Abstract
Core–shell structured CaCu3Ti4O12@TiO2 (CCTO@TiO2) nanofibers were prepared via a normal coaxial electrospinning technique with sol precursors. Polyimide (PI) nanocomposite films containing the core–shell structured CCTO@TiO2 nanofibers were fabricated by the solution casting method. The core–shell structure of the CCTO@TiO2 nanofibers was confirmed through transmission electron microscope. The percolation of the CCTO/TiO2 interfaces leads to much enhanced interfacial polarization of the CCTO@TiO2 nanofibers, which gives rise to substantially increased dielectric constant of the nanocomposites. Compared to the nanocomposites with CCTO nanofibers, the breakdown strength of the nanocomposites with CCTO@TiO2 nanofibers is also increased due to the charge shifting is limited to the interfacial zone of CCTO/TiO2 interfaces, instead of in the PI matrix to form a percolation path. For the nanocomposites with 5 vol% nanofibers, the dielectric constant of 5.55 was enhanced to 5.85 and the breakdown strength of 201 kV/mm was increased to 236 kV/mm by utilizing the TiO2 coated CCTO nanofibers, while the dielectric loss shows no obvious change. Meanwhile, the PI nanocomposite film filled with 1 vol% CCTO@TiO2 nanofibers exhibits a maximal energy density of 1.6 J/cm3. The core–shell structured nanofibers open up an effective way to optimize the dielectric properties of polymer nanocomposites with high energy density.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Polymeric matrix composites with high dielectric permittivity, low dielectric loss and high breakdown strength have received tremendous attentions because of their widely range of applications in energy storage, electronic fields and organic thin film transistors [1,2,3,4,5,6,7,8,9]. Compared with traditional inorganic dielectric materials, the polymeric matrix composites are more potential because of their excellent performance such as good flexibility, processability and dielectric strength. Polyimide (PI) is one of the candidates due to its good thermal stability, low moisture absorption, ease of film preparation and good mechanical properties [10, 11]. However, most polymers are originally low dielectric constant which fail to meet the requirement of dielectric performance. Thus, developing polymer matrix composites with high dielectric constant can be a promising alternative to solve the problem.
Generally, there are two possible ways to increase the dielectric permittivity of polymers. One strategy is to introduce conductive nanofillers (e.g., Ag particles, carbon fibers, carbon nanotube and polyaniline) into polymers [12,13,14,15,16,17,18]. Ultra-high dielectric constant can be achieved for these polymeric matrix composites but with a few defects such as a relatively high conductivity, high loss tangent and low breakdown strength when little concentration of conductive nanofillers were added. Another approach is adding ceramic nanofillers with high dielectric permittivity such as TiO2, ZrO2, BaTiO3 (BT), and CaCu3Ti4O12 (CCTO) into polymers [19,20,21,22,23,24,25,26,27,28,29,30]. Unfortunately, the ceramic/polymer composites need a large nanofiller loading (50 vol%) to get a high dielectric constant, which results in the composites lose their advantages such as low dielectric loss, high breakdown strength, flexibility and uniformity. The stored energy density (Uc) of a dielectric material is expressed as \({\text{Uc}}=\int EdD,\) where D is the electric displacement and E is the applied electric field [31]. Therefore, to increase the maximum Uc of dielectric materials, it is extremely important to develop dielectric materials with maximal applied electric field it can be applied and high dielectric permittivity which is related to the electric displacement.
Theoretical calculation and experimental studies have found the dielectric nanofibers with a high aspect ratio can increase the dielectric constant as well as breakdown strengths effectively at a low concentrations (1–5 vol%), which breaks the paradox of high dielectric constant with decreased breakdown strength [32,33,34,35]. The nanofibers could effectively reduce the surface energy and prevent their aggregation in the polymeric matrix composites, which contributes to the enhanced breakdown strengths. However, the breakdown strength of nanocomposites still decreased with high loading of nanofibers, due to the more structure imperfections and electric field concentration exist at the interface between the nanofibers and the polymeric matrix composites.
Interface polarization is the dominant mechanism of polarization in the polymeric matrix nanocomposites. To modulate the interface polarization, nanofibers with core–shell structure were imported to realize it. Generally, high permittivity nanofibers coated with an inorganic buffer layer with moderate permittivity, can effectively reduce the local electric field and moderate the interfacial polarization in the polymer matrix [36]. Recently, some studies showed that the nanofibers with core–shell structure have effectively increased the energy density of nanocomposites. Zhang et al. found the energy density of polyvinylidene fluoride (PVDF) nanocomposite can be enhanced to 31.2 J/cm3 by the TiO2 nanofiber embedded BaTiO3 nanoparticles [37]. Liu et al. demonstrated that the discharged energy density of PVDF nanocomposite film could be significantly improved to 7.1 J/cm3 by the incorporation of BaTiO3@Al2O3 [32]. Our group demonstrated that the energy density of the PI nanocomposites increased to 2.31 J/cm3 containing a low volume fraction of core–shell structured BaTiO3@SiO2 nanofibers. The energy density of PI nanocomposites could be markedly enhanced by introducing the SiO2 shell on the surface of BaTiO3 nanofibers [38].
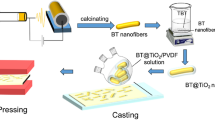
Along this line, a core–shell structure of CaCu3Ti4O12@TiO2 (CCTO@TiO2) nanofibers has been designed and prepared via coaxial electrospinning. The PI nanocomposite films consisting of the core–shell structured CCTO@TiO2 have been successfully synthesized by the solution casting method (Scheme 1). Compared with the CCTO/PI nanocomposites, the dielectric constant as well as the breakdown strength is highly enhanced in the CCTO@TiO2/PI nanocomposites. In addition, the nanocomposite with a 1 vol% of CCTO@TiO2 nanofibers raised the breakdown strength up to 299 kV/mm, which exhibits the maximal energy density of 1.6 J/cm3. This work shows the advantage of the core–shell nanofiber in enhancing the dielectric permittivity of polymeric matrix composites, and presents a novel strategy to efficiently increase the energy density.
2 Experimental
2.1 Preparation of the CCTO@TiO2 nanofibers
CCTO@TiO2 nanofibers were prepared by a normal coaxial electrospinning technique. Raw calcium nitrate terahydrate, copper acetate monohydrate and tetrabutyl titanate (TNBT) in a 1:3:4 target molar ratio were dissolved in ethanol with stirring to obtain a uniform precursor solution. Polyvinylpyrrolidone (M ~ 1,300,000) was used to control the sol viscosity of the solution. The TNBT sol solution was obtained through the same procedure. Then the two sol solutions were respectively transferred into two syringes. The coaxial electrospinning process was realized at electric field of 1.8 kV/cm. The as-electrospun nanofibers were calcined at 850 °C for 3 h to obtained CCTO@TiO2 nanofibers. To effectively improve the compatibility between the PI matrix and the nanofibers, dopamine was introduced into surface of the CCTO@TiO2 nanofibers. The CCTO nanofibers and CCTO@TiO2 nanofibers were respectively ultrasonic dispersed into 0.01 mol/L dopamine hydrochloride aqueous solution with stirring at 60 °C for 12 h. Then the dopamine-coated nanofibers were obtained by centrifugation and dried at 100 °C for 6 h.
2.2 Synthesis of polyamic acid (PAA)
The synthesis of PAA is shown in Scheme 2. Firstly, a certain amount of recrystallized diamino diphenyl ether (ODA) was added into the distilled NMP and stirred under the protection of nitrogen atmosphere and an ice bath. As ODA was fully dissolved in the NMP, pyromellitic dianhydride was added into the solution for three times. At last, the solution was stirred for 24 h to get the PAA solution with a content of 0.1 g/mL preserved in a desiccator for use.
2.3 Fabrication of the nanocomposite films
The nanocomposite films were prepared as follows. To form a homogeneous suspensions, the dopamine-coated CCTO@TiO2 nanofibers and PAA solution were proportionally ultrasonic dispersed in NMP, and then stirred for 24 h. The suspensions were poured into a glass mold and as-cast films were progressively dried at 60 °C for 2 h, 90 °C for 2 h, 120 °C for 2 h, 150 °C for 2 h, 180 °C for 2 h and 240 °C for 1 h. In the end, the films were transferred into a muffle furnace to completely imidize at 300 °C for 2 h. A series of PI based composite films (PI-1, PI-2, PI-3, PI-4 and PI-5) of different CCTO@TiO2 nanofibers contents (1, 2, 3, 4 and 5 vol%) were fabricated. For comparison, CCTO/PI composite films (PI–CCTO-1, PI–CCTO-2, PI–CCTO-3, PI–CCTO-4 and PI–CCTO-5) also were prepared by the same process as above. The thickness of the final nanocomposite films is ≈ 15 µm.
2.4 Characterization
X-ray diffraction (XRD) patterns were performed with a Bruker D8 Advance X-ray diffractometer using Cu Kα radiation (40 kV, 200 mA). Fourier transform Infrared (FTIR) transmission spectra were performed with a BRUKER-EQUINOX-55 IR spectrophotometer. The thermogravimetric analysis (TGA) was tested on TA Instruments SDT-Q600 with a heating rate of 20 °C/min and a nitrogen atmosphere with a gas flow rate of 100 mL/min. The microstructure of CCTO@TiO2 nanofibers was observed by a transmission electron microscope (TEM, Tecnai, and G2 T20). The morphologies of the cross-section and the in-plane of nanocomposite film were observed with a Hitachi S-4800 scanning electron microscope (SEM). X-ray photoelectron spectroscopy (XPS) measurements were performed with a Thermo Scientific K-Alpha using monochromatic Al Kα radiation. The dielectric permittivity and dielectric loss of the nanocomposite film were measured using a Hioki 3532-50 impedance analysis instrument (Ueda, Nagano, Japan) with a frequency range from 103 to 107 Hz at room temperature. Electric breakdown tests were carried out with the Dielectric Breakdown Voltage Test (HCDJC-50 kV, Beijing Hua Ce Testing Instrument Co., Ltd, China) at a climbing speed of 200 V/s1 and a limited current of 5 mA.
3 Results and discussion
3.1 Microstructure and characterization of CCTO@TiO2 nanofiber
The CCTO@TiO2 nanofibers were successfully prepared by the normal coaxial electrospinning method. Figure 1 demonstrates the XRD pattern of the CCTO@TiO2 nanofibers. According to the standard PDF card, the nanocomposite phases of perovskite CCTO and rutile TiO2 are well indexed. The morphology and structure of the CCTO@TiO2 nanofiber were further determined by the SEM and TEM images. The ceramic CCTO@TiO2 nanofibers are uniform in appearance and have a high aspect ratio, with a diameter of 350 nm and length of a few dozens of micrometer. As demonstrated in Fig. 2b, the CCTO@TiO2 nanofiber has an apparent core–shell structure, where the thickness of the outer shell layer is approximately 20 nm and the inner core layer is 300 nm.
For further improve the compatibility between CCTO@TiO2 nanofibers and pristine PI matrix, dopamine are employed as the surface functionalization of the CCTO@TiO2 nanofibers. This can be further evidenced by FTIR and XPS. Figure 3a presents the results of FTIR of raw CCTO nanofibers, CCTO@TiO2 nanofibers and dopamine-coated CCTO@TiO2 nanofibers. Successful modification by dopamine of CCTO@TiO2 nanofibers is confirmed by new absorbance peaks appeared at 1480 and 1260 cm, which respectively are correspond to aromatic C–C stretching vibrations and aromatic amine C–N stretching vibrations, however such peaks are not observed in the matter of unmodified CCTO@TiO2 nanofibers. Figure 3b exhibits the results of XPS of raw CCTO nanofibers, CCTO@TiO2 nanofibers and dopamine-coated CCTO@TiO2 nanofibers. Compared with CCTO and CCTO@TiO2 nanofibers, the peak of N 1s at about 400 eV which is owing to free –NH2 is observed at dopamine-coated CCTO@TiO2 nanofibers, affirming the successfully introduction of dopamine on the CCTO@TiO2 nanofibers surface.
3.2 Microstructure and characterization of CCTO@TiO2/PI nanocomposite films
The XRD patterns of PI, CCTO@TiO2/PI composites with the nanofibers contents of 1, 2, 3, 4 and 5 vol% are shown in Fig. 4. It can be found that the positions and relative intensities of the PI nanocomposite films are in good agreement with CCTO@TiO2 nanofibers after them were incorporated into PI matrix. As the volume fraction of CCTO@TiO2 nanofibers increasing, the peaks of the perovskite CCTO and rutile TiO2 became sharper and stronger, while the signal of PI reduced gradually. In addition, the XRD patterns of the PI based nanocomposites show the CCTO, TiO2, and PI diffraction peaks and clearly showed that the CCTO@TiO2 nanofibers are filled in the polymer matrix.
Figure 5 shows the morphologies and microstructures of the pristine PI and the 5 vol% CCTO@TiO2/PI nanocomposite film. As showed in Fig. 5a, b, the surface of pristine PI is neat and smooth and there is not pores or voids in the fracture surface. Figure 5c, d exhibit the CCTO@TiO2 nanofibers are randomly and uniformly distributed into the PI based nanocomposites, meanwhile there is not agglomeration and voids observed for the film. The majority of the embedded CCTO@TiO2 nanofibers remains in a high aspect ratio, which has a significant impact on the dielectric property.
3.3 Dielectric properties of the nanocomposite films
The dielectric properties of the PI nanocomposites as a function of frequency and the CCTO@TiO2 nanofibers volume fraction is researched. Figure 6 demonstrates the frequency dependence of dielectric properties of PI based nanocomposite films. Obviously, the dielectric permittivity gradually increases with the addition of CCTO@TiO2 nanofibers in the whole testing frequency range. For the content of CCTO@TiO2 nanofibers is 5 vol%, the dielectric constant of the nanocomposite film is 5.85, which is substantially enhanced by 70% over that of pristine PI (3.40). The dielectric constant of the PI nanocomposite films has a great improvement mainly originated from the introduction of high dielectric constant CCTO@TiO2 nanofibers.
In addition, for the high-performance dielectric materials, dielectric loss is another important parameter that needs to be considered. Figure 6b, d shows the dependence of frequency on the dielectric loss of PI based nanocomposites. The dielectric loss of the CCTO@TiO2/PI nanocomposite films is ranging from 0.0021 to 0.025. Even the loading content of CCTO@TiO2 nanofibers is increased to 5 vol%, the dielectric loss is only 0.025, which is quite low compared with other traditional nanocomposites.
To availably show the effect of the core–shell structure on the dielectric properties of the PI nanocomposites, CCTO nanofibers without TiO2 coating were fabricated. Figure 7 demonstrate the comparison of the dielectric permittivity and dielectric loss between nanocomposites with CCTO nanofibers and nanocomposites with CCTO@TiO2 nanofibers, respectively. It is clearly demonstrated that by creating the core–shell structure, the nanocomposites with CCTO@TiO2 nanofibers showed a significant increase in the dielectric permittivity meanwhile maintaining a low dielectric loss. For the nanocomposites with 5 vol% CCTO@TiO2 nanofibers, the dielectric constant of 5.55 was enhanced to 5.85 by utilizing the TiO2 coated CCTO nanofibers, while the dielectric loss shows no obvious change. The CCTO@TiO2 nanofibers are quite efficient at increasing the dielectric constant of the nanocomposites. The primary improvement is attributed to the percolation of the CCTO/TiO2 interfaces leads to much enhanced interfacial polarization of the CCTO@TiO2 nanofibers, which gives rise to substantially increased dielectric constant of the nanocomposites.
3.4 Breakdown strength and energy storage of the nanocomposite films
The energy density (Uc) is depended on the electric displacement (D) and applied electric field (E) of the dielectrics as: \({\text{Uc}}=\int EdD.\) The electric displacement (D) is determined by relative dielectric constants (εr) and the polarization (P) by D = P + εrε0E, where ε0 is the vacuum permittivity. For liner dielectrics, Uc =1/2 εrε0Eb2, where Eb is the breakdown strength of the dielectrics [39]. Thus, for dielectric materials, the breakdown strength is the most critical parameter in defining the energy density of nanocomposites in electrostatic capacitor application. Figure 8 exhibits the breakdown strength of PI based nanocomposites with varying contents of CCTO nanofibers and CCTO@TiO2 nanofibers. The results declare that the breakdown strength of the nanocomposites with CCTO@TiO2 nanofibers is higher than those of CCTO nanofibers at the same volume fraction. Such as, at 5 vol% of nanofibers, the breakdown strength of the composite with CCTO@TiO2 nanofibers is 236 kV/mm, about 1.2 times that of the CCTO nanofibers (201 kV/mm). Compared to the nanocomposites with CCTO nanofibers, the breakdown strength of the nanocomposites with CCTO@TiO2 nanofibers is enhanced due to the charge shifting is subjected to the interfacial zone of CCTO/TiO2 interfaces, instead of in the PI matrix to form a percolation path. Compared to pristine PI, increasing the content of CCTO@TiO2 nanofibers in PI nanocomposites results in continuous decrease of breakdown strength, such as for 5 vol% of CCTO@TiO2 nanofibers, the breakdown strength decreases to 236 kV/mm, which is generally related to the structural defects, such as voids or pores, caused by the introduction of increasing amounts of nanofibers.
The energy storage density of PI based composites containing various volume fractions of modified CCTO@TiO2 nanofibers as a function of breakdown strength is presented in Fig. 9. As the content of CCTO@TiO2 nanofibers increases, the maximum energy storage density of the composite films first increases obviously, and then decreases with the increase of CCTO@TiO2 nanofibers content in the composite films. The maximum energy storage density of the nanocomposites with 1 vol% of CCTO@TiO2 nanofibers reaches a value as high as 1.6 J/cm3 at 299 kV/mm, which is more than 14% increases compared with the pristine PI of 1.4 J/cm3 at 308 kV/mm.
3.5 Thermal properties of the nanocomposite films
Figure 10 shows TGA curves of the PI based composite films containing CCTO@TiO2 nanofibers at a nitrogen atmosphere. Thermal stability of the PI nanocomposite films is of prime importance for high temperature applications. As indicated in Fig. 10, the CCTO@TiO2/PI nanocomposite films have good thermal stability below 500 °C and there is not weight loss for all the PI nanocomposite films. Table 1 shows the weight loss temperatures (> 5% weight loss) of PI, PI-1, PI-2, PI-3, PI-4 and PI-5 are 565.8, 570.8, 576.6, 581.7, 590.6 and 593.4 °C, respectively. Therefore, it could be concluded that the CCTO@TiO2 nanofibers gives rise to greater thermal stability of the PI nanocomposite films. The CCTO@TiO2 nanofibers have larger specific surface area, potentially leading to the improved interfacial fiber/PI interaction which will restrict the thermal motion of PI chains, thus resulting in much higher thermal properties of the CCTO@TiO2/PI nanocomposite films.
4 Conclusions
In conclusion, CCTO@TiO2 nanofibers were successfully fabricated by the normal coaxial electrospinning method. The TEM images exhibited that TiO2 was homogeneously coated on the surface of CCTO nanofibers with an average thickness of 20 nm. The PI nanocomposite films consisting of 1D core–shell structured CCTO@TiO2 nanofibers have been successfully fabricated through the solution casting method. The SEM images demonstrated that the CCTO@TiO2 nanofibers were uniformly dispersed in the PI matrix. The TGA results indicated that the CCTO@TiO2/PI nanocomposite films have excellent thermal stability below 500 °C. The nanocomposites of CCTO@TiO2 nanofibers exhibit higher dielectric constant and enhanced breakdown strength compared to those of CCTO nanofibers. For the nanocomposites with 5 vol% CCTO@TiO2 nanofibers, the dielectric constant of 5.55 was enhanced to 5.85 and the breakdown strength of 201 kV/mm was increased to 236 kV/mm by utilizing the TiO2 coated CCTO nanofibers, while the dielectric loss shows no obvious change. In addition, the energy density of the composites with 1 vol% CCTO@TiO2 nanofibers exhibits a maximal energy density of 1.6 J/cm3, which is more than 14% increases compared with the pristine PI of 1.4 J/cm3. The core–shell structured nanofibers are effective in increasing the dielectric constant as well as the breakdown strength to enhance the energy storage density of polymer composites.
References
B. Chu, X. Zhou, K. Ren, B. Neese, M. Lin, Q. Wang, F. Bauer, Q.M. Zhang, Science 313, 334 (2006)
E.J. Barshaw, J. White, M.J. Chait, J.B. Cornette, J. Bustamante, F. Folli, D. Biltchick, G. Borelli, G. Picci, M. Rabuffi, IEEE Trans. Magn. 43, 223 (2007)
T. Tanaka, M. Kozako, N. Fuse, Y. Ohki, IEEE Trans. Dielectr. Electr. Insul. 12, 669 (2005)
Y. Hirose, K. Adachi, Macromolecules 39, 1779 (2006)
R.A.C. Amoresi, A.A. Felix, E.R. Botero, N.L.C. Domingues, E.A. Falcao, M.A. Zaghete, A.W. Rinaldi, Ceram. Int. 41, 14733 (2015)
E. Baer, L. Zhu, Macromolecules 50, 2239 (2017)
W. Xia, Z. Xu, F. Wen, Z. Zhang, Ceram. Int. 38, 1071 (2012)
Q. Li, G. Zhang, F. Liu, K. Han, M.R. Gadinski, C. Xiong, Q. Wang, Energy Environ. Sci. 8, 922 (2015)
H. Zhu, Z. Liu, F. Wang, J. Mater. Sci. 52, 50489 (2017)
Y. Chen, B. Lin, X. Zhang, J. Wang, C. Lai, Y. Sun, Y. Liu, H. Yang, J. Mater. Chem. A 2, 14118 (2014)
X. Fang, X. Liu, Z.K. Cui, J. Qian, J. Pan, X. Li, Q. Zhuang, J. Mater. Chem. A 3, 10005 (2015)
G.C. Psarras, Composites A 37, 1545 (2006)
C. Wu, X. Huang, X. Wu, J. Yu, L. Xie, P. Jiang, Compos. Sci. Technol. 72, 521 (2012)
W. Liu, J. Chen, D. Zhou, X. Liao, M. Xie, R. Sun, Polym. Chem. 8, 725 (2017)
C. Yang, Y. Lin, C.W. Nan, Carbon 47, 1096 (2009)
Z.M. Dang, M.S. Zheng, J.W. Zha, Small 12, 1688 (2016)
N.A. Almeida, P.M. Martins, S. Teixeira, J.A. Lopes da Silva, V. Sencadas, K. Kühn, G. Cuniberti, S. Lanceros-Mendez, P.A.A.P. Marques, J. Mater. Sci. 51, 6974 (2016)
S. Liu, M. Tian, L. Zhang, Y. Lu, T.W. Chan, N. Ning, J. Mater. Sci. 51, 2616 (2015)
L. Yao, Z. Pan, S. Liu, J. Zhai, H.H.D. Chen, ACS Appl. Mater. Interfaces 8, 26343 (2016)
H. Tang, H.A. Sodano, Appl. Phys. Lett. 102, 063901 (2013)
K. Yu, Y. Niu, Y. Zhou, Y. Bai, H. Wang, J. Am. Ceram. Soc. 96, 2519 (2013)
S. Liu, J. Wang, B. Shen, J. Zhai, Ceram. Int. 43, 585 (2017)
C. Zou, D. Kushner, S. Zhang, Appl. Phys. Lett. 98, 082905 (2011)
X. Zhang, S. Zhao, F. Wang, Y. Ma, L. Wang, D. Chen, C. Zhao, W. Yang, Appl. Surf. Sci. 403, 71 (2017)
T. Wang, X.H. Zhang, D. Chen, Y.H. Ma, L. Wang, C.W. Zhao, W.T. Yang, Appl. Surf. Sci. 356, 232 (2015)
P. Kim, N.M. Doss, J.P. Tillotson, P.J. Hotchkiss, M.J. Pan, S.R. Marder, J. Li, J.P. Calame, J.W. Perry, ACS Nano 3, 2581 (2009)
S. Liu, J. Zhai, J. Wang, S. Xue, W. Zhang, ACS Appl. Mater. Interfaces 6, 1533 (2014)
S. Luo, Y. Shen, S. Yu, Y. Wan, W.H. Liao, R. Sun, C.P. Wong, Energy Environ. Sci. 10, 137 (2017)
C. Zhang, Q. Chi, L. Liu, Y. Chen, J. Dong, T. Ma, X. Wang, Q. Lei, J. Mater. Sci.: Mater. Electron. 28, 2502 (2016)
J. Wang, X. Chao, G. Li, L. Feng, K. Zhao, J. Mater. Sci.: Mater. Electron. 28, 5435 (2016)
X. Zhang, Y. Shen, Q. Zhang, L. Gu, Y. Hu, J. Du, Y. Lin, C.-W. Nan, Adv. Mater. 27, 819 (2015)
S. Liu, J. Wang, B. Shen, J. Zhai, H. Hao, L. Zhao, J. Alloys Compd. 696, 136 (2017)
Z. Pan, L. Yao, J. Zhai, D. Fu, B. Shen, H. Wang, ACS Appl. Mater. Interfaces 9, 4024 (2017)
X. Lin, P. Hu, Z. Jia, S. Gao, J. Mater. Chem. A 4, 2314 (2016)
Z. Pan, J. Zhai, B. Shen, J. Mater. Chem. A 5, 15217 (2017)
S. Liu, S. Xue, B. Shen, J. Zhai, Appl. Phys. Lett. 107, 032907 (2015)
X. Zhang, Y. Shen, B. Xu, Q. Zhang, L. Gu, J. Jiang, J. Ma, Y. Lin, C.W. Nan, Adv. Mater. 28, 2055 (2016)
J.C. Wang, Y.C. Long, Y. Sun, X.Q. Zhang, H. Yang, B. Lin, Appl. Surf. Sci. 426, 437 (2017)
Z.M. Dang, J.K. Yuan, S.H. Yao, R.J. Liao, Adv. Mater. 25, 6334 (2013)
Acknowledgements
This study was financially supported by National Natural Science Foundation of China (21304018 and 21374016), Jiangsu Provincial Natural Science Foundation of China (BK20130619 and BK20130617) and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, J., Long, Y., Sun, Y. et al. Fabrication and enhanced dielectric properties of polyimide matrix composites with core–shell structured CaCu3Ti4O12@TiO2 nanofibers. J Mater Sci: Mater Electron 29, 7842–7850 (2018). https://doi.org/10.1007/s10854-018-8783-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-018-8783-z