Abstract
High performance dielectric materials are highly required for practical application for energy storage technologies. In this work, high-k pristine and modified calcium copper titanate having nominal formula Ca0.95Nd0.05Cu3Ti4−xZrxO12 (x = 0.01, 0.03 & 0.10) were synthesized and characterized for structural and dielectric properties. Single phase formation of the synthesized compositions was confirmed by X-ray diffraction patterns and further analysed using Rietveld refinement technique. Phase purity of the synthesized ceramics was further confirmed by Energy-dispersive X-ray Spectroscopy (EDX) analysis. SEM images demonstrated that grain size of the modified CCTO ceramics was controlled by Zr4+ ions due to solute drag effect. Impedance spectroscopy was employed to understand the grain, grain boundaries and electrode contribution to the dielectric response. Nyquist plots were fitted with a 2R-CPE model which confirms the non-ideality of the system. Substitution of specific concentration of Nd and Zr improved the dielectric properties of high dielectric permittivity (ε′ ~ 16,902) and minimal tanδ (≤ 0.10) over a wide frequency range. The giant ε′ of the investigated system was attributed to internal barrier layer capacitance (IBLC) effect and reduced tanδ accredited to enhanced grain boundaries resistance due to substitution of Zr4+ ions at Ti4+ site.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In the present age of science, there is increasing attention to miniaturization of electronic devices with efficient performance. For this, continuous interest to develop high performance dielectric materials useful for technological applications. Most of the ferroelectric materials such as barium titanate and lead zirconate titante exhibits excellent dielectric properties, but change in crystal structure and space group with temperature make them undesirable for many practical applications. Recently, CaCu3Ti4O12 (CCTO) has been very much famous material due to its high dielectric permittivity (ε′ ~ 104) in single and polycrystalline ceramics, nearly frequency independent up to1 MHz and without any structural changes in the temperature range100–400 K [1]. These extraordinary features make CCTO more technical, but one serious drawback is that the loss tangent (tanδ ≥ 0.10) is too high which hinder its many commercial applications. So, investigate to origin of high tanδ value and reduce to this value is an urgent issue that should be intensively performed. Generally, solid-state reaction technique is used to synthesize the CCTO from CaCO3, CuO, and TiO2 at high temperatures that accompains many drawbacks like heterogeneity of precursor materials and long reaction time [2, 3]. Another drawbacks is formation of secondary phases during the synthesis because of limited atomic diffusion through micrometer-sized grains.the presence of secondary phases increase the disorder at the grain boundaries and leads high tanδ values [4, 5]. It is well known that the electric properties can be remarkably enhanced when ceramic exihibits homogeneous microstructure. Other effective synthesis methods such as; wet chemistry route, sol–gel, solution combustion synthesis, sonochemical-assisted route, co-precipitation [6,7,8,9,10,11,12] and Pechini sol–gel method are available which can improve CCTO characteristics, especially dielectric loss. The another factor that contributing to high tanδ in CCTO is the high DC conductivity (σdc) at lower frequencies which follows the relationship \(\tan \delta \approx {\sigma _{dc}}/\omega {\varepsilon _0}\varepsilon ^{\prime}\), where ε′ is the dielectric permittivity at lower frequencies [13]. As per IBLC model, the CCTO has electrically heterogeneous microstructure consisting of semiconducting grains isolated by insulating grain boundaries (GBs) and the best approach to minimise the dielectric loss in CCTO is to control the overall insulating properties of the bulk ceramics that might be achieve by increasing GBs layer resistance or via endorsing dense GBs networks [14, 15]. Ion doping in CCTO also found to be effective technique that strongly controls the morphology, particle size, electrical conductivity of grain (conducting/semiconducting nature) and GBs characteristics (thickness, permittivity and resistance) [14, 16]. Each dopant has its own characteristics and affect the microstructure (sintering kinetics and grain growth) as well as modifies the electrical properties of the host system [17]. Therefore, the selection of dopant should be done in such a way that it should tune the grain and GBs characteristics to attain best dielectric results. Rare earth elements (REEs) are lithophile elements that exihibits stable oxidation state (+ 3) (except for Ce+4 and Eu2+ in some environments). The stable valence/oxidation state allow for liberal substitution of the REEs into various crystal lattices to alter the existing various properties [18]. To improve the dielectric properties of CCTO, plenty of works have been done on modified CCTO with rare earth ions such as; La3+ [19,20,21], Eu3+ [19, 22], Y3+ [19, 23], Gd3+ [14, 19, 23], Yb3+ [24], Sm3+ [25] and the dielectric losses were found to be quite low. For example, La3+ substitution into CCTO reduced tanδ value to be less than 0.03, but simultaneously it worsens other dielectric parameter (large decrease in ε′ values) also [20]. Recently, Xu et al. reported that co-substitution of rare earth and transition metal (Y + Zr) in CCTO greatly improved the dielectric properties [26]. A very low value of tanδ ~ 0.039 alongwith high ε′ ~ 10,196) was achieved at room temperature at 1 kHz. Nd element exist in Nd3+ state and have comparable ionic radii to the Ca2+ ions among all the rare earth elements. Wang et al. [27] studied the effects of Nd-substitution on microstructures and dielectric characteristics of CCTO ceramic and observed that both ε′ and dielectric loss were greatly affected with Nd concentration. Later on, Wang et al. [28] synthesized Nd doped Ca1−3x/2NdxCu3Ti4O12 ceramics and a moderate ε′ value (~ 11,200) with low tanδ (~ 0.12 at 10 kHz) was achieved at room temperature. It is well known that Zr4+ cation is more stable than Ti4+ cation and substitution of Zr4+ at Ti4+ ions in perovskite oxide ceramics leads to outstanding dielectric properties for useful applications [29,30,31,32]. With this motivation, both Nd3+ and Zr4+ were simultaneously doped into CCTO matrix and characterised to understand the electrical properties. There are several reports on on separately Nd and Zr doped CCTO ceramics, but, none about the co-substitution of Nd3+ and Zr4+ ions. Recently, we have studied the composition Ca0.95Nd0.05Cu3Ti3.95Zr0.05O12 co-doped with Nd and Zr and a giant dielectric permittivity (ε′ ~ 19,426) with minimal dielectric loss (tanδ ~ 0.067 at 1 kHz) was achieved [33]. So, the aim of the present work is to explore the concentration of both the ions to get even better dielectric properties. Nd and Zr co-substituted CCTO compositions have been synthesized and effect of concentration of both ions on the microstructure and dielectric properties is investigated in detail.
2 Experimental
Polycrystalline pure and modified CCTO have nominal compositions Ca0.95Nd0.05Cu3Ti4−xZrxO12 (x = 0.01, 0.03, & 0.10) abbreviated as (CCTO, CCTO1, CCTO2& CCTO3) were synthesized via solid state method. The starting chemicals CaCO3 (99%), CuO (99%), Nd2O3 (99%), TiO2 (99.9%) and ZrO2 (99.9%) were taken in stoichiometric ratio, ball milled and then calcined at 1000 °C for 12 h in a conventional furnace. The mixture was dried at hot plate for half an hour. PVA as a binder was added to the mixture to make the pellet of thickness 1–2 mm and diameter ~ 10 mm using uniaxial hydraulic pressing with a pressure of 20 MPa. Sintering was done at 1080 °C for 6 h. X-ray diffraction (XRD) pattern was collected at room temperature using Rigaku Miniflex-II X-Ray Diffractometer with CuKα (λ = 1.541 Å) radiation in a wide range of 2θ (10° < 2θ < 80°). Simulations of collected XRD data by the Rietveld profile method using Fullprof software package [34]. Microstructure of fractured surface was observed by scanning electron microscope (Semtrac Mini, Nikkiso, Japan). The elemental composition was confirmed by EDX technique. For electroding, both flat surfaces of the pellets were painted with air-drying conducting silver paste to study the electrical properties. Dielectric measurement was performed using HIOKI 3532-50 LCR HITESTER in the frequency range 100 Hz–5 MHz at room temperature.
3 Result and discussion
3.1 Structural and microstructure analysis
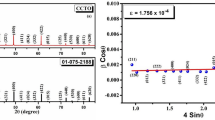
X-ray diffraction patterns along with a magnified view of most intense diffraction peak (~ 2θ = 34°) for all the modified CCTO compositions are shown in Fig. 1. The well defined diffraction peaks without any secondary phase indicate that substituted dopants occupies their assigned site and did not influenced the crystal symmetry. Further, XRD data were analysed to calculate the lattice parameters for all compositions using Rietveld refinement. CCTO are known to have double perovskite cubic structure described by the space group Im3 [1].The refinement of the collected XRD data was performed using cubic crystal symmetry with space group Im3 Initially, the zero point shift, unit cell and background and then all input parameters required for the simulation were refined. Pseudo-Voigt function was employed to fit the diffraction peak profile. Rietveld refined X-ray pattern of all prepared compositions are shown in Fig. 2. A low value of goodness of fit (χ2) factor for all the compositions indicates good fitting between the theoretical and experimental data and refined structural parameters are listed in Table 1. A small decrease in lattice parameters and unit cell volume was observed for CCTO1 ceramic as compared to pure CCTO might be due to substitution of effective small ionic radii of Nd3+ (1.27 Å) at Ca2+ (1.35 Å) site. For CCTO2 and CCTO3 ceramics the trend is reversed because of the dominance effect of Zr4+ ions at Ti4+ site. The average crystallite size (D) was estimated by Debye Scherrer formula, for highly intense diffraction peak (at around ~ 2θ = 34°).
where, K ~ 0.9 (constant), θ is the Bragg’s angle and β is Full Width Half Maxima (in Radian). The average crystallite size obtained from the XRD analysis is reported in Table 2. Theoretical X-ray density (dx) for all the samples was calculated by the following equation;
where all the parameters have their usual meanings. The experimental bulk density (dexp) for all the samples was calculated by Archimedes principle using Xylene as buoyant liquid. The porosity was also calculated using the equation;
The calculated values of all the parameters are summarized in Table 2.
Figure 3a–d shows the SEM micrographs of the fractured surface of all the CCTO compositions. Images clearly indicated that the presence of dopants strongly influenced the microstructure. The mean grain size of the doped CCTO was decreased with increase in subsequent concentrations of Zr4+ ions. For undoped CCTO, the abnormal grain formation was appeared which usually occurs due to presence of CuO/Cu2O liquid phase formation at grain boundaries due to high temperature sintering process [35]. With subsequent doping of Nd and Zr in CCTO lattice, the grain growth is suppressed and normal grain matrix starts to appear. These observations indicate that the grain growth of the system was strongly controlled by of Zr4+ ions due to solute drag effect. As per solute theory substituted aliovalent ion with larger radii than host ion strongly segregate at grain boundaries and restricts the grain boundary movement. The similar microstructure observation was also reported earlier [36]. The average grain size for all the samples are listed in Table 2. The chemical composition and purity of product was confirmed through EDX technique. As illustrated in Fig. 4a–e, pure CCTO ceramic composed of three different elements, Ca, Cu and Ti whereas, Nd and Zr co-doped CCTO ceramics have five different elements, Ca, Nd, Cu, Ti, and Zr. Furthermore, no impurity peaks were seen which indicate high purity of as-prepared CCTO ceramics.
3.2 Impedance spectroscopy
In the present section, we undertook the impedance analysis for the synthesized ceramics for revealing the effect of dopants on electrical properties and to recognize the various transport mechanisms existing within the material. Figure 5 shows the simulated complex impedance plane plots at 303 K for pure and doped CCTO compositions.The Nyquist plots elucidate that all samples exhibit single semicircular arc in the measured frequency range, which may be resultant of two or more relaxation process, respectively. Generally, high frequency semicircular arc identifies as bulk contribution, whereas the arc at intermediate frequencies ascribes as GBs response, while an electrode interface effect commonly appears at very low frequency domain. For all the synthesized ceramics, a single semicircular arc appears and the diameter of arc continuously increases with subsequent doping of Zr4+ ions in CCTO lattice. To facilitate the exact information about the contribution of grain and grain boundaries to the total dielectric response, impedance data were simulated using two R-CPE equivalent circuit model. CPE has been adopted instead of conventional capicatance (C) to accommodate the non-ideality of the system, which might be due to multiple relaxation time, non-uniform current distribution and surface roughness [37]. The frequency dependence of real and imaginary part of the impedance of the equivalent circuit can be expressed as [38].
where Rg and Cg are grain resistance and capacitance, respectively, and Rgb and Cgb are the corresponding quantities for grain boundary.
The capacitance of CPE can be calculated using the relationship;
where the value of n represents ideality (Debye or non-Debye behaviour) of the system. The calculated parameters [resistances (Rg, Rgb) and CPEs (Qg, Qgb)] of fitted equivalent circuit are listed in Table 3. Further, looking at table results it is clear that grain resistance continuously decreases and GBs resistance increases with subsequent doping of Zr4+ ions. The GBs resistance value is approximately 10 times of magnitudes of grains resistance which signifies that the system are electrically heterogenous and approaches to IBLC structure. Therefore, impedance results suggest that the dielectric response of the investigated system may be accredited to IBLC effect.
3.3 Dielectric properties
Figure 6a, b represents the frequency dependent behaviour of ε′ and tan δ at room temperature for all the compositions. It is clear that all the sample exhibits low frequency dielectric dispersion with high value of ε’ is attributed due to Maxwell–Wanger effect or can be understood with Koop’s theory [39, 40]. The impedance spectroscopy also identifies the co-existence of insulating regions (GBs) with less resistive regions (grains) results to the dielectric heterogeneity. In the low frequency domain, the charge carriers cannot migrate easily through highly resistive grain boundaries and piles up in interfacial layers gives rise to strong polarization and result in enhanced values of ε′. At high frequencies the decrease in ε’ is observed due to lack of ability of electric dipoles to switch with the applied electric field leading to a lower dipolar response. According to Koop’s model, at lower frequencies high energy is required by the charge carriers to move across the resistive GBs and leads to high tanδ values. Pure CCTO exhibits frequency independent plateau with giant ε′ value and a sudden drop in ε′ over 400 kHz might be due to the conductive nature of grains [41]. The giant value of ε′ ~ 25,345 at 1 kHz for undoped CCTO drops to ε′ ~ 16,902 for CCTO3 by addition of Nd3+and Zr4+ ions in lattice, but the high frequency relaxation shifted toward higher frequencies which is a desirable feature for device applications. The decrease in dielectric permittivity may attributed to decrease in grain size which can be explained by IBLC model introduced by Adams et al. [42]. As per this model, the dielectric permittivity can be correlated to the average grain size and thickness of grain boundary is given by the relationship as follow;
where tg represents the mean grain size and tgb corresponds to the grain boundary thickness. Equation (1) suggests that the effective dielectric permittivity is directly related to grain size and inversely related to grain boundary thickness. Due to the large number of grain boundaries embedded in the pool of the grains in polycrystalline materials, it is very difficult to analyse the impact of grain boundary thickness of ε′ and hence the grain size effect dominates to determine the effective dielectric permittivity. Therefore, the observed dielectric behaviour of the present system can be explained by the grain size effect. Further, the charge compensation is required for lattice neutralization due to replacement of Ca2+ by Nd3+ ions in CCTO matrix. This charge compensation may preserve the large value of ε′ and prevents the proportionate drop in dielectric constant values with a decrease in grain size. The investigated system was valuable only their dielectric losses were also controlled. As shown in Fig. 5b, the tanδ spectra are similar for all the compositions, but values of tanδ are different significantly. Especially, for the CCTO3 composition, the value of tanδ is less than 0.10 in wide frequency range 1–200 kHz with the minimal value of 0.031 at 2.1 kHz at room temperature which is 85% lower than that of undoped CCTO. Zr4+ has larger ionic radii than Ti4+, the significant size mismatch between these cations cause a strong driving force that segerate Zr4+ ions at grain boundaries which enhances the GBs resistance. This was primarily due to enhanced GB density occurring from reduction in the mean grain size by the solute drag effect. The trend of Rgb is well supported with observed tanδ spectra and enhanced Rgb values are responsible for low loss over a broad range of frequencies (inset Fig. 5b). The low value of tanδ with high ε′ value in wide frequency region is greatly desired for energy storage technologies. Therefore, the studied system can be very useful for practical applications.
4 Conclusion
Nd and Zr modified CCTO ceramics were successfully synthesized by conventional solid state route. The XRD analysis confirmed that all the modified CCTO ceramics remained cubic in structure. The variation in lattice parameters and unit cell volume analyzed by Rietveld refinement of XRD data surely indentified that the substituted dopants successfully incorporated in CCTO lattice. Analysis of SEM images revealed that microstructure was strongly affected by Nd and Zr co-substitution and its direct impact reflected on dielectric properties. A huge value of dielectric permittivity (ɛr ~ 16,902) with nominal dielectric losses (tan δ ~ 0.067 at 1 kHz) was observed in a broad frequency range from 103 to 105 Hz for CCTO3 ceramic. Thus, it is concluded that the both dopant ions have played an independent role to improve the dielectric performance of CCTO system. A′ site Ca substitution by Nd may be attributed to enhancement in dielectric permittivity, and Zr ions suppressed the grain growth and yielded a high resistive GBs network that strongly favours the improved the dielectric performance of CCTO system.
References
M.A. Subramanian, D. Li, N. Duan, B.A. Reisner, A.W. Sleight, High dielectric constant in ACu3Ti4O12 and ACu3Ti3FeO12 phases. J. Solid State Chem. 151, 323–325 (2000)
M.M. Ahmad, E. Al-Libidi, A. Al-Jaafari, S. Ghazanfar, K. Yamada, Mechanochemical synthesis and giant dielectric properties of CaCu3Ti4O12. Appl. Phys. A 116, 1299–1306 (2014)
V.P.B. Marques, A. Ries, A.Z. Simoes, M.A. Ramrez, J.A. Varela, E. Longo, Evolution of CaCu3Ti4O12 varistor properties during heat treatment in vacuum. Ceram. Int. 33, 1187–1190 (2007)
S. G-Fritsch, T. Lebey, M. Boulous, B. Durand, Dielectric properties of CaCu3Ti4O12 based multiphased ceramics. J. Eur. Ceram. Soc. 26, 1245–1257 (2006)
M.A. de la Rubia, P. Leret, A. Del Campo, R.E. Alonso, A.R. Lopez-Garcia, J.F. Fernandez, J. De Frutos, Dielectric behaviour of Hf-doped CaCu3Ti4O12 ceramics obtained by conventional synthesis and reactive sintering. J. Eur. Ceram. Soc. 32, 169–1699 (2012)
W.M. Hua, Z. Fu, W.Q. Li, Y. Chao, Synthesis of CaCu3Ti4O12 powders and ceramics by sol–gel method using decanedioic acid and its dielectric properties. J. Cent. South Univ. 19, 3385–3389 (2012)
N. Banerjee, S.B. Krupanidhi, Low temperature synthesis of nano-crystalline CaCu3Ti4O12 through a fuel mediated autocombustion pathway. Curr. Nanosci. 6, 432–438 (2010)
M. Eghbali-Arani, A. Sobhani-Nasab, M. Rahimi-Nasrabadi, F. Ahmadi, S. Pourmasoud, Ultrasound-assisted synthesis of YbVO4 nanostructure and YbVO4/CuWO4 nanocomposites for enhanced photocatalytic degradation of organic dyes under visible light. Ultrason. Sonochem. 43, 120–135 (2018)
S. Pourmasoud, A. Sobhani-Nasab, M. Behpour, M. Rahimi-Nasrabadi, F. Ahmadi, Investigation of optical properties and the photocatalytic activity of synthesized YbYO4 nanoparticles and YbVO4/NiWO4 nanocomposites by polymeric capping agents. J. Mol. Struc. 1157, 607–615 (2018)
M. Salavati-Niasari, F. Soofivand, A. Sobhani-Nasab, M. Shakouri-Arani, M. Hamadanian, S. Bagheri, Facile synthesis and characterization of CdTiO3 nanoparticles by Pechini sol-gel method. J. Mater. Sci.: Mater. Electron. 28, 14965–14973 (2017)
A. Sobhani-Nasab, A. Ziarati, M. Rahimi-Nasrabadi, M.R. Ganjali, A. Badiei, Five-component domino synthesis of tetrahydropyridines using hexagonal PbCrxFe12–xO19 as efficient magnetic nanocatalyst. Res. Chem. Intermed. 43, 6155–6165 (2017)
S.S. Hosseinpour-Mashkani, A. Sobhani-Nasab, Investigation the effect of temperature and polymeric capping agents on the size and photocatalytic properties of NdVO4 nanoparticles. J. Mater. Sci.: Mater. Electron. 28, 16459–16466 (2017)
S. Vangchangyia, E. Swatsitang, P. Thongbai, S. Pinitsoontorn, T. Yamwong, S. Maensiri, V. Amornkitbamrung, P. Chindaprasirt, Very low loss tangent and high dielectric permittivity in pure-CaCu3Ti4O12 ceramics prepared by a modified sol-gel process. J. Am. Ceram. Soc. 95, 1497–1500 (2012)
R. Kashyap, O.P. Thakur, R.P. Tandon, Study of structural, dielectric and electrical conduction behaviour of Gd substituted CaCu3Ti4O12 ceramics. Ceram. Int. 38, 3029–3037 (2012)
L. Feng, X. Tang, Y. Yan, X. Chen, Z. Jiao, G. Cao, Decrease of dielectric loss in CaCu3Ti4O12 ceramics by La doping. Phys. Status Solidi A 203, R22–R24 (2006)
C.H. Mu, P. Liu, Y. He, J.P. Zhou, H.W. Zhang, An effective method to decrease dielectric loss of CaCu3Ti4O12 ceramics. J. Alloys Compd. 471, 137–141 (2009)
R. Kumari, N. Ahlawat, A. Agarwal, S. Sanghi, M. Sindhu, S. Rani, Effect of doping of alkaline metal ions on structural and electrical properties of Bi0.8M0.2FeO3-modified Na0.5Bi0.5TiO3 ceramics (M = Ca,Sr, and Ba. J. Alloys Compd. 747, 712–720 (2018)
B. Castor, B. James Hedrick, in Industrial Minerals and Rocks, ed. by J. E. Kogel, N. C. Trivedi, J. M. Barker (Society for mining, Metallurgy, and Exploration, United State, 2006), pp. 769–792
L. Liu, L. Fang, Y. Huang, Y. Li, D. Shi, S. Zheng, S. Wu, C. Hu, Dielectric and nonlinear current–voltage characteristics of rare–earth doped CaCu3Ti4O12 ceramics. J. Appl. Phys. 110, 094101 (2011)
L. Feng, X. Tang, Y. Yan, X. Chen, Z. Jiao, G. Cao, Decrease of dielectric loss in CaCu3Ti4O12 ceramics by La doping. Phys. Status Solidi (a) 203, R22 (2006)
B.S. Prakash, K.B.R. Varma, Microstructural and dielectric properties of donor doped (La3+) CaCu3Ti4O12 ceramics. J. Mater. Sci. Mater. Electron. 17, 899–907 (2006)
T. Li, Y. Xue, Z. Chen, F. Chang, Dielectric characteristics and positron annihilation study of Eu2O3-doped CaCu3Ti4O12 ceramics. Mater. Sci. Eng. B 158, 58–62 (2009)
C. Mu, H. Zhang, Y. Liu, Y. Song, P. Liu, Rare earth doped CaCu3Ti4O12 electronic ceramics for high frequency applications. J. Rare Earths 28, 43–47 (2010)
J. Boonlakhorn, P. Thongbai, B. Putasaeng, T. Yamwong, S. Maensiri, Very high-performance dielectric properties of Ca1–3x/2Yb x Cu3Ti4O12 ceramics. J. Alloys Compd. 612, 103–109 (2014)
J. Li, B. Fu, H. Lu, C. Huang, J.W. Sheng, Dielectric properties of Sm-doped CaCu3Ti4O12 ceramics. Ceram. Int. 39, S149-S152 (2013)
Z. Xu, H. Qiang, Y. Chen, Z. Chen, Microstructure and enhanced dielectric properties of yttrium and zirconium co-doped CaCu3Ti4O12 ceramics. Mater. Chem. Phys. 191, 1–5 (2017)
Y. Wang, L. Ni, X.M. Chen, Effects of Nd-substitution on microstructures and dielectric characteristics of CaCu3Ti4O12 ceramics. J. Mater. Sci.: Mater. Electron. 22, 345–350 (2012)
M.H. Wang, B. Zhang, F. Zhou, C. Yao, Microstructure and dielectric properties of Nd doped CaCu3Ti4O12 synthesized by sol–gel method. J. Sol-Gel. Sci. Technol. 69, 281–287 (2014)
S. Mahajan, O.P. Thakur, C. Prakash, K. Sreeniwas, Effect of Zr on dielectric, ferroelectric and impedance properties of BaTiO3 ceramic. Bull. Mater. Sci. 34, 1483–1489 (2011)
K.M. Sangwan, N. Ahlawat, R.S. Kundu, S. Rani, S. Rani, N. Ahlawat, Improved dielectric and ferroelectric properties of Mn doped Barium zirconium titanate (BZT) ceramics for energy storage applications. J. Phys. Chem. Solids 117, 158–166 (2018)
Q.G. Chi, L. Gao, X. Wang, J.Q. Lin, J. Sun, Q. Lei, Effects of Zr doping on the microstructures and dielectric properties of CaCu3Ti4O12 ceramics. J. Alloys Compd. 559, 45–48 (2013)
K.M. Sangwan, N. Ahlawat, R.S. Kundu, S. Rani, S. Rani, N. Ahlawat, Influence of Mn doping on electrical conductivity of lead free BaZrTiO3 perovskite ceramic. Cer. Int. https://doi.org/10.1016/j.ceramint.2018.03.039
S. Rani, N. Ahlawat, R.S. Kundu, R. Punia, S. Kumar, K.M. Sangwan, N. Ahlawat, Structural and dielectric properties of Ca0.95Nd0.05Cu3Ti3.95Zr0.05O12 ceramic. Ferroelectrics 516, 156–166 (2017)
J.R. Carvajal, Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B 192, 55–69 (1993)
D.W. Kim, T.G. Kim, F.S. Hong, Low firing of CuO-doped anatase. Mater. Res. Bull. 34, 771–781 (1999)
L.J. Liu, Y.M. Huang, Y.H. Li, D.P. Shi, S.Y. Zheng, S.S. Wu, L. Fang, C.Z. Hu, Dielectric and non-Ohmic properties of CaCu3Ti4O12 ceramics modified with NiO, SnO2, SiO2, and Al2O3 additives. J. Mater. Sci. 47, 2294–2299 (2012)
S. Rani, N. Ahlawat, R. Punia, K.M. Sangwan, S. Rani, Dielectric relaxation and conduction mechanism of complex perovskite Ca0.90Sr0.10Cu3Ti3.95Zn0.05O12 ceramic. Ceram. Int. 44, 5996–6001 (2018)
J. Liu, C. Duan, W.N. Mei, R.W. Smith, J.R. Hardy, Dielectric properties and Maxwell–Wanger relaxation compounds ACaCu3Ti4O12 (A = Ca,Bi2∕3,Y2∕3,La2∕3). J. Appl. Phys. 98, 093703 (2005)
J.C. Maxwell, Electricity and Magnetism, 1st edn. (Oxford University Press, Oxford, 1929)
C.G. Koops, On the dispersion of resistivity and dielectric constant of some semiconductors at audiofrequencies. Phys. Rev. 83, 121–124 (1951)
T.B. Adams, D.C. Sinclair, A.R. West, Giant barrier layer capacitance effects in CaCu3Ti4O12 ceramics. Adv. Mater. 14, 1321 (2002)
T.B. Adams, D.C. Sinclair, A.R. West, Characterization of grain boundary impedances in fine-and coarse-grained CaCu3Ti4O12 ceramics. Phys. Rev. B 73, 94124–94126 (2009)
Acknowledgements
Authors are thankful to Department of Science and Technology, New Delhi for providing XRD facilities (FIST scheme). One of the author is thankful to the University Grants Commission, New Delhi for providing financial assistance through major research project (F. No. 41-853/2012 (SR)).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rani, S., Ahlawat, N., Sangwan, K.M. et al. Structural investigation and giant dielectric response of CaCu3Ti4O12 ceramic by Nd/Zr co-doping for energy storage applications. J Mater Sci: Mater Electron 29, 10825–10833 (2018). https://doi.org/10.1007/s10854-018-9150-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-018-9150-9