalakshmi, Designed synthesis of Au/Ag/Pd trimetallic nanoparticle-based catalysts for sonogashira coupling reactions. Langmuir 26(14), 12225–12229 (2010)
A. Singh, A. Singh, S. Singh et al., Preparation and characterization of nanocrystalline nickel ferrite thin films for development of a gas sensor at room temperature. J. Mater. Sci. 27(8), 8047–8054 (2016)
A.M. Alwan, A.B. Dheyab, Room temperature, CO2 gas sensors of AuNPs/mesoPSi hybrid structures. Appl. Nanosci.7(7),335–341, (2017)
V.G. Litovchenko, T.I. Gorbanyuk, V.S. Solntsev, Mechanism of adsorption-catalytic activity at the nanostructured surface of silicon doped with clusters of transition metals and their oxides. Ukr. J. Phys. 62, 605–614 (2017)
D.I. Potemkin, D.K. Maslov, K. Loponov, P.V. Snytnikov, Y.V. Shubin, P.E. Plyusnin, D.A. Svintsitskiy, V.A. Sobyanin, A.A. Lapkin, Porous nanocrystalline silicon supported bimetallic Pd–Au catalysts: preparation, characterization, and direct hydrogen peroxide synthesis. Front. Chem. J. 6, 85 (2018)
A.M. Alwan, D.A. Hashim, M.F. Jawad, Optimizing of porous silicon alloying process with bimetallic nanoparticles. Gold Bull. 51(13), 175–184 (2018)
A. Jabbar, A.M. Alwan, A.J. Haider, Modifying and fine controlling of silver nanoparticle nucleation sites and SERS performance by double silicon etching process. Plasmonics 13(4), 1171–1182 (2018)
L.A. Wali, K.K. Hasan, A.M. Alwan, Rapid and highly efficient detection of ultra-low concentration of Penicillin G by gold nanoparticles/porous silicon SERS active substrate. Spectrochimica Acta A 206, 31–36 (2019)
A.M. Alwan, A.A. Yousif, L.A. Wali, The growth of the silver nanoparticles on the mesoporous silicon and macroporous silicon: a comparative study. Indian J. Pure Appl. Phys. 55, 813–820 (2017)
F. Razia, A.I. Zada, F. Rahimia, Investigation of hydrogen sensing properties and aging effects of Schottky like Pd/porous Si. Sens. Actuators B 146, 53–60 (2010)
A.M. Alwan, A.A. Yousif, L.A. Wali, A Study on the morphology of the silver nanoparticles deposited on the n-type porous silicon prepared under different illumination types. Plasmonics 13(4), 1191–1199 (2018)
R. Hoehn, C. Herzig, Direct determination of thermodynamic activities of Au in the Systems Au–Pd and Au–Ag–Pd. Z. Metallkd. 77, 291–297 (1986)
L. Wang, Y. Yamauch, Strategic synthesis of trimetallic Au@Pd@Pt core–shell nanoparticles from poly(vinylpyrrolidone)-based aqueous solution toward highly active electrocatalysts. Chem. Mater. 23, 2457–2465 (2011)
L. Nahar, A.A. Farghaly, R.J. Alan, Esteves, I.U. Arachchige, Shape controlled synthesis of Au/Ag/Pd nanoalloys and their oxidation-induced self-assembly into electrocatalytically active aerogel monoliths. Chem. Mater. 29, 7704–7715 (2017)
A.M. Alwan, L.A. Wali, A. Yousif, Optimization of AgNPs/mesoPS active substrates for ultra–low molecule detection process. Silicon 10(5), 2241–2251 (2018)
H. Xua, J. Wang, B. Yana, S. Lia, C. Wangb, Y. Shiraishi, P. Yang, Y. Du, Facile construction of fascinating trimetallic PdAuAg nanocages with exceptional ethylene glycol and glycerol oxidation activity. R. Soc. Chem. 9, 17004–17012 (2013)
R.A. Ismail, A.M. Alwan, A.S. Ahmed, Preparation and characteristics study of nano-porous silicon UV photodetector. Appl Nanosci 7, 9–15 (2017)
S. Bykkam, M. Ahmadipour, S. Narisngam, V.R. Kalagadda, S.C. Chidurala, Extensive studies on X-ray diffraction of green synthesized silver nanoparticles. Adv. Nanopart 4, 1 (2015)
L. Yang, X. Li, X. Tuo, T.T. Van Nguyen, X. Luo, M. Hong, Alloy nanoparticle plasmon resonance for enhancing broadband antireflection of laser-textured silicon surfaces. Optics Express, 19, S4 (2011)
A.M. Alwan, Calculation of energy band gap of porous silicon based on the carrier transport mechanisms. Eng. Tech. 25, 10 (2007)
M. Alwan, O.A. Abdulrazaq, Aging effect on the photosynthesized porous silicon. Int. J. Modern Phys. B 22(4), 417–422 (2008)
M.V. Nikolic, S.M. Radic, V. Minic, M. Ristic, The dependence of the work function of rare earth metals on their electron structure. Microelectron. J. 27(1), 93–96 (1996)
S. Sadr, M.E. Azim-Araghi, M. Rahimi, R.S. Dariani, Effect of the gold electrode annealing on gas sensing properties of nano-and microstructures of macroporous silicon. Indian J. Pure Appl. Phys. 51, 860–863 (2013)
S. Salameh, M.A. van der Veen, M. Kappl, J.R. van Ommen, Contact forces between single metal oxide nanoparticles in gas-phase applications and processes. Langmuir 33(10), 2477–2484 (2017)
A.M. Alwan, R.A. Abbas, Effects of the porous silicon morphology on the gas sensor performance. Int. J. Eng. Sci. Res. Technol. 59, 204–217 (2017)
M.S. Gorji, K.A. Razak, K.Y. Cheong, Shottky barrier height engineering of Al contacts on Si by embedded Au nanoparticles. Microelectron. Eng. 133, 110–119 (2015)
Acknowledgements
The author would like to thank the Department of Applied Sciences - University of Technology, Nanotechnology and Advanced Materials Research Center - University of Technology, and Razi Metallurgical Research Center - Iran for their facility and assistance to use the SEM (MIRA3 TESCAN) device and conducting the EDS analyses. The financial support is also acknowledged.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors have an active participation in research.
Corresponding author
Ethics declarations
Conflict of interest
There’s no financial/personal interest or belief that could affect our objectivity.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alwan, A.M., Hashim, D.A. & Jawad, M.F. CO2 gas sensor based on macro porous silicon modified with trimetallic nanoparticles. J Mater Sci: Mater Electron 30, 7301–7313 (2019). https://doi.org/10.1007/s10854-019-01043-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-019-01043-3