Abstract
A new type of red-emitting Eu3+ and Sm3+ co-doped Ca3Sr3(VO4)4 phosphor was synthesized by the combustion method. The photoluminescence properties and microstructure were investigated by photoluminescence spectroscopy, X-ray powder diffraction, and scanning electron microscopy. All samples were found to match perfectly with the rhombohedral structure and belong to the R3c space group. The photoluminescence emission intensity of the optimal phosphor Ca3Sr3(VO4)4:0.05Eu3+, 0.09Sm3+ at 619 nm was significantly enhanced compared with those of Ca3Sr3(VO4)4:Eu3+ samples and commercial Y2O3:Eu3+ at an excitation wavelength of 393 nm, as a result of the energy transfer from Sm3+ to Eu3+. The energy transfer process between Sm3+ and Eu3+ is discussed and interpreted by considering the energy level diagram. Furthermore, the CIE chromaticity coordinate of Ca3Sr3(VO4)4:0.05Eu3+, 0.09Sm3+ was closer to the standard red-emitting point (x = 0.67, y = 0.33) than Y2O3:Eu3+. The luminescence performance of Ca3Sr3(VO4)4:Eu3+, Sm3+ upon excitation by near-UV radiation makes it a promising red phosphor for manufacturing white-light-emitting diodes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Rare-earth ions have been extensively used in various devices, such as scintillators [1], fluorescent lamps [2], and white LEDs [3]. Among these applications, white LEDs and Hg-free fluorescent lamps are considered to be eco-friendly. White LEDs have many merits, such as high efficiency, long operational lifetime, and compactness [4]. One conventional method to fabricate white light is using a yellow phosphor (typically YAG:Ce3+ [5, 6]) in combination with a blue-emitting InGaN LED chip. However, this approach leads to a high color temperature and is an imperfect match in terms of the color rendering in the red region, and it does not satisfy the requirements for low-color-temperature illumination [7]. Another approach is combining semiconductor chips of tri-color phosphors to fabricate white LEDs excited by one UV or near-UV (NUV) chip [8, 9]. The main advantages of this method are avoiding light loss in the process of white light emitting and controlling the emission intensity of different colors to obtain the desired output color [10,11,12]. However, the most common commercially available red-emitting phosphors, such as Y2O2S:Eu3+ and Y2O3:Eu3+, are inefficient and chemically unstable. Therefore, new inorganic red-emitting phosphors need to be developed to combine with an appropriate NUV LED for signaling or illumination applications. In addition, the majority of commercial red-emitting phosphors at present use rare-earth elements as the positive ion in the host lattices (e.g., Y3+, La3+, Ga3+), which means that the cost of red-emitting phosphors is much higher than those of yellow-emitting and blue-emitting phosphors.
Recently, solid-state phosphors based on vanadates doped with rare-earth ions have attracted increasing attention because they can be excited by a broad range of wavelengths and have stable chemical characteristics [13, 14]. In the vanadate group (VO4 3−), four oxygen ions are coordinated to a V5+ ion in a structure with tetrahedral symmetry, which is considered to be an effective luminescent center [15]. Because of the occurrence of energy transfer, the photoluminescence intensity of vanadate-based phosphors could be improved by doping trivalent rare-earth ions into the vanadate hosts. In particular, vanadate-based phosphors with doped Eu3+ have been investigated because they can be applied extensively in the solid-state luminescence industry [13]. Choi et al. [16] reported the solid-state synthesis of Ca3Sr3(VO4)4:Eu3+ and Ca3Sr3(VO4)4:Eu3+, M+ (M = Li, Na, K) red-emitting phosphors and studied the effects of Eu3+ concentration, the type of charge compensation, and their concentration on emission intensity. Sun et al. [17] reported the use of another solid-state reaction to synthesize Ca3Sr3(VO4)4:Sm3+, Na+ red-emitting phosphors. Comparing to conventional solid state reaction, the combustion method can be reacted at relatively low temperature [18]. It also has many advantages such as short reaction time, high efficiency of energy conservation and homogeneous grain size. To the best of our knowledge, there have been no reports of Ca3Sr3(VO4)4:Eu3+ red-emitting phosphors using Sm3+ co-doping for white LEDs. Sm3+ can be applied as the sensitizer in Eu3+-doped phosphors and the excitation bands of Eu3+-doped samples can be strengthened and broadened by Sm3+ doping in the range of ultraviolet–near ultraviolet (UV–NUV) [19]. Moreover, it has been reported that the energies of the 4G5/2 energy level of Sm3+ and the 5D0 energy level of Eu3+ are similar [20]. Thus, it seemed plausible that Sm3+ could transfer absorbed energy to Eu3+ in Ca3Sr3(VO4)4 phosphors and that the emission intensity could be improved significantly.
In this paper, the combustion method has been applied to synthesize Ca3Sr3(VO4)4:Eu3+, Sm3+ phosphors for the first time, to the best of our knowledge. In addition, Ca3Sr3(VO4)4:Eu3+ and commercial Y2O3:Eu3+ phosphors have also been synthesized by the same method for comparison. The photoluminescence properties of Sm3+ and Eu3+ single-doped and co-doped samples were investigated and the relevant mechanism is analyzed.
2 Experimental
2.1 Sample preparation
The Ca3Sr3(VO4)4:Eu3+, Ca3Sr3(VO4)4:Sm3+, and Ca3Sr3(VO4)4:Eu3+, Sm3+ phosphors were synthesized by the combustion method. The starting materials were high-purity europium oxide (Eu2O3), high-purity samarium oxide (Sm2O3), analytical reagent (AR) grade calcium carbonate (CaCO3), AR grade strontium carbonate (SrCO3), AR grade ammonium metavanadate (NH4VO3), nitric acid (HNO3), and citric acid (C6H8O7·H2O). The appropriate stoichiometric ratio of Eu2O3 and Sm2O3 was dissolved in 1 mL HNO3 with 15 mL distilled water to afford a homogeneous solution. Then, CaCO3, SrCO3, C6H8O7·H2O, and NH4VO3 were added to the solution with heating and stirring at a temperature of 70–80 °C. After stirring this solution for around 30 min, we obtained the blue sol precursor. This was placed into a furnace at a defined temperature for 1 h under an air atmosphere and then allowed to cool to room temperature.
2.2 Characterization
The crystal structures of the samples were determined by X-ray diffraction with Cu Kα radiation (Philips X’Pert Pro MPD, Cu Kα, 40 kV, 40 mA, λ = 1.5418 Å). The sample morphologies and particle sizes were investigated using a JEX-100CX scanning electron microscope with a running voltage of 10 kV. Excitation and emission spectra of all of the synthesized samples were recorded using a Hitachi F-7000 photoluminescence spectrometer. The fluorescence lifetime of the obtained samples was investigated using a fluorescence spectrophotometer (Fluorolog-3-Tau, Jobin Yvon Inc., USA). The absolute PL quantum yields (QYs) of powder samples were determined on a FLS980 (Eidinburgh) equipped with an integrating sphere. The fluorimeter have been corrected for the wavelength dependence of the sensitivity of the detectors and throughput of the monochromators. All samples were measured at room temperature.
3 Results and discussion
3.1 Synthesis temperature
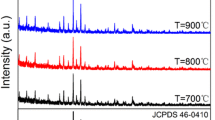
To determine the optimum synthesis temperature, this parameter was varied while other experimental conditions were kept the same. The phase, degree of crystallinity, and photoluminescence properties of the samples obtained using various synthesis temperatures were investigated. The powder XRD patterns of the Ca3Sr3(VO4)4:Eu3+ phosphors obtained at four different synthesis temperatures (700, 800, 900, 1000 °C) are shown in Fig. 1. It can be seen that the diffraction peaks for all samples matched perfectly with the standard diffraction peaks for Ca3Sr3(VO4)4 (PDF card: JCPDS 52-0468), which has a rhombohedral structure. These results confirmed that all of the Ca3Sr3(VO4)4 samples synthesized here after firing at the various temperatures for 1 h were highly pure and belonged to the R3c space group, without the occurrence of other obvious mixed phases, and that Eu3+ entered into the crystal lattice of Ca3Sr3(VO4)4 without affecting the host structure. Taking the ion sizes of Sr3+ (0.118 nm), Ca3+ (0.1 nm), and Eu3+ (0.094 nm) and valence states into consideration, Eu3+ ions can easily substitute for Ca3+ ions in the Ca3Sr3(VO4)4 phosphor due to the similar ion radii. A small shifting of the predominant diffraction peaks around 29.6° (2\({\theta }\)) in the XRD patterns of the Ca3Sr3(VO4)4:Eu3+ samples was observed as shown in Fig. 1 right side. This further confirmed that the Eu3+ ions successfully replaced the Ca3+ ions in the host lattice. The shifting in the XRD patterns was due to the ionic radii difference between the Eu3+ ion and Ca3+ ion. With the increase of temperature from 700 to 900 °C, the intensity of diffraction peaks enhanced orderly and increasingly sharp and symmetrical, which indicates the enhancement of crystalline. It could be also observed from the magnified XRD predominant diffraction peaks in Fig. 1 that the XRD peak width declined orderly. Meanwhile, with the increase of the synthesis temperature from 700 to 1000 °C, the FWHM of the intense diffraction peaks (0, 2, 10) decreased orderly (0.190 at 700 °C, 0.169 at 800 °C, 0.155 at 900 °C, and 0.149 at 1000 °C), which can be calculated by the software of Jade. The FWHM results mean that the crystallinity and the grain size of samples were enhanced successively. The FWHM of the intense diffraction peaks were used to calculate the average crystallite sizes obtained at different synthesis temperatures by using the Scherrer equation:
where D is the average crystallite size, K (K = 0.89) is the Scherrer constant, \(\lambda\)is the wavelength of the X-ray, \(\beta\)is the FWHM of the intense diffraction peaks (0, 2, 10) and \(\theta\) is the diffraction angle of the intense diffraction peaks. According to this equation, the average crystallite sizes of Ca3Sr3(VO4)4:Eu3+ phosphors synthesized at 700, 800, 900, 1000 °C were 47.55, 53.49, 58.26 and 60.64 nm, respectively. Compared with the solid-state reaction method, highly purified and crystalline Ca3Sr3(VO4)4:Eu3+ phosphors can be obtained by the citric acid assisted solution combustion method at relatively lower synthetic temperatures [18]; the reason for this is that the precursor of the citric acid assisted sol combustion method is the sol system, and it is possible that the strong exothermic action can occur quickly and easily at synthesis temperatures of 700–900 °C [21].
The synthesis temperature affects the grain size and this in turn affects the photoluminescence properties of phosphors [22, 23]. Figure 2 shows the SEM images of the samples synthesized at the various temperatures. The sample synthesized at 700 °C (Fig. 2a) did not exhibit a homogenous size distribution and some particles gathered together, because the synthesis temperature was too low and the combustion reaction was insufficient. The samples synthesized at 800–1000 °C (Fig. 2b–d) exhibited homogenous size distributions and the particle size grew quickly with the increasing synthesis temperature. The grain diameters of the samples were approximately 200–300 nm at 700 °C, 400–500 nm at 800 °C, 600–800 nm at 900 °C, and over 1000 nm at 1000 °C.
Figure 3 shows the emission spectra of the Ca3Sr3(VO4)4:Eu3+ phosphors synthesized at the various temperatures, revealing that the synthesis temperature had little influence on the overall peak positions and shapes of the spectra of the four samples, although the relative peak intensities showed some differences. In particular, it can be seen that for the samples obtained at different synthesis temperatures, the intensities of the main emission peak at 619 nm were different. From 700 to 900 °C, higher synthesis temperatures led to stronger emission spectra, and the red emission intensity was strongest at a synthesis temperature of 900 °C. Upon further increasing the synthesis temperature to 1000 °C, the red emission intensity decreased.
The reason for this phenomenon is that the crystallization of Ca3Sr3(VO4)4 structure is improved with the increasing temperature in the range of 700–900 °C and the grain boundary gradually decreased, and high temperature favors the doping Eu3+ ions into Ca3Sr3(VO4)4 lattice, which is consistent with the results shown in Figs. 1 and 2. What is more, as the particle size increases with the increasing temperature in the range of 700–900 °C, stable site for the active ion could be increased increasing emission intensity. However, upon further increasing the synthesis temperature to 1000 °C, agglomeration of active ions at the elevated temperature is the possible reason why the emission intensity decreased. Figure 4 shows the Decay curves of the Ca3Sr3(VO4)4:Eu3+ samples obtained at different synthesis temperatures. The monitoring wavelength is at 619 nm with a 393 nm excitation. The decay curves of Ca3Sr3(VO4)4:Eu3+ phosphors are well fitted by the second order exponential function:
where τ1, and τ2 are the luminescence decay times. The value of decay time can be calculated by the following equation [24]:
The values of decay time of Ca3Sr3(VO4)4:Eu3+ phosphors synthesized at 700, 800, 900, 1000 °C were 578, 555, 558, 576 μs, respectively. It can be seen that the sintering temperature only has a slight influence on the luminescence lifetime, which indicates that the sintering temperature can effectively improve the emission intensity of samples in the range of 700–900 °C, but not change their luminescence lifetime. Therefore, the best photoluminescence properties for the Eu3+-doped Ca3Sr3(VO4)4 phosphors were obtained at a synthesis temperature of 900 °C.
3.2 Photoluminescence spectra of Eu3+/Sm3+-doped Ca3Sr3(VO4)4
Figure 5 shows the photoluminescence (excitation and emission) spectra of Ca3Sr3(VO4)4:Eu3+. The emission spectrum of Ca3Sr3(VO4)4:Eu3+, using an excitation of 393 nm, exhibited several emission peaks centered at 594, 619, 651, and 705 nm, which correspond to the 5D0→7FJ (J = 1, 2, 3, 4) transitions of Eu3+, respectively. Of these peaks, the strongest peak was that situated at 619 nm derived from the 5D0→7F2 transition, because Eu3+ occupies the D 2d site without inversion symmetry [25]. Upon monitoring the 5D0→7F2 transition of Eu3+, the region of excitation was found to extend from 200 to 500 nm, which included a broad charge transfer band (CTB) and a number of small sharp peaks. The CTB was centered at 285 nm, which is mainly from the allowed transition 1A1→1T2 with the charge transfer states (CTS) of VO3− [26]. Multiple sharp peaks could be observed from 350 to 500 nm, which was due to the f–f inner-shell transitions of Eu3+, regardless of the concentration of Eu3+, and these sharp peaks located at 360, 380, 393, 415, and 463 nm were assigned to the 7F0→5D4, 5L7, 5L6, 5D3, and 5D2 transitions of Eu3+, respectively. The most intense peak in the UV–NUV region was located at 393 nm, which represents the 7F0→5L6 transition of Eu3+. Figure 6 shows the emission spectra of different concentrations of Eu3+ single-doped Ca3Sr3(VO4)4 phosphors. It is evident that the emission intensity of Eu3+ single-doped Ca3Sr3(VO4)4 phosphors consistently increased with increasing percentage of Eu3+ up to 5 mol%, and then declined when the concentration of Eu3+ was above 5 mol%. Consequently, Ca3Sr3(VO4)4:0.05Eu3+ exhibited the strongest emission intensity.
The luminescence characteristics of Ca3Sr3(VO4)4:Sm3+ were also investigated and the results are shown in Fig. 7. The emission spectrum show four emission peaks located at 565, 605, 651, and 712 nm upon excitation at 393 nm. The highest peak centered at approximately 605 nm is due to the 4G5/2→6H7/2 transition of Sm3+. Meanwhile, the three other peaks at 565, 651, and 712 nm were ascribed to the 4G5/2→6H5/2, 4G5/2→6H9/2, and 4G5/2→6H11/2 transitions of Sm3+, respectively. Upon monitoring the 4G5/2→6H7/2 transition of Sm3+, the PLE spectrum was made up of a CTB from 200 to 350 nm and some sharp peaks extending from 350 to 500 nm; these small peaks were attributed to the electronic transitions of 6H5/2→4L17/2 (361 nm), 6H5/2→6P5/2 (376 nm), 6H5/2→4F7/2 (405 nm), 6H5/2→(6P,4P)5/2 (416 nm), 6H5/2→4G9/2 (439 nm), and 6H5/2→4I11/2 (476 nm). The most intense peak was that located at 405 nm.
3.3 Photoluminescence spectra of Eu3+ and Sm3+ co-doped Ca3Sr3(VO4)4
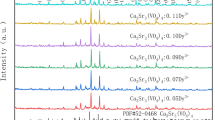
Based on the analysis of the photoluminescence properties of Eu3+ and Sm3+ single-doped Ca3Sr3(VO4)4, Sm3+ and Eu3+ co-doped Ca3Sr3(VO4)4 samples were synthesized and their photoluminescence properties were analyzed. The emission spectra of Ca3Sr3(VO4)4:0.05Eu3+, ySm3+ (y = 0, 0.01, 0.03, 0.05, 0.07, 0.09, 0.11) are shown in Fig. 8. The emissions from the 5D0→7F2 transition of Eu3+ and the 4G5/2→6H9/2 transition of Sm3+ are changed similar with Sm3+ increasing when excited at 393 nm. This supports the theory that Sm3+ can transfer the absorbed energy to Eu3+. Moreover, the PL intensity of the Sm3+ and Eu3+ co-doped phosphors at 619 nm was much higher than samples without Sm3+ doping, confirming that the 393 nm excitation energy can be absorbed by Sm3+ and then subsequently transferred to Eu3+. Upon fixing the Eu3+ concentration at 5 mol% and increasing the Sm3+ concentration to 9 mol%, the luminescence intensity reached its maximum, and decreased at higher or lower concentrations of Sm3+. This phenomenon can be explained by the concentration quenching effect because Sm3+ can transfer energy to different sites of Sm3+ in the host lattice [18].
In this research, all of the Eu3+ and Sm3+ co-doped Ca3Sr3(VO4)4 phosphors can transmit bright red light if using UV or NUV lights as the excitation source. As Fig. 8 shows, the PLE spectrum monitoring the emission at 619 nm consisted of many excitation peaks in the range of 350–500 nm, which were located at 380, 393, 405, and 464 nm, corresponding to the 7F0→5L7 transition of Eu3+, the 7F0→5L6 transition of Eu3+, the 6H5/2→4F7/2 transition of Sm3+, and the 7F0→5D2 transition of Eu3+, respectively. It can be observed that the excitation spectrum of the Sm3+ and Eu3+ co-doped Ca3Sr3(VO4)4 phosphor was remarkably different from the samples without Sm3+ doping, because there is a new excitation peak located at approximately 405 nm. Meanwhile, this co-doped phosphor also has a CTB from 200 to 350 nm that is centered at 285 nm, which is mainly due to the allowed transition 1A1→1T2 with the CTS of VO3− [26]. Meanwhile, we cannot exclude the existence of CTB of Sm3+→O2− and Eu3+→O2− in the spectrum, as these CTBs are also situated in this region [27]. Upon excitation at 393 nm, the emission spectra consist of four main emission peaks at 565, 594, 619, 651, and 705 nm, which correspond to the 4G5/2→6H5/2 of Sm3+, 5D0→7F1 of Eu3+, 5D0→7F2 of Eu3+, 4G5/2→6H9/2 of Sm3+, and 4G5/2→6H11/2 of Sm3+, respectively. The highest emission peak, which is located at 619 nm, is due to a transition of Eu3+. As shown in Fig. 9, by comparing Eu3+ and Sm3+ co-doped samples and single-doped samples with the same concentration of Eu3+ and under otherwise identical conditions, it is obvious that the Sm3+ single-doped sample had no emission intensity at 619 nm but exhibited its strongest emission peak at 609 nm under excitation at 393 nm, whereas the emission intensity of the Ca3Sr3(VO4)4:Eu3+, Sm3+ phosphor at 619 nm was much stronger than that of the Ca3Sr3(VO4)4:Eu3+ phosphor, which proves that the luminescence mechanism of the Eu3+ and Sm3+ co-doped Ca3Sr3(VO4)4 phosphor consists of Eu3+ luminescence and Sm3+ passing energy to Eu3+. The excitation peak at 405 nm can be divided into two excitation peaks by using Gaussian fitting, owing to the peak of the 7F0→5L6 transition of Eu3+ partially overlapping with the peak of the 4G5/2→6H7/2 transition of Sm3+. That is, the excitation of Eu3+-doped samples around 393 nm can be broadened and strengthened by co-doping with Sm3+, which is beneficial for application to NUV LEDs.
The lifetime of Eu3+:5D0 and Sm3+:4G5/2 with varying Sm3+ content is shown in Fig. 10a, b respectively. The monitoring wavelength is at 619 and 609 with a 393 nm excitation. The decay curves of Ca3Sr3(VO4)4:0.05Eu3+, ySm3+ (0 ≤ y ≤ 0.11) phosphors are well fitted by the second order exponential function:
a The decay curves of Ca3Sr3(VO4)4:0.05Eu3+, ySm3+ (0 ≤ y ≤ 0.11) phosphors (λex = 393 nm and λem = 619 nm) and the relation between lifetime and Sm3+ ion doping concentration. b The decay curves of Ca3Sr3(VO4)4:0.05Eu3+, ySm3+ (0 ≤ y ≤ 0.11) phosphors (λex = 393 nm and λem = 609 nm) and the relation between lifetime and Sm3+ ion doping concentration
where τ1, and τ2 are the luminescence decay times. The value of decay time can be calculated by the following equation [24]:
It can be seen from Fig. 10 that the lifetime of Ca3Sr3(VO4)4:0.05Eu3+, ySm3+ (0 ≤ y ≤ 0.11) phosphors decreases with increasing of Sm3+ from 0 to 11 mol%. The result indicates that energy transfer occurs between Eu3+ and Sm3+ ions.
Figure 11 shows the energy transfer process between Sm3+ and Eu3+. As discussed previously, when the excitation wavelength is 393 nm, Sm3+ can be excited from its ground state 6H15/2 to its corresponding excited state 4F7/2, and Eu3+ can be excited from its ground state 7F0 to its corresponding excited state 5L6. After the excited electrons of Eu3+ and Sm3+ return to their respective ground states, the Sm3+ can transfer its absorbed energy to the Eu3+. Furthermore, a channel that can transfer energy between the 4G5/2 energy level of Sm3+ and the 5D0 energy level of Eu3+ exists, because these two energy levels are very close. The 4F7/2 energy level of Sm3+ can be reached upon excitation with 393 nm NUV light, and the excited electron of Sm3+ automatically returns to its lowest excited energy level 4G5/2. Subsequently, energy transfer can occur between the 4G5/2 level of Sm3+ and the 5D0 level of Eu3+ by resonance. This explains why the emission is improved in the Eu3+ and Sm3+ co-doped Ca3Sr3(VO4)4 phosphor. One of the decay channels of Sm3+ can be described as follows [18]:
where τ s is the lifetime of the sensitizer in the presence of the activator, τ s0 is the lifetime of sensitizer in the absence of the activator, P NR is the probability of nonradiative transitions, and P T is the probability that Sm3+ can transfer energy to Eu3+. Exchanging τ s/τ s0 for I s/I s0 and taking no account of the influence of P NR, the efficiency of the energy transfer from Sm3+ to Eu3+ can be described as follows:
where I s0 is the luminescence intensity of the sensitizer in the absence of the activator Eu3+, which can be measured from the emission intensity of Ca3Sr3(VO4)4:0.09Sm3+ at 651 nm, and I s is the luminescence intensity of the sensitizer in the presence of the activator Eu3+, which can be measured from the emission intensity of Ca3Sr3(VO4)4:0.05Eu3+, 0.09Sm3+ at 651 nm. According to this approach, the energy transfer efficiency of the optimal sample is 37%. For the purpose of confirming the type of interaction between Sm3+ and Eu3+, we calculated the distance between Sm3+ and Eu3+ using the following equation:
where V is the volume of the unit cell (4085.8 Å3), which can be obtained from the refinement result, z is the sum concentration of Eu3+ and Sm3+, and N (N = 10.5) is the number of Ca3Sr3(VO4)4 ions in the unit cell. According to this equation, the distance between Eu3+ and Sm3+ in the various co-doped Ca3Sr3(VO4)4:0.05Eu3+, ySm3+ phosphors (y = 0.01, 0.03, 0.05, 0.07, 0.09, 0.11) was estimated to be 23.14, 21.02, 19.52, 18.37, 17.45, and 16.68 nm, respectively. However, the typical critical distance of the exchange interaction between Eu3+ and Sm3+ is 0.5 nm, which is much smaller than the distance between Eu3+ and Sm3+ in the co-doped samples. Under normal conditions, the exchange interaction, multipole interaction, and radiation reabsorption may occur by transferring nonradiative energy among different ions [28]. To sum up, the exchange interaction hardly occurred during the process of energy transfer between Sm3+ and Eu3+ in the host lattice. In addition to this, a broad overlap of fluorescence activator and sensitizer can induce the radiation absorption. Considering the spectral characteristics of the co-doped samples, the type of energy transfer should be multipole interaction. What is more, based on the energy level difference, the phonon assisted energy transfer can be also occurred.
The emission spectra of Ca3Sr3(VO4)4:0.05Eu3+, Ca3Sr3(VO4)4:0.05Eu3+, 0.09Sm3+, and the commercial phosphor Y2O3:0.05Eu3+ at an excitation wavelength of 393 nm were compared, as shown in Fig. 12. It is clear that the PL intensity of Ca3Sr3(VO4)4:0.05Eu3+ was much lower than that of Ca3Sr3(VO4)4:0.05Eu3+, 0.09Sm3+, and the integrated PL intensity of Ca3Sr3(VO4)4:0.05Eu3+, 0.09Sm3+ ranging from 550 to 750 nm was improved by about 1.6 times compared to that of Ca3Sr3(VO4)4:0.05Eu3+. The integrated emission intensities of Ca3Sr3(VO4)4:0.05Eu3+, 0.09Sm3+ and commercial Y2O3:0.05Eu3+ were also compared, which revealed that the integrated emission intensity of Ca3Sr3(VO4)4:0.05Eu3+, 0.09Sm3+ was enhanced by around 2.4 times compared to that of Y2O3:0.05Eu3+ in the 550–750 nm range. In addition, the quantum efficiency of Ca3Sr3(VO4)4:0.05Eu3+, Ca3Sr3(VO4)4:0.05Eu3+, 0.09Sm3+, and the commercial phosphor Y2O3:0.05Eu3+ were also compared in Table 1, the results show that Ca3Sr3(VO4)4:0.05Eu3+, 0.09Sm3+ has the highest quantum efficiency. This comparison leads to the conclusion that the Ca3Sr3(VO4)4:0.05Eu3+, 0.09Sm3+ phosphor has excellent photoluminescence properties for application in white-light-emitting diodes.
Figure 13 shows the CIE chromaticity coordinates of the National Television System Committee (NTSC) standard for red phosphors (x = 0.67, y = 0.33) compared with Ca3Sr3(VO4)4:0.05Eu3+ (0.6448, 0.3548), commercial Y2O3:0.05Eu3+ (0.649, 0.343), and Ca3Sr3(VO4)4:0.05Eu3+, 0.09Sm3+ (0.6595, 0.3402). These values indicate that the CIE chromaticity coordinate of Ca3Sr3(VO4)4:0.05Eu3+, 0.09Sm3+ is closer to the NTSC standard for red phosphors than other commercial phosphors. The Eu3+ and Sm3+ co-doped Ca3Sr3(VO4)4 phosphor could be used as a potential red-emitting phosphor for application in near-UV or blue-based white LEDs.
4 Conclusion
A series of Ca3Sr3(VO4)4:Eu3+ and Ca3Sr3(VO4)4:Eu3+,Sm3+ phosphors were synthesized by a citric acid assisted sol combustion method. After firing for 1 h, all samples were matched perfectly to Ca3Sr3(VO4)4 (PDF card: JCPDS 52-0468), which has a rhombohedral structure and belongs to the space group of R3c. The photoluminescence properties of Eu3+ and Sm3+ single-doped and co-doped Ca3Sr3(VO4)4 phosphors in the near-UV region were investigated. As a sensitizer, Sm3+ absorbs the excitation energy and transfers it to the Eu3+ ions. The integrated intensity of the optimal sample Ca3Sr3(VO4)4:0.05Eu3+, 0.09Sm3+ was enhanced to 1.6 times and 2.4 times compared to Ca3Sr3(VO4)4:0.05Eu3+ and Y2O3:0.05Eu3+, respectively, indicating that it possesses excellent photoluminescence properties for application to NUV LEDs. Furthermore, the CIE chromaticity coordinate of Ca3Sr3(VO4)4:0.05Eu3+, 0.09Sm3+ was found to be closer to standard for red light than other commercial phosphors. Moreover, this research proves that Sm3+ can be employed to broaden and strengthen the absorption around 393 nm for Eu3+-doped samples. Finally, the Sm3+ and Eu3+ co-doped Ca3Sr3(VO4)4 phosphors may be applied as potential red-emitting luminescent materials for white-lighting devices under UV or NUV excitation.
Change history
19 September 2017
The original version of the article unfortunately contained an error in Fig. 5. The corrected version is published with this erratum (Fig. 5). The original version of the article unfortunately contained an error in Fig. 8. The corrected version is published with this erratum (Fig. 8).
References
C. Michail, N. Kalyvas, I. Valais, S. David, I. Seferis, A. Toutountzis, A. Karabotsos, P. Liaparinos, G. Fountos, I. Kandarakis, On the response of GdAlO3: Ce powder scintillators. J. Lumin. 144, 45–52 (2013)
G.C. Brainard, W. Coyle, M. Ayers, J. Kemp, B. Warfield, J. Maida, C. Bowen, C. Bernecker, S. Lockley, J.P. Hanifin, Solid-state lighting for the International Space Station: tests of visual performance and melatonin regulation. Acta Astronaut. 92, 21–28 (2013)
K.J. Chen, H.C. Chen, C.C. Lin, C.H. Wang, C.C. Yeh, H.H. Tsai, S.H. Chien, M.H. Shih, H.C. Kuo, An investigation of the optical analysis in white light-emitting diodes with conformal and remote phosphors structure. J. Display Technol. 9, 915–920 (2013)
L. Chen, C.-C. Lin, C.-W. Yeh, R.-S. Liu, Light converting inorganic phosphors for white light-emitting diodes. Materials 3, 2172–2195 (2010)
J. Zhang, W. Zhuang, X. Xing, L. Wang, Y. Li, Y. Zheng, Y. Liu, Y. Hu, Blue-shift of spectrum and enhanced luminescent properties of YAG:Ce3+ phosphor induced by small amount of La3+ incorporation. J. Alloys Compd. 674, 93–97 (2016)
H.-J. Reyher, N. Hausfeld, M. Pape, J. Baur, J. Schneider, Attribution of the near-UV absorption bands of YAG:Ce to Ce3+-ions by MCD and ODMR. Solid State Commun. 110, 345–349 (1999)
Y. Pan, M. Wu, Q. Su, Tailored photoluminescence of YAG:Ce phosphor through various methods. J. Phys. Chem. Solids 65, 845–850 (2004)
J. Liu, Z.C. Wu, S.P. Kuang, White-light from NUV InGaN LED chip precoated with blue/yellow phosphors. Optoelectron. Advan. Mater.-Rapid Commun. 7, 343–345 (2013)
X. Zhang, Y. Chen, L. Zhou, M. Gong, NUV chip based white LED using thermal stable Eu2+-activated phosphors and borate phosphor. Mater. Lett. 93, 390–392 (2013)
H. Wu, X.M. Zhang, C.F. Guo, R. Xu, M.M. Wu, Q. Su, Three-band white light from InGaN-based blue LED chip precoated with green/red phosphors. IEEE Photonics Technol. Lett. 17, 1160–1162 (2005)
V. Sivakumar, U.V. Varadaraju, An orange-red phosphor under near-UV excitation for white light emitting diodes. J. Electrochem. Soc. 154, J28–J31 (2007)
Z.L. Wang, H.B. Liang, L.Y. Zhou, H. Wu, M.L. Gong, Q. Su, Luminescence of (Li0.333Na0.334K0.333) Eu(MoO4)2 and its application in near UV InGaN-based light-emitting diode. Chem. Phys. Lett. 412, 313–316 (2005)
A. Huignard, T. Gacoin, J.P. Boilot, Synthesis and luminescence properties of colloidal YVO4:Eu phosphors, Chemistry of Materials. 12 (2000) 1090–1094
S. Neeraj, N. Kijima, A.K. Cheetham, Novel red phosphors for solid state lighting; the system Bi(x)Ln(1–x)VO(4):Eu3+/Sm3+ (Ln = Y, Ga). Solid State Commun. 131, 65–69 (2004)
A.R. Dhobale, M. Mohapatra, V. Natarajan, S.V. Godbole, Synthesis and photoluminescence investigation of the white light emitting phosphor, vanadate garnet, Ca2NaMg2V3O12 co-doped with Dy and Sm. J. Lumin. 132, 293–298 (2012)
S. Choi, Y.M. Moon, K. Kim, H.K. Jung, S. Nahm, Luminescent properties of a novel red-emitting phosphor: Eu3+-activated Ca3Sr3(VO4)4. J. Lumin. 129, 988–990 (2009)
J. Sun, R. Sun, J. Sun, H. Du, Photoluminescence studies on a new red emitting Sm3+-doped alkaline-earth vanadate phosphors Ca3Sr3(VO4)4:Sm3+, Na3+. Optoelectron. Adv. Mater.-Rapid Commun. 5, 215–219 (2011)
A. Senouci, J. Frene, H. Zaidi, Wear mechanism in graphite–copper electrical sliding contact. Wear 225–229, 949–953 (1999)
F. Zhang, W. Zhang, Z. Zhang, Y. Huang, Y. Tao, Luminescent characteristics and energy transfer of a red-emitting YVO4:Sm3+, Eu3+ phosphor. J. Lumin. 152, 160–164 (2014)
M. Ye, G. Zhou, L Zhou, D. Lu, Y. Li, X. Xiong, K. Yang, M. Chen, Y. Pan, P. Wu, Z. Wang, H. Liu, Q. Xia, Luminescent properties and energy transfer process of Sm3+-Eu3+ co-doped MY2(MoO4)4 (M = Ca, Sr and Ba) red-emitting phosphors. Solid State Sci. 59, 44e51 (2016)
K. Qiu, J. Li, J. Li, X. Lu, Y. Gong, J. Li, Luminescence property of Ca3(VO4)2:Eu3+ dependence on molar ratio of Ca/V and solution combustion synthesis temperature. J. Mater. Sci. 45, 5456–5462 (2010)
K. Tonooka, O. Nishimura, Effect of calcinations temperature on the luminescent properties of Tb-doped borosilicate glasses. J. Mater. Sci. 34, 5039–5044 (1999)
I. Ahemen, F.B. Dejene, B. Viana, P. Aschehoug, E. Odoh, Effect of annealing temperature and ambient on the structure and optical properties of Eu3+-doped ZnS nanocrystals. Mater. Chem. Phys. 184, 250–260 (2016)
M. Vijayakumar, K. Mahesvaran, D.K. Patel, S. Arunkumar, K. Marimuthu, Structural and optical properties of Dy3+ doped aluminofluoroborophosphate glasses for white light applications. Opt. Mater 37, 695–705 (2014)
Y. Zeng, K. Qiu, Z. Yang, Y. Bu, W. Zhang, J. Li, Enhanced red emission of NaSrVO4:Eu3+ phosphor via Bi3+co-doping for the application to white LEDs. Ceram. Int. 43, 830–834 (2017)
K.Y. Kim, S.J. Yoon, K. Park, Synthesis and photoluminescence properties of red-emitting Ca3 – 3x/2(VO4)2:xEu3+ phosphors. J. Lumin. 160, 78–84 (2015)
F. Zhang, Y. Wang, Y. Tao, VUV spectroscopic properties of Ba2Gd2Si4O13:Re3+(Re3 +=Ce3+, Tb3+, Dy3+, Eu3+, Sm3+). Mater. Res. Bull. 48, 1952–1956 (2013)
J. Su, X. Mi, J. Sun, L. Yang, C. Hui, L. Lu, Z. Bai, X. Zhang, Tunable luminescence and energy transfer properties in YVO4:Bi3+, Eu3+ phosphors. J. Mater. Sci. 52, 782–792 (2017)
Acknowledgements
We would like to acknowledge the financial support from the Key Scientific and Technological Research and Development Program (Grant No. 2017GZ0400), Sichuan Province, P.R. China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tang, Q., Qiu, K., Li, J. et al. Synthesis and photoluminescence enhancement of Ca3Sr3(VO4)4:Eu3+ red phosphors by Sm3+ doping for white LEDs. J Mater Sci: Mater Electron 28, 18686–18696 (2017). https://doi.org/10.1007/s10854-017-7818-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-017-7818-1