Abstract
Electrodeposition of gold nanoparticles (Au-NPs) decorated single polypyrrole nanowire (Ppy-NW) for arsenic (As) detection was studied in this paper. Arsenic is a toxic element, which exists in variety of chemical forms. Arsenic forms combined with groundwater and soil is leading to toxicity and creating health risks, hence the requirement of its detection arises. The present study is to develop a highly sensitive Au-NPs decorated Ppy-NW based sensor for detecting the trace amounts of arsenic (As3+). The chemiresistive method was adopted for detecting the sensitivity of the arsenic traces. A 200 nm in diameter Ppy nanowires were developed using anodic aluminum oxide (AAO) template based synthesis and decorated with Au-NPs and further tested for arsenic sensing. The response of arsenic with the sensors was recorded by chemiresistive method with varying potential between ±100 mV. The sensitivity of Au-NPs decorated Ppy-NW towards arsenic (for two set of As conc. range) was observed to be 0.22 and 4.38 µM−1 and the detection limit of the sensor was observed to be 0.32 µM
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Arsenic is the twentieth most abundant element in the earth’s crust. Arsenic is a very toxic element that is available in variety of chemical forms. Arsenic species are found in environment as arsenic(III) and arsenic(V) oxy-acids. Its high toxicity is the reason for the need of its effective measurement. Adverse health issues such as cardiovascular effect, respiratory problems, skin, liver, kidney cancer and pigmentation changes upon exposer to arsenic [1,2,3]. Major source of arsenic intake by humans in its inorganic form is by means of the potable water. More arsenic consumption leads to chronic arsenic ingestion called arsenicosis. Arsenic detection in potable water is extremely difficult because no difference observes in taste, odor or appearance with and without arsenic content. There are several methods of arsenic detection. Some of these methods include atomic absorption spectroscopic method, inductively coupled plasma method (ICP) which could be further subdivided into mass spectroscopy and atomic emission spectroscopy, silver diethyldithiocarbamate method (SDDC), colorimetric assays test [4,5,6], ASV equipment ESTCP [7], and laser induced breakdown spectroscopy [8,9,10,11]. Besides all the method the electrochemical method for arsenic detection i.e. cyclic voltammetry, potentiometry are finest despite of its limited applications. An electrochemical method is cost effective and gives more accurate results as compared to other methods which are expensive and require the use of sophisticated equipments. The most promising technique is anodic stripping voltammetry (ASV), which consists of a specified period of pre-concentration of As(0) produced from reduction of As(III) and deposited on the electrode surface followed by anodic stripping of As(0). Many researchers have revealed that the use of different forms of gold electrode materials is the key to the ASV detection of arsenic. The determination of arsenic by ASV and differential pulse anodic stripping voltammetry (DPASV) at various electrode materials like HMDE, platinum and gold, of which gold was found to be most suitable because of its sensitivity and high hydrogen over-voltage [12]. In addition to solid gold electrode, macro-sized gold film electrodes [13, 14] have also been used and the re-plating and washing [15] and reactivation of electrode surface [16] before each measurement improved the reproducibility. Besides, Bodewig et al. [17] and Sun et al. [18] investigated arsenic determination on gold rotating disc electrodes to remove produced hydrogen bubbles mechanically. The effect of ultrasonic and infrasonic sounds on electroanalytical arsenic detection on gold electrodes was reported by Simm et al. [19, 20]. The determination of arsenic with square wave anodic stripping voltammetry (SWASV) [21] using a micro-fabricated electrode array consisting of 564 gold ultra-microelectrodes were reported by Feeney and Kounaves [22, 23]. Similarly, Dai et al. [24] applied a gold nanoparticle-modified electrode for the ASV detection of arsenic.
Hydride generation with detection by atomic fluorescence spectrometry or inductively coupled plasma high-resolution mass spectrometry (ICP-HR-MS) [25, 26] and graphite furnace atomic absorption spectrometry (GFAAS) [27, 28] provides accurate and reproducible results in laboratory, however this method is not suitable for detection into the field. Several research works has been reported about arsenic detection using electrochemical methods. Kamenev et al. [29] investigated in detail the determination of arsenic(III) using anodic stripping voltammetry at graphite electrode modifying with Au and Cu. Authors have reported that arsenic is deposited at potential −0.7 and −0.8 V with 0.1 M HCl and 0.05 M complexone III Y as supporting electrolytes respectively. The reported LOD for arsenic with graphite electrode modified with gold was 17 g L−1 and with that of a Cu-modified graphite electrode was 47 g L−1. Simm et al. [30] compared gold, platinum and silver as viable electrode substrates for arsenic detection using ASV with optimum conditions of 0.1 M HNO3 and −0.6 V as the deposition potential with 120 s deposition time. These conditions provide for a LOD 6.3 × 10−7 M. When employing ultrasound, an increase in mass-transfer reduced the LOD to 1.4 × 10−8 M. Cepria et al. [31] developed the electrochemical screening method for detecting arsenic in the soil by physically immobilizing soil micro particles in graphite carbon paste. The technique shows an LOD of 5 mg kg−1 of total arsenic concentration. Commercially available colorimetric assays offer rapid response but not necessarily correct results in on-site conditions. Thus, there is a need for a rapid and accurate sensor is clear. Electrochemical techniques have been proven to be able to give reliable results in laboratory conditions and have potential for a further development to look into a handheld, low cost analytical device capable of fulfilling the requirements of a rapid and accurate sensor.
In the present study, we have developed a chemiresistive Au-NPs decorated single Ppy-NW device for the detection of arsenic in the potable water. As the surface area of nanowire and nanoparticle is more there is a possibility to enhance the sensitivity of the sensor as well as rapid and accurate detection too. The Ppy-NW device was fabricated using potentiostatic method and decorated with Au-NPs using cyclic voltammetry (CV). Furthermore, the device was characterized by I–V characteristic for the sensing behavior of the device towards various arsenic concentrations, scanning electron microscopy (SEM), energy dispersive spectroscopy (EDAX).
2 Experimental
2.1 Materials and methods
To develop polypyrrole nanowire, AAO membranes of 200 nm pore size and 60 µm thickness was purchased from Whatman International Ltd. (Maidstone, England). Pyrrole was obtained from Sigma-Aldrich (St. Louis, MO, USA). Lithium perchloric acid (LiClO4), sulphuric acid (H2SO4), hydrogen peroxide (H2O2) [Fisher Scientific (Fair Lawn, New Jersey, USA)], gold colloid solution (HAuCl4) (Sigma Aldrich), phosphoric acid, arsenic trioxide As2O3 (analytical reagent grade), NaOH, HNO3, were used in the present study. All the reagents were prepared in deionized water.
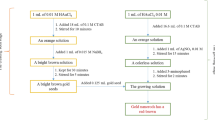
Ppy nanowires were electrochemically synthesized inside an alumina template with 200 nm pore diameter and about 60 µm thicknesses (Whatman International Ltd., Maidstone, UK), the one side of template was coated with gold as a seed layer using thermal evaporation system (High Hindvacc—12A4D) and assembled the single Ppy nanowire on the gold patterned electrode to make the sensor device. The detailed discussion about the synthesis of Ppy nanowire is mentioned in our earlier reported work [32]. The arsenic sensor was fabricated as shown in Scheme 1. The synthesized Ppy nanowire was then aligned on a 16-pair electrode patterned on Si/SiO2 substrate as mentioned in earlier reported work [32]. The synthesized single Ppy nanowire is aligned on the electrode patterned using electrophoretic alignment method with removal of excess Ppy nanowire physically using 25 µm (diameter) gold wires (Scheme 1a). The aligned Ppy nanowire was then anchored using mask-less electrodeposition using chronoamperometry method (Biologic SP 150) with the electrolyte solution HAuCl4 at −0.5 V applied potential versus Ag/AgCl electrode (Scheme 1b). The SEM image of aligned and anchored Ppy-NW is shown in Fig. 1.
The Ppy-NW was decorated with Au-NPs (Scheme 1c) to do this, the anchored Ppy-NW was inserted into an aqueous solution containing 0.1 M KCl and 0.5 mM HAuCl4 and then Au-NPs were deposited using five cycles of cyclic voltammetry (CV) between +0.2 to −0.5 V versus Ag/AgCl reference electrode along with platinum counter electrode in a three-electrode electrochemical arrangement (Fig. 2). The scan rate was fixed at 50 mV s−1. The composition of PPY-NW and gold nanoparticles composites was characterized by the SEM and EDAX mapping as shown in Fig. 2 (inset). The presence of pyrrole and gold element are clearly observed. In addition, the peak of Si element in EDAX observed as the device was prepared on to Si/SiO2 wafer.
For arsenic sensing, stock solution was prepared by dissolving 1.320 g of arsenic trioxide, As2O3 (analytical reagent grade) in 100 mL of deionized distilled water containing 4 g NaOH and acidify the solution with 20 mL conc. of HNO3. A combination of stock and buffer solution is used for varying As concentration from 0.1 to 70 µM. The sensor was tested each time using 4 µL solution drop for various molar concentrations.
3 Result and discussion
To study the response of sensor towards various As concentration, Ppy-NWs were synthesized by electrodeposition in 200 nm pore diameter AAO templates and then assembled on prefabricated gold microelectrodes decorated with gold nanoparticles. Sensor resistances were measured in terms of their current–voltage (I–V) responses using a Potentiostat/Galvanostat (Model SP-150, BioLogic, France) under wet conditions [with a 4 µL drop containing 0.1 M phosphate buffer (PB) at pH 4 on the nanowire].
The voltage was varied by ±500 mV across pair of contact electrodes and the current was recorded. The nanowire resistance was measured as the inverse of the slope of I–V near zero voltage in the linear range of ±200 mV. Resistance changes of the sensor upon exposure to different concentrations of arsenic of various (two step) concentrations ranging from 0.1–8 µM (first step) and 10–70 µM (second step) in 0.1 M PB (pH 4) were measured in wet condition (with a 4 µL drop of As in PB on the nanowire) after the sensor was incubated in respective analyte solution for 5 min followed by washing with PB. Figure 3 shows the I–V characteristic of the Au-NPs/Ppy-NW sensor response towards the various As concentration. The Fig. 3 (inset) shows the I–V response for without alignment (bare), after alignment (AA) and After Au-NPs (AG) decorated polypyrrole nanowire thereby confirming the alignment of nanowire and gold deposition. It is observed that, with increase in As concentration there is increase in the current response [33] owing to the oxidation of As(III) to As(V) on Au-NP. The resistance was calculated by taking the 1/slope of IV characteristics for eight different Au-NPs/Ppy-NW sensors individually. As 1/slope is equal to volt over current i.e. V/I which is equal to resistance (R). The resistance value of eight different sensor for each concentration was then calculated and normalized with blank PB solution to find the sensing behavior of the sensor with standard deviation. Figure 4 shows the calibration plot of eight different sensor responses towards various As concentration. It is found that the change in conductance for various concentration of arsenic for two set of As conc. range i.e. (a) 0.1–8 µM and (b) 10–70 µM are near about 0.22 and 4.38 µM−1 showing the sensitivity of the Au-NPs/Ppy-NW sensors with correlation coefficient i.e. 0.9738 and 0.9493 respectively. Further the sensor has got lowest detection limit of about 0.32 µM. Since it is observed that the sensors have got two distinct linear regions indicates a change in the nucleation kinetics/mechanism. The arsenic sensing trend reported is in good agreement with the previously published work [34]. Table 1 shows the comparative analysis of the reported work with our results, it clearly shows that our results stood better in respect of sensitivity and LOD that could be due to the change in sensor dimension, here we have used the nanowire as a base material and the gold nanoparticle as a sensing material that are known to be high surface area as per the dimension factor, which lead to enhance the sensor sensitivity with rapid detection and accuracy [39].
4 Conclusion
We have demonstrated a highly sensitive single Ppy nanowire decorated with gold nanoparticle chemiresistive sensor device for the rapid and label-free detection of arsenic. The electrochemical, electrical conductivity and SEM–EDAX studies reveal that the successfully synthesized Au-NPs/Ppy-NW sensor provides a good sensitivity towards the various arsenic concentrations. The sensor has got the excellent sensitivity i.e. 0.22 and 4.38 µM−1. The lowest detection limit of the sensor was recorded to be 0.32 µM.
References
P.B. Tchounwou, B.A. Wilson, A. Ishaque, Rev. Environ. Health 14, 1 (1999)
P.L. Goering, H.V. Aposhian, M.J. Mass, M. Cebrian, B.D. Beck, M.P. Waalkes, Toxicol. Sci. 49, 5 (1999)
B.K. Mandal, K.T. Suzuki, Talanta 58, 201 (2002)
D.G. Kinniburgh, W. Kosmus, Talanta 58, 165 (2002)
M.M. Rahman, D. Mukherjee, M.K. Sengupta, U.K. Chowdhury, D. Lodh, C.R. Chanda, S. Roy, M.D. Selim, Q. Quamruzzaman, A.H. Milton, S.M. Shahidullah, Environ. Sci. Technol. 36, 5385 (2002)
A. Hussam, M. Alauddin, A.H. Khan, S.B. Rasul, A.K. Munir, Environ. Sci. Technol. 33, 3686 (1999)
S.B. Rasul, A.K.M. Munir et al., Environmental Security Technology Certification Program, Heavy Metals Analyzer, http://www.estcp.org/projects/compliance/199606v.cfm
R.T. Wainner, R.S. Harmon, A.W. Miziolek, K.L. McNesby, P.D. French, Spectrochim. Acta B 56, 777 (2001)
A. Koskelo, D.A. Cremers, Los Alamos National Lab NM (United States) (1994). http://www.iaea.org/inis/collection/NCLCollectionStore/_Public/26/032/26032331.pdf
E.R. Cespedes, S.H. Lieberman, B.J. Nielsen, G.E. Robitaille, Army Engineer Waterways Experiment Station Vicksburg MS; EPA Science Inventory Record ID 18871, ADA369242 (1999)
B.T. Fisher, H.A. Johnsen, S.G. Buckley, D.W. Hahn, Appl. Spectrosc. 55, 1312 (2001)
G. Forsberg, J.W. O’Laughlin, R.G. Megargle, S.R. Koirtyihann, Anal. Chem. 47, 1586 (1975)
P.H. Davis, G.R. Dulude, R.M. Griffin, W.R. Matson, E.W. Zink, Anal. Chem. 50, 137 (1978)
E.A. Viltchinskaia, L.L. Zeigman, D.M. Garcia, P.F. Santos, Electroanalysis 9, 633 (1997)
U. Greulach, G. Henze, Anal. Chim. Acta 306, 217 (1995)
T. Navratil, M. Kopanica, J. Krista, Chem. Anal. 48, 265 (2003)
F.G. Bodewig, P. Valenta, H.W. Nürnberg, Anal. Chem. 311, 187 (1982)
Y.-C. Sun, J. Mierzwa, M.H. Yang, Talanta 44, 1379 (1997)
A.O. Simm, C.E. Banks, R.G. Compton, Electroanalysis 17, 335 (2005)
A.O. Simm, C.E. Banks, R.G. Compton, Anal. Chem. 76, 5051 (2004)
E. Munoz, S. Palmero, Talanta 65, 613 (2005)
R. Feeney, S.P. Kounaves, Anal. Chem. 72, 2222 (2000)
R. Feeney, S.P. Kounaves, Talanta 58, 23 (2002)
X. Dai, O. Nekrassova, M.E. Hyde, Anal. Chem. 76, 5924 (2004)
M. Leermakers, W. Baeyens, M. De Gieter, B. Smedts, C. Meert, H.C. De Bisschop, R. Morabito, P. Uevauviller, TrAC Trends Anal. Chem. 25, 1 (2006)
B. Klaue, J.D. Blum, Anal. Chem. 71, 1408 (1999)
K. Anezaki, I. Nakatsuka, K. Ohzenki, Anal. Sci. 15, 829 (1999)
N. Hata, H. Yamada, I. Kasahara, S. Taguchi, Analyst 124, 23 (1999)
A.I. Kamenev, S.E. Orlov, A.B. Lyakhov, J. Anal. Chem. 56(9), 850 (2001)
A.O. Simm, C.E. Banks, R.G. Compton, Electroanalysis 17(19), 1727 (2005)
C.S. Hamida, F. Laborda, J.R. Castillo, J. Appl. Electrochem. 37(10), 1171 (2007)
D.J. Shirale, M.A. Bangar, W. Chen, N.V. Myung, A. Mulchandani, J. Phys. Chem. C 114, 13375 (2010)
A.K. Sakira, I.T. Som, E. Ziemons, B. Dejaegher, D. Mertens, P. Hubert, J.-M. Kauffmann, Electroanalysis 27, 309 (2015)
M. Khairy, D.K. Kampouris, R.O. Kadara, C.E. Banks, Electroanalysis 22(21), 2496 (2010)
J.A. Sánchez, B.L. Rivas, S.A. Pooley, L. Basaez, E. Pereira, .I.P. Paintrand, C. Bucher, G. Royal, E. Saint-Aman, J. Moutet, Electrochim. Acta 55, 4876 (2010)
D. Yamada, T.A. Ivandini, M. Komatsu, A. Fujishima, Y. Einaga, J. Electroanal. Chem. 615(2), 145 (2008)
E. Mafakheri, A. Salimi, R. Hallaj, A. Ramazani, M.A. Kashi, Electroanalysis 23, 2429 (2011)
G. Hignett, J.D. Wadhawan, N.S. Lawrence, D.Q. Hung, C. Prado, F. Marken, R.G. Compton, Electroanalysis 16(11), 897 (2004)
A. Mulchandani, N.V. Myung, Curr. Opin. Biotechnol. 22(4), 502 (2011)
Acknowledgements
Authors are thankful to the SERB DST, New Delhi, India for the financial assistance under Young Scientist scheme (SR/FTP/PS-041/2012) and under Empowerment and Equity Opportunities for Excellence in Science (EMEQ) (SB/EMEQ-208/2013), Prof. Ashok Mulchandani, Department of Chemical and Environmental Engineering, University of California, Riverside, 92521 CA, USA for providing the 16-pair electrode pattern silicon chip and Prof. J. V. Sali, Organic Photo-Voltaic Lab, Department of Electronics, NMU, Jalgaon for providing the Thermal Evaporation System.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salunke, R.S., Kasar, C.K., Bangar, M.A. et al. Electrodeposition of gold nanoparticles decorated single polypyrrole nanowire for arsenic detection in potable water: a chemiresistive sensor device. J Mater Sci: Mater Electron 28, 14672–14677 (2017). https://doi.org/10.1007/s10854-017-7332-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-017-7332-5