Abstract
Metal-free 1D polyaniline nanotubes/carbon nitride (PNCN) hollow composites are fabricated by self-assembly chemisorption and solvent evaporation method for the first time. The results show that carbon nitride (g-C3N4) is successfully covered onto polyaniline (PANI) nanotubes. Due to similar electronic structures of nitrogen-rich materials of PANI and g-C3N4, the combination of two π-conjugated system not only benefits the separation of photogenerated charge carriers but also increases the light absorption range of g-C3N4. The compact contact of the PANI nanotubes and g-C3N4 nanosheets as well as their matched energy level is the determinant factor for interfacial heterojunction. Specially, the hollow 1D “Janus” heterojunction impels photogenerated charge pairs to separate and transfer to opposite directions in a short diffusion path, with the additional benefits of exposure of more active sites and transmission of reactants for catalytic surface reactions. As expected, PNCN composites are photoactive and exhibit enhanced photocatalytic performance under visible-light irradiation over pure g-C3N4 and PANI nanotubes. In addition, PNCN composites possess strong adsorption capacity. This versatile 1D PNCN hollow composites prepared from a cost-effective and green process have potential applications in catalysis, drug delivery system, sensors, and energy storage and conversion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Semiconductor photocatalysis as a high-profile technology has been widely appreciated in clean energy production and environmental issues [1]. As a “green” method, semiconductor photocatalysts can harvest sunlight, activate with reactive charge carriers, split the water molecules into hydrogen or degrade organic pollutants, and simultaneously stay its own stable [2,3,4]. One of the greatest stumbling blocks hindering broad applications of solar energy utilization is the high cost and vulnerability of the metal-based photocatalyst as well as the sluggish photocatalytic reaction. Thus, the ongoing search for high-efficiency metal-free catalysts with sustainability and scalability has attracted much attention.
Binary graphic carbon nitrides (g-C3N4) polymer is sustainable material containing nonmetallic element of carbon and nitrogen, only [5]. Graphene-like g-C3N4 is comprised of extended π-conjugated planar layers packed through van der Waals force as in the structure of graphite due to the aromatic nature of the component blocks (tri-s-triazine rings) [6]. Theoretically, it has been proposed as an attractive visible light driven photocatalyst with a narrow band gap (2.7 eV) [7]. Considering the high thermal and chemical stabilities of g-C3N4 against oxidation together with its remarkable semiconductor photoelectronic properties and low cost of mass production, g-C3N4 based materials are potentially close to an ideal candidate for a solar energy conversion system [8, 9]. However, there are many drawbacks of the g-C3N4 for photocatalysis, which include: (1) low separation efficiency of photogenerated charge pairs, (2) an optical band gap of 2.7 eV, namely, limited absorption band below 460 nm in solar spectra, and (3) the conventional g-C3N4 synthesized by thermopolymerization of nitrogen-containing precursors suffered from small specific surface area [10, 11]. To this end, massive efforts have been devoted to develop photocatalytic performance of g-C3N4. The band gap has been finely engineered by either doping (e.g., B, S, Fe, and P) or copolymerization that allows for the tuning of sub-band positions at the molecular level [10]. Additional modification of the functional g-C3N4 materials is carried out by combined with other semiconductors such as Ag3PO4, BiVO4, CdS and TiO2 to construct heterojunctions, which can, in principle, promote the kinetics of charge diffusion at the interfaces [12,13,14,15]. Conventional metallic materials are unavoidably limited to the confining region of the absorption spectrum, especially poor in the visible-light region, which usually leads to weak photocatalytic activity under visible-light irradiation.
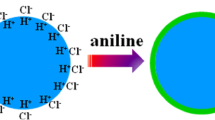
A conductive polymer of choice, polyaniline (PANI), similar to chemical composition of g-C3N4 (mainly carbon and nitrogen as well), is thought to have excellent carrier mobility due to delocalized π-conjugated structures and high absorption coefficient in the visible-light range [16]. In particular, PANI in its partially doped states acts as not only an electron donor upon photoexcitation, but also a good hole conductor [17]. Our previous work and multiple attempts have proved that the PANI is a promising candidate to enhance the photocatalytic activity, such as PANI–TiO2, PANI–CdS, and PANI–BiVO4 [17,18,19]. However, these obtained PANI nanostructures are usually irregular aggregates of amorphous structures and stacked onto the surface of substrate by in situ polymerization aniline, which may be harmful to the stability of PANI result from volume swelling and shrinking of PANI during the charge transfer with a doping and dedoping process [20]. It is, therefore, scientifically promising to perform the stable g-C3N4 nanosheets onto 1D PANI nanotubes (PANI NTs), not only to protect the tender PANI but also to obtain light-harvesting semiconductor nanotubes with directional charge transport properties as building blocks for artificial photocatalysis system. Such anisotropic 1D architectures are known to have a long axis to absorb incident sunlight yet a short radial distance for separation of photogenerated charge carriers (Fig. 1 top right), which results from promoting charge separation and charge carrier collection into opposite spatial regions [21]. In the meantime, heterogeneous photocatalysis reacts on separately inside and outside of this “Janus” heterojunction taking into consideration of photoactive charge diffusion at each ends and its hollow structure.
In this paper, we, for the first time, present a metal-free 1D PANI nanotubes/C3N4 (PNCN) hollow composites fabricated by a facile solvent evaporation and chemisorption method. PANI NTs were chosen as the host templates and g-C3N4 nanosheets were promising access to uniformly loading on, for the flexible feature of exfoliated thin nanosheets. Such well-designed configuration with intimate contact with π-conjugated system has believed in availing to a fast charge transport in 1D heterojunction structure. The component structures and optical properties of these 1D PNCN hollow composites were comprehensively characterized and investigated. The photocatalytic degradation activity and adsorption performance were also investigated. As expected, PNCN composites exhibit enhanced photocatalytic performance and photostability under visible-light irradiation. In addition, PNCN composites possess strong adsorption capacity. The possible photocatalytic mechanism of the PNCN heterojunction is also proposed. It is suggested that the present work may have potential applications in high-efficiency and high stability photocatalyst.
2 Experimental
2.1 Materials
Melamine was used as received by Aladdin Chemistry Co. The aniline monomer (Chuandong Chemical Reagent Co.) was distilled under reduced pressure. Ammonium persulfate (APS), succinic acid (SA), ammonium oxalate (AO), isopropanol (IPA), benzoquinone (BQ), methanol and ethanol were all acquired from Chuandong Chemical Reagent Co. (Chengdu, China) and used without further treatment. Distilled water was used throughout.
2.2 Synthesis
First, PANI NTs were synthesized by a self-assembly process [22]. Briefly, the aniline monomer (0.13 M) and SA (0.065 M) were dissolved in distilled water and stirred magnetically at room temperature for 0.5 h to form a uniform solution. An aqueous solution of the APS oxidant (0.13 M) was added to the above solution. The mixture was allowed to react for 12 h. PANI NTs were obtained by washing with ethanol and water to remove oligomers and dried. PANI NTs were disposed further by de-doping in 1 M NaOH solution and re-doping in 1 M HCl solution. The bulk g-C3N4 used in this study was prepared by heating melamine to 500 °C for 2 h, and then to 520 °C for another 2 h in the air according to the literature [23]. The typical preparation of PNCN photocatalysts was as follows: a certain amount of g-C3N4 was added into methanol and then placed in an ultrasonic bath to exfoliate into a homogeneous suspension. The PANI NTs was added into the above solution and stirred for 24 h until volatilization of the solvent. A celadon powder was obtained after drying in a vacuum oven at 60 °C. Different mass ratios of C3N4 in PNCN photocatalysts at 10, 20, 30, 40, and 50% were synthesized and named as PNCN-10, PNCN-20, PNCN-30, PNCN-40 and PNCN-50, respectively.
2.3 Characterization
X-ray diffraction (XRD) patterns were performed in a Bruker D8 advance X-ray powder diffractometer with Cu (Kα) radiation. Fourier transformation infrared spectra (FT-IR) of the samples were carried out from KBr sample pellets on a FT-IR spectrophotometer (NICOLET-5700). The morphology of the hybrids was observed on field-emission scanning electron microscope (FE-SEM, JEOLJSM-6335F) and transmission electron microscopy [TEM, JEM-1200EX (120KV)]. Diffuse reflectance UV–Visible spectra were recorded on a Shimadzu-2501PC spectrometer using BaSO4 as a reference. The photoluminescence (PL) spectra were obtained using a Varian CaryEclipse 500 with an excitation wavelength at 380 nm. The Brunauer–Emmett–Teller (BET) specific surface area of the samples was characterized by nitrogen adsorption with Micromeritics 3020. X-ray photoelectron spectroscopy (XPS) measurements were done on ESCALAB 250 instrument with Al Kα source. Electrochemical impedance spectroscopy (EIS) plots were measured at amplitude of 5 mV and the frequency ranges from 10,000 to 0.01 Hz.
2.4 Visible-light photocatalytic activity and stability behavior
Visible-light photocatalytic activity of the prepared composites were examined by the degradation of Rhodamine B (RhB) as target in order to compare with other systems. A 300 W Xe lamp equipped with a 420 nm cutoff filter was used as visible light source. The reaction suspension containing a 0.1 g photocatalyst and 200 mL 1 × 10−5 mol L−1 RhB solution. Prior to irradiation, the suspension was magnetically stirred in the dark for 60 min to ensure the equilibrium of adsorption and desorption. Then, 3 mL solution was taken out at given intervals and centrifuged to remove the photocatalysts. The concentration of RhB solution was analyzed by recording the absorbance in the UV–Vis spectrum. To further test the stability of the as-prepared photocatalyst, the photocatalyst was recycled and reused five times in the decomposition of RhB under the same conditions.
3 Results and discussion
3.1 Structure and morphology characterization of PNCN composites
The overall process of the construction of PNCN composites is displayed in Fig. 1. Two kinds of building blocks, PANI NTs and exfoliated g-C3N4 nanosheets, were prepared beforehand by easy-to-get methods. Then g-C3N4 nanosheets will spontaneously coat onto the nanotubes outside.
The crystal structures of the as-prepared samples are shown in Fig. 2a. For pure g-C3N4, two distinct diffraction peaks at 13.0° and 27.3° are indexed for the (100) and (002) facets according to its standard pattern (JCPDS 87-1526), corresponding to in-plane structure of tri-s-triazine units and the interlayer stacking of nanosheets [24]. Compared with g-C3N4, the (002) diffraction peak of all PNCN photocatalysts move from 27.3° to 27.7° (the corresponding d changed from 0.326 to 0.322 nm), and the interlayer stacking peak intensity of (002) is significantly reduced. The phenomenon is in line with relating research that the gallery distance between the layers is reduced which clearly demonstrates the layered g-C3N4 has undergone the process of exfoliation into nanosheets and then recombination again [25]. A board characteristic peak of PANI at 18.3° is found in the XRD patterns of PNCN composites, and displays weakened intensity following the nanosheets loading on [26]. It implies that PANI NTs is covered by g-C3N4 nanosheets. As well, in that same reason, the intensity of (002) diffraction peak significant augments.
The morphological and structural features of the PNCN composites are further examined by SEM and TEM. Figure 2a shows that g-C3N4 consist of the stacked nanosheets with a random size of 0.1–1 µm. The morphology of PANI that is uniform 1D nanotubes can be visualized by SEM and TEM image (Figs. 2b, 3), and the well-formed PANI NTs have a neat and smooth surface (the inset in Fig. 2b). Through TEM observation of Fig. 3b, the outside diameter and inner diameter are about 100 and 13.6 nm, respectively. It is pointed out that the presence of ducts benefits exposure of more active sites and transmission of reactants for catalytic surface reactions. From SEM image of PNCN composites in Fig. 2c, it can be seen clearly that there is a smooth coating layer on the surface of PANI. Furthermore, nearly no obvious bulk g-C3N4 but even nanosheets distribute on PANI NTs, which implies that g-C3N4 have been exfoliated into nanosheets and the soft nanosheets intend to cover on PANI NTs with intimate contact to achieve a minimum surface energy. As shown in Fig. 3b, the thickness of the C3N4 layer coated is about 15.9 nm, and the interface boundary of the core–shell structures is distinctly different from the PANI NTs core.
The chemical bonding structures of the resulting PNCN composites are characterized by FT-IR spectroscopy. Figure 4a shows the FT-IR spectra of PANI NTs, g-C3N4 and PNCN-40. For PANI and g-C3N4, the bands in the 1200–1450 cm−1 region are corresponding to their coexisted C–N stretching vibration modes. Beyond that, the peak at 1572 cm−1 is attributed to the PANI quinonoid rings of C=N stretching vibrations while the g-C3N4 band of which is located in 1637 cm−1. The others characteristic peaks of PANI can be assigned as follows: C=C stretching vibration of benzenoid ring occurs at 1502 cm−1. The bands at 827 and 1151 cm−1 are associated with aromatic C–H out-of-plane and in-plane bending vibration, respectively [27]. In the spectrum of pure g-C3N4, the characteristic peaks appear at 1577 cm−1, which can be assigned to the typical stretching modes of C, N heterocycles. Moreover, the mode of triazine units at 805 cm−1 is observed [28]. For PNCN composite, both signals are observed, suggesting the successful introduction of g-C3N4 onto PANI NTs. Meanwhile, the significant shifts of the characteristic bands confirm the interfacial interactions between PANI and g-C3N4 [29].
XPS is used to explore the chemical environment of valence states and constituent elements on the surface of the sample. The survey XPS spectrum (Fig. 4b) finds that all the products are metal-free and the elements are only C, N with small amounts of adsorbed O. The high resolution XPS spectra of C 1s and N 1s of g-C3N4, PNCN-40 and PANI are conducted in Fig. 7c, d, respectively. The C 1s and N 1s spectrum of pure PANI display an asymmetrical profile as observed in other publications [28]. The C 1s spectra of g-C3N4 (Fig. 7c) show two distinct peaks at 283.5 (C1) and 287.1 eV (C2), identified as the graphitic carbon and sp2-hybridized carbon (N–C=N) in the N-containing network. In the N 1s spectrum of g-C3N4 (Fig. 7d), four de-convoluted peaks at 397.4 (N1), 398.7 (N2), 399.7 (N3), 403 eV (N4) are corresponded to sp2-bonded nitrogen (C–N=C), tertiary nitrogen (N–(C)3), quaternary N in the aromatic cycles and the π excitations in the conjugated structure, respectively [30]. Compared to g-C3N4, the binding energy of N1 and N2 shift along with the disappearing of N4 for the π-excitations after the incorporation of g-C3N4. It implies that there may be strong interaction via conjugative π-electrons between g-C3N4 and PANI, which could improve the charge separation efficiency in the system [31].
The specific surface areas and pore structures of the PNCN samples were obtained from the nitrogen adsorption–desorption measurements at 77 K. As shown in Fig. 5a, PNCN samples exhibit similar nitrogen adsorption–desorption isotherms and are identified as type-IV with hysteresis loops in the relative pressure range of 0.4–1.0, indicating the presence of mesoporous. According to the IUPAC classification, all of the hysteresis loops can be categorized as type H3, implying the existence of slit-like pores, which originates in aggregation of layered structure on the fistulous PANI NTs [32]. The pore size distribution was calculated from the desorption branch of the nitrogen isotherm using the Barrett–Joyner–Halenda (BJH) method. As shown in the Fig. 5b, pore size distribution obviously centralize at around 10–25 nm, which is in agreement with TEM observations (Fig. 3b). In addition, the BET specific surface areas (SBET), pore volumes, and average pore sizes of PNCN samples are summarized in Table 1. When the C3N4 content is increased, the SBET and pore volumes of the samples significantly become smaller, e.g., the SBET and pore volume of PNCN-10 is 3.5 and 9.4 times higher than that of PNCN-50.
a Nitrogen adsorption–desorption isotherms b pore size distributions calculated by the BJH method for PNCN-10, PNCN-30, and PNCN-50. c The photocatalytic activities of various photocatalysts for degradation of RhB under visible light irradiation. d The adsorption and photodegradation of RhB e kinetic constants k over various photocatalysts. f Recyclability of the PNCN-40 composite for the photocatalytic degradation of RhB under visible light irradiation
3.2 Photocatalytic activity and adsorption performance
The photocatalytic activities of PNCN samples were evaluated by the decomposition of RhB dye solution under visible-light irradiation, and the results are shown in Fig. 5c, d. To highlight the role of the heterojunctions and the building structure, the physical mixture of PANI NTs and g-C3N4, referred to as PANI + C3N4 reference, with a 40 wt% of C3N4, is also performed.
Prior to irradiation, the suspensions of catalysts and RhB were stirred in dark to establish the adsorption–desorption equilibrium between photocatalysts and dyes. As can be seen in Fig. 5c and Table 1, the initial adsorption value of RhB over PNCN (10–50) composites are reached by 66.2, 64.8, 53.5, 52.3, 42.6% after the guaranteed adsorption–desorption equilibrium. The results show the excellent adsorption properties of PNCN. In comparison to negligible 5.2% adsorption of g-C3N4, we can find that PANI NTs play an important role in adsorption capacity of PNCN samples. This may be attributed to that the 1D templates of PANI NTs, as compared to 2D or 0D nanostructures provide a better anchoring surface and uniquely penetrating cavity for adsorbing molecules. In addition, PANI + C3N4 displays a slight higher adsorption value of 58.7% compared to that of the synthesized PNCN-40 (52.3%). Not only that, adsorption value of PNCN composite gradually decline following C3N4 nanosheets accumulating, which is easily understood by the fact that excessive cladding of nanosheets on the surface of PANI NTs leads to slump of the SBET for PNCN samples. Evidently, the SBET of the PNCN samples dramatically decrease from 42.4 m2 g−1 (PNCN-10) to 11.8 m2 g−1 (PNCN-50). And yet for all that, the adsorption value of PNCN-50 remains at a high level of 42.6%. Note such high adsorption value with a small SBET is seldom ever reported for 1D nanostructured materials [25, 33]. Thereby, the adsorption ability of 1D PANI NTs or its based materials is not worn down easily. It is said that this kind of attractive nanomaterials with an excellent adsorption capacity will have considerable applications in wastewater treatment fields to remove organic pollutants.
After the absorption process, the photocatalytic degradation activities of PNCN samples were investigated. Under visible light irradiation, the degradation rate of pure g-C3N4 towards RhB (14.1%) is slow due to low surface areas and a cut-off absorption band below 460 nm. However, the PNCN composites exhibit enhanced photocatalytic activities considering the prominent adsorption capacity and the wide visible light absorption of PANI. Furthermore, the physically mixed PANI + C3N4 shows quite lower photocatalytic degradation of 14.5% than the corresponding 42.7% for PNCN-40. Consequently, the enhanced photocatalytic activity of the PNCN is also closely related to the formed heterostructure with compact contact that is supposed to improve the separation and transfer of photo-generated charges, between PANI and C3N4 [34]. Especially, there is an optimal amount ratio of C3N4 nanosheets loading on and the PANI NTs with regard to photocatalytic efficiency. The highest photocatalytic activity of PNCN heterostructure is achieved when the loading of C3N4 is 40 wt%, with the degradation rate as high as 42.7%, which is higher than those from the PNCN 10–30 and 50 (Table 1). When the C3N4 content is above its optimal value, excessive loading of C3N4 (50 wt%) blocks visible light absorption. While low loading of C3N4 (10–30 wt%) meaning too much PANI may lead to insufficient charge transfer between the core of PANI NTs and the shell of C3N4 nanosheets, which is caused by recombination of the photogenerated electron–hole pairs in PANI [30]. In addition, a comparison experiment with the commercial photocatalyst P25 under visible-light irradiation has been conducted. It can be seen that photocatalytic activity of 1D PNCN hollow composite is better than that of P25. The photocatalytic reaction rate constant k is displayed in Fig. 5e and Table 1, based on a pseudo-first-order reaction model. In the composites, PNCN-40 has the highest rate of 1250 and 462.5% enhancement compared to that of pristine g-C3N4 and P25. Figure 5f exhibits the stable recycling photocatalytic reaction of PNCN-40, there is no obvious decrease in photocatalytic degradation activity even after five cycles. Overall, the removal rate of PNCN-40 at 150 min (about 3 h) is 95%. This degradation capacity is competitive with the previously reported metal-free C3N4-based composite [31, 35].
3.3 Optical properties
Apart from adsorption property and specific surface area, it is generally accepted, light-absorption ability and photogenerated charge pairs separation efficiency holds great obligation on the photocatalytic activity [36]. The optical absorption of the obtained photocatalysts are examined with diffuse reflectance spectroscopy (DRS) in Fig. 6a. The absorption edge of the g-C3N4 presented a characteristic band at 460 nm, confirming that it mainly absorbs UV light. For PANI NTs, the absorption spectrum covered full-spectrum, exposing an obvious absorption in UV and visible light regions. The former is ascribed to electron transition between the benzenoid segments, and the latter arises from the formation of polarons in quinoid segments [37]. Further analysis through a Tauc equation demonstrates that the energy gap (Eg) of PANI NTs and g-C3N4 can be estimated to be 2.62 and 2.71 eV (Fig. 6b). As expected, the PNCN composites exhibited strong absorption in the visible region, especially longer than 460 nm compared with the pure g-C3N4. The results prove that PNCN composites have an efficient visible-light harvesting ability and this is expected to enhance the photocatalytic behaviors.
3.4 Photocatalytic mechanism
To elucidate the photocatalytic mechanisms and examine the role of photogenerated active species in photocatalytic process, a series of trapping experiments were carried out by using 1 mM AO, BQ and IPA as effective scavengers for h+ (holes), ·O2 − and ·OH radicals, respectively [38, 39]. Obviously, the process of absorption rises superior to all the scavengers (Fig. 7a). It is noteworthy that the degradation rate of RhB, to some extent, was suppressed by the radical scavengers in the order AO > BQ > IPA. So, the results indicate that h+ and ·O2 − play the major factor in the photodegradation of dyes.
The EIS measurements are recorded to explore the interfacial charge behaviors between PANI NTs and g-C3N4 nanosheets. As seen in Fig. 7b, PNCN-40 exhibited a much smaller arc radius than that of PANI NTs and g-C3N4, implying the lower charge transfer resistance [40]. That is, PNCN heterostructure avails to interfacial charge carrier transfer and separation photoinduced charge pairs. To further verify the effective charge separation by PNCN heterostructure, EIS tests of PANI + C3N4 reference were also conducted. The arc radius of the Nyquist plot of PANI + C3N4 is much larger compared with PNCN-40, which indicates that the well-organized heterostructure is formed in PNCN composite.
The photoluminescence emission spectroscopy is a well-known study in revealing the recombination of free carriers. Figure 7c shows the PL spectra of g-C3N4, PNCN-40, PANI + C3N4 and PANI photocatalysts under an excitation wavelength of 380 nm at room temperature. The pure g-C3N4 has a strong emission peak around 460 nm. After introduction of PANI NTs, the intensity of PL emission peak of PNCN-40 apparently suppresses, which suggests that rate of electron–hole recombination is held down [41]. However, it can be seen that PANI + C3N4 reference does not perform well in separation of charge pairs. This agrees with the EIS results in that the charge transfer of PANI + C3N4 is rigid. The intensity of PANI is very low possibly because few photo-induced charge pairs are generated under the same irradiation condition [30]. Therefore, the EIS and PL results demonstrate that effective separation of charge pairs achieves in PNCN composite.
On the basis of these results we conclude the possible mechanism of photocatalytic degradation process for PNCN heterostructure under visible light and illustrate in Fig. 8. The Eg values of g-C3N4 and PANI have been determined to be 2.65 and 2.71 eV, both of which can be photo-motivated to produce electrons (e−) and holes (h+) under visible light. It is evaluating in a number of studies that ascertained that the conduction band (CB) and valence band (VB) potential of g-C3N4 is about − 1.12 and 1.53 eV versus normal hydrogen electrode (vs. NHE), while the lowest unoccupied molecular orbital (LUMO) and highest occupied molecular orbital (HOMO) of PANI is about − 2.14 and 0.57 eV (vs. NHE) [31]. Accordingly, the energy band of g-C3N4 matches a well staggered alignment with PANI as shown in Fig. 8. Moreover, PANI acts as high mobility of charge carriers and has excellent transporting capacity of holes. When irradiated by visible light, the photogenerated h+ transfer from C3N4 toward the PANI while e− run in reverse, which means a good separation of charge can be achieved consequently. If so, e− will accumulate in the CB of C3N4 with potential of − 1.12 eV, having the ability of reducing oxygen to form ·O2 − due to the more negative potential than E0(O2/·O2 −) = − 0.33 eV (vs. NHE) [42]. Since the HOMO of PANI is more negative than E0(·OH/H2O) = + 2.27 eV (vs. NHE), ·OH cannot generate from h+ accumulated in HOMO of PANI [43]. It suggests that ·OH is mostly from e− in CB of C3N4 or ·O2 − [44]. According to the aforementioned discussion, the h+ oxidize the RhB directly at PANI surface. On the other hand, e− in g-C3N4 react with O2 to produce increasing ·O2 −. And the remainder e− resulted in the decreasing of ·OH.
Taking into account the overall course of photocatalysis in PNCN composite, the excellent performance depends on the elaborately constructed structure of rolling g-C3N4 nanosheets on PANI nanotubes. Firstly, this 1D hollow “Janus” heterojunction drives e− and h+ to flow in opposite directions, followed by executing photoreaction with h+ inside and ·OH outside. Secondly, the diffuse length of charge is short in the direction that is perpendicular to tube wall. Thirdly, a novel adsorption and hollow structure deriving from PANI nanotubes are more preferable for dye molecule photoreaction by reducing diffuse resistance. Finally, good stability of PNCN demonstrates that a thin layer of stable g-C3N4 nanosheets, as protecting shell, can well inhibit disablement of PANI.
4 Conclusion
In summary, we have successfully fabricated metal-free 1D PNCN hollow composites with different g-C3N4 nanosheets contents by chemisorption and then solvent evaporation. The photocatalytic rate of PNCN-40 reaches the maximum value in the degradation of RhB dye under visible-light irradiation. At the same time, the adsorption capacity of which keeps at a higher level. In our study, the enhanced photocatalytic activity may be closely related to adsorptive enrichment, strong absorption in the visible region and forceful inhibition of charge pairs recombination in 1D PNCN hollow composites. According to characterization and discussion, the well-matched band structures and integrated intimate contact with π-conjugated systems between g-C3N4 nanosheets and PANI nanotubes is the decisive factor for the formation of interfacial heterojunction, which plays significant roles in photogenerated charge carriers separation and transfer. Besides, the protect layer of g-C3N4 nanosheets improve the photostability of PNCN composites. This metal-free 1D PNCN hollow composites, with high visible-light photocatalytic activity and excellent photostability, may also a promising candidate for possible practical utilization in H2 production, sensors and drug delivery system.
References
X. Liu, J. Iocozzia, Y. Wang, X. Cui, Y. Chen, S. Zhao, Z. Li, Z. Lin, Energy Environ. Sci. 10, 402 (2017)
J.C. Colmenares, ChemSusChem 7, 1512 (2014)
C. Han, N. Zhang, Y.J. Xu, Nano Today 11, 351 (2016)
H. Wang, L. Zhang, Z. Chen, J. Hu, S. Li, Z. Wang, J. Liu, X. Wang, Chem. Soc. Rev. 43, 5234 (2014)
S. Cao, J. Low, J. Yu, M. Jaroniec, Adv. Mater. 27, 2150 (2015)
Y. Zheng, J. Liu, J. Liang, M. Jaroniec, S.Z. Qiao, Energy Environ. Sci. 5, 6717 (2012)
J. Zhang, X. Chen, K. Takanabe, K. Maeda, K. Domen, J.D. Epping, X. Fu, M. Antonietti, X. Wang, Angew. Chem. Int. Ed. 49, 441 (2010)
X. Wang, S. Blechert, M. Antonietti, ACS Catal. 2, 1596 (2012)
G. Dong, Y. Zhang, Q. Pan, J. Qiu, J. Photochem. Photobiol. C 20, 33 (2014)
L. Ge, C. Han, X. Xiao, L. Guo, Y. Li, Mater. Res. Bull. 48, 3919 (2013)
Q. Liang, J. Jin, C. Liu, S. Xu, C. Yao, Z. Chen, Z. Li, J. Mater. Sci. Mater. Electron. 28, 11279 (2017)
H. Katsumata, T. Sakai, T. Suzuki, S. Kaneco, Ind. Eng. Chem. Res. 53, 8018 (2014)
C. Li, S. Wang, T. Wang, Y. Wei, P. Zhang, J. Gong, Small 10, 2783 (2014)
F. Jiang, T. Yan, H. Chen, A. Sun, C. Xu, X. Wang, Appl. Surf. Sci. 295, 164 (2014)
J. Yu, S. Wang, J. Low, W. Xiao, Phys. Chem. Chem. Phys. 15, 16883 (2013)
Q.Z. Yu, M. Wang, H.Z. Chen, Z.W. Dai, Mater. Chem. Phys. 129, 666 (2011)
H. Zhang, Y. Zhu, J. Phys. Chem. C 114, 5822 (2010)
T. Zhou, S. Tan, Y. Guo, L. Ma, M. Gan, H. Wang, X. Sun, H. Wang, J. Alloy. Compd. 652, 358 (2015)
M. Shang, W. Wang, S. Sun, J. Ren, L. Zhou, L. Zhang, J. Phys. Chem. C 113, 20228 (2009)
H. Cao, X. Zhou, Y. Zhang, L. Chen, Z. Liu, J. Power Sources 243, 715 (2013)
S. Liu, Z.R. Tang, Y. Sun, J.C. Colmenares, Y.J. Xu, Chem. Soc. Rev. 44, 5053 (2015)
L. Zhang, Y. Long, Z. Chen, M. Wan, Adv. Funct. Mater. 14, 693 (2004)
Y. Li, R. Jin, Y. Xing, J. Li, S. Song, X. Liu, M. Li, R. Jin, Adv. Energy Mater. 6, 1601273 (2016)
G. Dong, L. Zhang, J. Mater. Chem. 22, 1160 (2012)
C. Pan, J. Xu, Y. Wang, D. Li, Y. Zhu, Adv. Funct. Mater. 22, 1518 (2012)
Y. Yang, M. Wan, J. Mater. Chem. 12, 897 (2002)
K. Pandiselvi, H. Fang, X. Huang, J. Wang, X. Xu, T. Li, J. Hazard. Mater. 314, 67 (2016)
L. Ge, C. Han, J. Liu, J. Mater. Chem. 22, 11843 (2012)
S. Zhang, L. Zhao, M. Zeng, J. Li, J. Xu, X. Wang, Catal. Today 224, 114 (2014)
K. Li, X. Xie, W.D. Zhang, Carbon 110, 356 (2016)
W. Jiang, W. Luo, R. Zong, W. Yao, Z. Li, Y. Zhu, Small 12, 4370 (2016)
D. Zheng, G. Zhang, X. Wang, Appl. Catal. B 179, 479 (2015)
J. Zhang, Y. Wang, J. Jin, J. Zhang, Z. Lin, F. Huang, J. Yu, ACS Appl. Mater. Interfaces 5, 10317 (2013)
Y. Zou, J.W. Shi, D. Ma, Z. Fan, L. Lu, C. Niu, Chem. Eng. J. 322, 435 (2017)
Y. Xu, H. Xu, L. Wang, J. Yan, H. Li, Y. Song, L. Huang, G. Cai, Dalton Trans. 42, 7604 (2013)
Y. Zhu, Y. Wang, Q. Ling, Y. Zhu, Appl. Catal. B 200, 222 (2017)
T. Guo, L. Wang, D.G. Evans, W. Yang, J. Phys. Chem. C 114, 4765 (2010)
L. Ye, J. Liu, Z. Jiang, T. Peng, L. Zan, Appl. Catal. B 142, 1 (2013)
S. Meng, X. Ning, T. Zhang, S.F. Chen, X. Fu, Phys. Chem. Chem. Phys. 17, 11577 (2015)
C.Y. Wang, X. Zhang, H.B. Qiu, G.X. Huang, H.Q. Yu, Appl. Catal. B 205, 615 (2017)
S. Kumar, A. Baruah, S. Tonda, B. Kumar, V. Shanker, B. Sreedhar, Nanoscale 6, 4830 (2014)
X. Wang, S. Li, H. Yu, J. Yu, S. Liu, Chemistry 17, 7777 (2011)
S. Zhang, J. Li, X. Wang, Y. Huang, M. Zeng, J. Xu, J. Mater. Chem. A 3, 10119 (2015)
H. Zhang, L.H. Guo, L. Zhao, B. Wan, Y. Yang, J. Phys. Chem. Lett. 6, 958 (2015)
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 51501068).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, T., Fu, S., Ma, L. et al. Conjugated system in metal-free 1D polyaniline nanotubes/carbon nitride hollow composites with strong adsorption and enhanced visible-light photocatalytic activities. J Mater Sci: Mater Electron 29, 4266–4275 (2018). https://doi.org/10.1007/s10854-017-8373-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-017-8373-5