Abstract
Fe3O4/CoWO4 magnetic nanocomposites with spherical morphology were synthesized by simple method. The crystal structures, morphology and chemical properties of the as-synthesized nanoparticles were characterized using scanning electron microscopy, transmission electron microscopy, energy dispersive X-ray, X-ray diffraction, and vibrating sample magnetometer techniques. The photocatalytic activity of Fe3O4/CoWO4 magnetic nanocomposites was investigated by degradation of rhodamine B (RhB) in aqueous solution under visible light irradiation. The results indicate that about 98% of RhB were degraded after 180 min. The antimicrobial activity of Fe3O4/CoWO4 nanocomposites were investigated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
It is well known that environmental pollution due to the rapid industrial expansion and human population growth is one of the most important challenges facing all leaving beings worldwide [1,2,3,4].
At the moment the nanoparticles and nanocomposites materials are widely used in construction of high-area-density storage devices, environmental chemistry, photocatalyst, construction of electrochemical sensors, pharmaceutical sciences, medicine and etc. [5,6,7,8,9,10,11,12].
Among the different methods for synthesizing nanoparticles and nanocomposites, the precipitation route has been regarded as one of the most convenient and practical techniques, because it not only enables to avoid special instruments and severe preparation condition, but also provides good control over purity, homogeneity, composition, phase and microstructure of resultant products [13,14,15].
The literature review showed that there is not any report about preparation and investigation photocatalytic activities of the Fe3O4/CoWO4 nanocomposites. Due to low band gap of CoWO4, it seems that the binary Fe3O4/CoWO4 nanocomposite could have remarkable activity under visible-light irradiation. Hence, a series of novel magnetically separable Fe3O4/CoWO4 nanocomposites were prepared through a precipitation process followed by a calcination step at 500 for 1 h and they were characterized in detail by energy dispersive analysis of X-rays (EDX), scanning electron microscopy (SEM), UV–Vis diffuse reflectance spectroscopy (UV–Vis DRS), transmission electron microscopy (TEM), X-ray diffraction (XRD) and vibrating sample magnetometery (VSM) techniques. In addition, photocatalytic activity of the prepared samples was evaluated by photodegradation of rhodamine B (RhB), under visible light irradiation. The results showed that the Fe3O4/CoWO4 (30%) nanocomposite has much greater activity for degradation of these pollutants under visible-light irradiation than the Fe3O4 samples.
2 Experimental
2.1 Synthesis of Fe3O4 nanoparticles
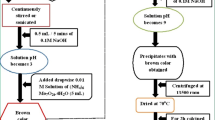
The magnetic nanoparticles were synthesized through a co-precipitation method according to the previously published work [16]. Typically, 0.4 mmol of FeCl2·4H2O and 0.6 mol of FeCl3·6H2O were dissolved in 80 mL of degased HCl (0.8 M) by vigorous stirring. Afterward, 400 mL of degased NH3 (1.5 M, 25 wt%) solution was added to the above solution drop by drop for 45 min under ultrasonic. After that, the Fe3O4 nanoparticles were formed forth with. Then, the black precipitate separated with a magnet. In the final step, the products were washed with deionized water and dried at 70 °C for 4 h.
2.2 Synthesis of Fe3O4/CoWO4 nanocomposites
The Fe3O4 were obtained in the last step were dispersed in 40 mL of deionized water for 20 min solution C. To do so, CoNO3·6H2O were dissolved in 30 mL of water according Table 1 which we called solution A. Another solution, solution B, was formed by adding 1 mmol of Na2WO4 to 30 mL of water. Next, solution A was added to solution B with vigorous and constant stirring to make the third solution, solution C. In the final step, the mixture was separated by centrifuged and washed with deionized water and dried at room temperature for 2 h and calcined in temperature 500 °C for 1 h. All reaction conditions are listed in Table 1.
2.3 Photocatalytic activity test
The degradation of RhB was investigated to calculate the photoactivity of Fe3O4/CoWO4 nanocomposites. The experiments were carried out in a photocatalytic reactor. A 100 mL beaker contains 50 mL of 3 × 10−5 M RhB 0.1 g of catalyst at room temperature. The suspension was constantly stirred for about 30 min in the dark to make sure adsorption–desorption balance between the photocatalyst and dyes. Then, the mixture was placed inward the photoreactor which the vessel was 40 cm away from the visible source of 400 W Osram lamp under magnetic stirring. In reaction time, the mixed suspensions were gathered for UV–Vis analysis. The reaction was continued by a UV–Vis spectrometer. The photocatalytic degradation percent was measurement as follow (Eq. 1) [17]:
where C0 and Ct are the UV–Visible absorbance value of dyes pollutant solution before and after destruction.
2.4 Materials and characterization
The chemicals (Na2WO4, Co(NO3)2·6H2O, FeCl2·4H2O and FeCl3·6H2O) were obtained from Merck Co. and were used as received. The magnetic properties, at room temperature, were evaluated with an alternating gradient force magnetometer (AGFM) instrument (Meghnatis Daghigh Kavir Co.; Kashan Kavir; Iran) by scanning the magnetic field between ± 10,000 Oe. The XRD patterns were acquired using a Philips, XRD instrument with anNi-filtered CuKα radiation. The SEM images were recorded using a LEO 1455VP instrument after coating a very thin layer of Pt (using a BAL-TEC SCD 005 sputter coater) on the samples, to induce conduction to the sample surface, prevent charge accumulation, and help obtain improved contrast. DRS spectrum of the as-produced Fe3O4/CoWO4 nanocomposites has been detected with the aid of UV–Vis spectrophotometer (Shimadzu, UV-2550, Japan).
2.5 Antimicrobial activity
2.5.1 Microbial strains
The samples were tested against a set of 11 microorganisms. Following microbial strains were provided by Iranian Research Organization for Science and Technology (IROST) and used in this research: Pseudomonas aeruginosa (ATCC 27853), Escherichia coli (ATCC 10536), Bacillus subtilis (ATCC6633), Staphylococcus aureus (ATCC 29737), Klebsiella pneumonia (ATCC 10031), Staphylococcus epidermidis (ATCC 12228), Shigella dysenteriae (PTCC 1188), Proteus vulgaris (PTCC 1182), Salmonella paratyphi-A serotype (ATCC 5702), Candida albicans (ATCC 10231) and Aspergillus niger (ATCC 16404). Bacterial strains were cultured overnight at 37 °C in nutrient agar (NA) and Fungi were cultured overnight at 30 °C in sabouraud dextrose agar (SDA).
2.5.2 Disk diffusion assay
Determination of antimicrobial activity of Fe3O4/CoWO4 nanocomposites was accomplished by agar disk diffusion method (Clinical and Laboratory Standard Institute (CLSI)). Fe3O4/CoWO4 nanocomposites were disperse in DMSO to a final concentration of 30 mg/mL and filtered by 0.45 µm Millipore filters for sterilization. Antimicrobial tests were carried out using the disk diffusion method and employing 100 µL of suspension containing 108 CFU/mL of bacteria, 106 CFU/mL of yeast and 104 spore/mL of fungi spread on the nutrient agar (NA), sabouraud dextrose (SD) agar and potato dextrose (PD) agar mediums, respectively.
The disks (6 mm in diameter) impregnated with 10 µL of sample solutions (300 µg/disk) and DMSO (as negative control) were placed on the inoculated agar. The inoculated plates were incubated for 24 h at 37 °C for bacterial strains and 48 h and 72 h at 30 °C for yeast and mold isolates, respectively. Gentamicin (10 µg/disk), and rifampin (5 µg/disk) were used as positive controls for bacteria and nystatin (100 I.U.) for fungi. The diameters of inhibition zones were used as a measure of antimicrobial activity and each assay was repeated twice [18].
2.5.3 Micro-well dilution assay
Microorganisms sensitive to antibiotics in disk diffusion assay were studied for their minimal inhibition concentration (MIC) values using micro-well dilution assay method [19]. The inocula of the microbial strains were prepared from 12 h broth cultures and suspensions were adjusted to 0.5 McFarland standard turbidity. The antibiotics were first diluted to the highest concentration (500 µg/mL) to be tested, and then serial twofold dilutions were made in a concentration range from 7.8 to 500 µg/mL in 10 mL sterile test tubes containing brain heart infusion (BHI) broth for bacterial strains and sabouraud dextrose (SD) broth for fungi. The 96-well plates were prepared by dispensing 95 µL of the cultures media. A 100 µL aliquot from the stock solutions of the samples initially prepared at the concentration of 500 µ/mL was added into the first well. Then, 100 µL from their serial dilutions was transferred into six consecutive wells. The last well containing 195 µL of the cultures media without the test materials and 5 µL of the inoculum on each strip was used as the negative control. The final volume in each well was 200 µL. Gentamicin and rifampin for bacteria and nystatin for fungi were used as standard drugs for positive control in conditions identical to tests materials. The plates were covered with sterile plate sealers. Contents of each well were mixed on plate shaker at 300 rpm for 20 s and then incubated at appropriate temperatures for 24 h. Microbial growth was determined by the presence of a white pellet on the well bottom and confirmed by plating 5 µL samples from clear wells on NA medium. The MIC value was defined as the lowest concentration of the plant extracts required for inhibiting the growth of microorganisms. All tests were repeated two times [19].
3 Results and discussion
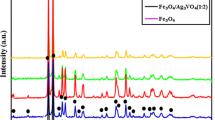
XRD patterns of Fe3O4 nanostructures are shown in Fig. 1. The spectrum of bare CoWO4 sample shows a series of diffraction peaks at the position of 30.82° ((111) line), and 35.43° ((112) line) which is in good agreement with the standard JCPDS file of Fe3O4 tetragonal phase (space group I41/a, JCPDS No. 75-0033). The XRD patterns of Fe3O4/CoWO4 nanocomposites have been displayed in Fig. 2. As our results suggest, prepared nanocomposites are pure and have two phases. The first is the phase of Fe3O4 and CoWO4 with crystal structure of tetragonal (JCPDS 75-0033) and space group of I41/amd and the second is tetragonal (JCPDS 15-0867) with space group of I41/a, respectively. From XRD data and Scherrer equation the crystallite diameter (Dc) of Fe3O4 nanostructures and Fe3O4/CoWO4 nanocomposites, samples 1–2, calculate to be 21.1, and 22.3 nm, respectively [19].
In which β is the breadth of the noticed diffraction line at its half intensity maximum, K is the so-called shape factor, which commonly takes a value of approximately 0.9, and λ is the wavelength of X-ray source applied in XRD.
The process led to the formation of fine and uniform Fe3O4 and Fe3O4/CoWO4, which can be attributed to the creation of extremely high local temperatures and pressures of over 5000 K and 20 MPa, and high heating and cooling rates of over 1010 Ks−1 due to the so-called acoustic cavitation, which leads to the formation of a wide range of nanostructured materials [20,21,22]. Figures 3 and 4 illustrate SEM images of samples Fe3O4 and Fe3O4/CoWO4, respectively. All sample shows is sphere-like nanostructures which were consisted of nanoparticles with average particle size of 50–55 nm.
Transmission electron microscopy (TEM) technique was used to further examine the morphology of nanocomposites. The as-prepared nanocomposites with the size of nearly 45–50 nm in the presence of ultrasonic wave have been shown in Fig. 5.
The purity of Fe3O4/CoWO4 nanocomposites was confirmed by EDS technique. As illustrated in Fig. 6 (sample No. 2), the Fe3O4/CoWO4 nanocomposites are composed of Fe, W, Co, and O elements. Furthermore, no impurity peaks were observed which indicates high purity level of as-prepared Fe3O4/CoWO4 nanocomposites.
The magnetization curve patterns of Fe3O4/CoWO4 nanocomposites were indicated by VSM (Fig. 7). The saturation magnetization of Fe3O4/CoWO4 nanocomposites were measured to be 10.12 emu/g, The results show, that samples are superparamagnetic. Although the magnetism of photocatalytic was reduced, the photocatalyst is still sufficiently strong to magnetically separable when a magnetic field was applied. The Fe3O4/CoWO4 nanocomposites indicate still high magnetization, that photocatalyst suitable for magnetically separable by a magnetic field and separation of photocatalyst from solution.
A blank test (without catalyst) revealed that no dye degradation without using Fe3O4/CoWO4 nanocomposites as photocatalysts after 100 min under UV light, but the degradation rates were considerably increased in the presence of photocatalysts.
According to photocatalytic yield calculated by Eq. (1), the percent degradation of rhodamine B for Fe3O4/CoWO4 nanocomposites were about 98%. The percent degradation for Fe3O4 was about 66% after 180 (Fig. 8). The results show that the photocatalytic activity was enhanced after CoWO4 particles were deposited on the surface of the Fe3O4 for degradation of RhB. Therefore, when the Fe3O4/CoWO4 nanocomposites were used as photocatalyst for degradation of dye molecules, the photocatalytic efficiency of this nanostructure was better than Fe3O4 NPs. These results demonstrated a high degree of the Fe3O4/CoWO4 nanocomposites (Sample No. 2) to be employed as a favorable, suitable, and new type of photocatalyst under visible light for elimination of Rh B dye. The composition procedure of contaminants for the Fe3O4/CoWO4 nanocomposites has been shown in Scheme 1 [23,24,25]. To the antimicrobial activity of Fe3O4/CoWO4 nanocomposites was evaluated against a set of 11 microorganisms and their potency were assessed qualitatively and quantitatively by the presence or absence of inhibition zones, zone diameters and MIC values. The results are given in Table 2 and indicate that, at tested concentrations, Fe3O4/CoWO4 nanocomposites has no antimicrobial activity against tested microorganisms.
4 Conclusions
In this research, we have synthesized a novel Fe3O4/CoWO4 photocatalyst by means of an easy and prompt route based on the precipitation method. The VSM results showed that samples presented a high degree of superparamagnetism. TEM revealed the size distribution of Fe3O4/CoWO4 nanocomposites. Observed Fe3O4/CoWO4 nanocomposites were approximately spherical. Results show that Fe3O4/CoWO4 nanocomposites is synthesized homogenously, without further impurity. Prepared nanocomposite shows excellent photocatalytic behavior toward Rh B under visible light. To the antimicrobial activity of Fe3O4/CoWO4 nanocomposites was evaluated against a set of 11 microorganisms were assessed qualitatively.
References
H.O. Seo, C.W. Sim, K.D. Kim, Y.D. Kim, D.C. Lim, Chem. Eng. J. 183, 381 (2012)
W. Yu, J. Shi, L. Wang, X. Chen, M. Min, L. Wang, Y. Liu, Aquac. Fish. 2(1), 34 (2017)
T. Reemtsma, M. Jekel, Organic Pollutants in the Water Cycle: Properties, Occurrence, Analysis and Environmental Relevance of Polar Compounds, (Wiley, Weinheim, 2006)
B. Zhao, L. Gao, W. Liao, B. Zhang, Geo-spat. Inf. Sci. 20, 309 (2017)
J. Amania, M. Malekia, A. Khoshrooa, A. Sobhani-Nasabb, M. Rahimi-Nasrabadi, Anal. Biochem. 548, 53–59 (2018)
A. Sobhani-Nasab, A. Ziarati, M. Rahimi-Nasrabadi, M.R. Ganjali, A. Badiei, Res. Chem. Intermed. 43 (11), 6155–6165
X. Guan, S. Liao, J. Bai, F. Wang, Z. Li, Q. Wen, J. He, T. Chen, Geo-spat. Inf. Sci. 20, 299 (2017)
A. Sobhani-Nasab, M. Rangraz-Jeddy, A. Avanes, M. Salavati, J. Mater. Sci. Mater. Electron. 26, 9552–9560 (2015)
H.R. Naderi, A. Sobhani-Nasab, M. Rahimi-Nasrabadi, M.R. Ganjali, Appl. Surf. Sci. 423, 1025 (2017)
A. Sobhani-Nasab, Z. Zahraei, M. Akbari, M. Maddahfar, S.M. Hosseinpour-Mashkani, J. Mol. Struct. 1139, 430–435 (2017)
A. Keler, J.M. Krisp, L. Ding, Geo-spat. Inf. Sci. 20, 333 (2017)
A. Khoshroo, L. Hosseinzadeh, A. Sobhani-Nasab, M. Rahimi-Nasrabadi, H. Ehrlich, J. Electroanal. Chem. 823, 61–66 (2018)
S. Pourmasoud, A. Sobhani-Nasab, M. Behpour, M. Rahimi-Nasrabadi, F. Ahmadi, J. Mol. Struct. 1157, 607 (2018)
S.S. Hosseinpour-Mashkani, A. Sobhani-Nasab, J. Mater. Sci. Mater. Electron. 28(21), 16459 (2017)
M. Rahimi-Nasrabadi, F. Ahmadi, A. Fosooni, J. Mater. Sci. Mater. Electron. 28, 537–542 (2017)
Y.F. Shena, J. Tanga, Z.H. Nie, Y.D. Wang, Y. Ren, L. Zuo, Sep. Purif. Technol. 68, 312 (2009)
S.M. Pourmortazavi, M. Rahimi-Nasrabadi, M. Khalilian-Shalamzari, H.R. Ghaeni, S.S. Hajimirsadeghi, J. Inorg. Organomet. Polym. Mater. 24, 333 (2014)
CLSI, Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fifth Informational Supplement. CLSI document M100-S25 (Clinical and Laboratory Standards Institute, Wayne, 2015)
M. Gulluce, M. Sokmen, F. Sahin, A. Sokmen, A. Adiguzel, H. Ozer, J. Sci. Food Agric. 84, 735 (2004)
A. Sobhani-Nasab, M. Rahimi-Nasrabadi, H. RezaNaderi, V. Pourmohamadian, F. Ahmadi, M.R. Ganjali, H. Ehrlich, Ultrason. Sonochem. 45, 189 (2018)
M. Eghbali-Arania, A. Sobhani-Nasabb, M. Rahimi-Nasrabadic, F. Ahmadid, S. Pourmasoud, Ultrason. Sonochem. 43, 120 (2018)
M. Ghiyasiyan-Arani, M. Salavati-Niasari, S. Naseh, Ultrason. Sonochem. 39, 494 (2017)
H. Yang, L. Yu, J. For. Res. 28(2), 395 (2017)
M. Shekofteh-Gohari, A. Habibi-Yangjeh, Ceram. Int. 43, 3063–3071 (2017)
M. Eghbali-Arani, A. Sobhani-Nasab, M. Rahimi-Nasrabadi, S. Pourmasoud, J. Electron. Mater. 47, 3757 (2018)
Acknowledgements
Authors are grateful to council of University of Kashan (7532666514) for providing financial support to undertake this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maddah, B., Jookar-Kashi, F. & Akbari, M. Facile precipitation synthesis of pure Fe3O4/CoWO4 nanocomposites and investigation of their photocatalyst and antimicrobial activity. J Mater Sci: Mater Electron 29, 13723–13730 (2018). https://doi.org/10.1007/s10854-018-9502-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-018-9502-5