Abstract
In the present study, povidone-SiO2 nano-composite dielectric film was introduced to replace SiO2 gate dielectric film. The organic and inorganic particles homogeneously dispersed in nano-composite film. The structure of nano-composite film was affected by annealing temperatures. By increase in annealing temperature up to 200 °C, wt% of carbon, oxygen and nitrogen increased and wt% of silicon decreased. At 240 °C, the organic phase desorbed and nano-composite structure degraded. The annealing temperature of 150 °C was suitable for adhesion between two phases. The cross-linked structure of dielectric film annealed at 150 °C led to decrease in leakage current.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The usage of SiO2 gate dielectric in Si field-effect transistors (Si-FET) has been limited due to some problems such as increased leakage current [1,2,3,4,5]. The leakage current density of around 10−2 A cm−2 at −2.5 V was reported based on SiO2 dielectric thin film [6]. Many high-K oxides were studied as a replace SiO2 gate dielectric. HfTaO, HfO2 and ZrxLa(1−x)Oy gate dielectrics caused the leakage current density of around 10−1 A cm−2 at 2 V [7], 10−4 A cm−2 at −6 V [8] and 10−5 A cm−2 at 5 V [3], respectively.
The organic materials as a gate dielectric have advantages consisting light weight, flexibility and low cost fabrication process [9,10,11,12,13]. However, organic gate dielectric films commonly lead to increased leakage current. The leakage current density of around 102, 101 and 101 A cm2 at −10 V were reported for poly (methylmethacrylate) (PMMA), poly (4-vinyl phenol) (PVP) and poly(vinly alcohol) (PVA) [9]. Recently the composite organic–inorganic materials have received great attention due to combined characteristics of two phases improving insolating properties [12,13,14,15,16,17,18,19,20,21,22]. Also the organic particles significantly prevent the aggregation of inorganic particles [23] led to the formation of cross-linked structure. It is well known cross-linked dielectric film reduces gate leakage [24, 25].
The purpose of this article is to prevent aggregation of SiO2 nanoparticles by polymeric nanoparticles and cross-linking agent. In order to find the optimal temperature for formation interactions between two phases, povidone-SiO2 films on the Si (p) substrate were annealed at 150, 200 and 240 °C. The changes created in structure of povidon-SiO2 nano-composite dielectric film (via different annealing temperatures) were examined by Fourier transform infrared (FTIR), energy dispersive X-ray spectroscopy (EDS) and EDS mapping images. The values of capacitance and leakage current of dielectric films were measured on the Au/povidone-SiO2/Si (p) structure at room temperature.
2 Experimental
The sol–gel method was used for synthesize of povidone-SiO2 nano-composite. In solution I, povidone as an organic material (Wt 40,000 Aldrich) was solved in ethanol (ETOH, 99.99% Aldrich). In solution II, tetraethyl orthosilicate (TEOS, 99.999% Aldrich) as a SiO2 precursor, 3-trimethoxysilyl propyl metacrylate (TMSPM, 97% Merk) as a cross-linking agent, ETOH and deionized water were stirred at 300 rpm at room temperature for I hour. Two solutions were mixed and stirred at 500 rpm at 70 °C (with a 1:1:0.05 ratio of povidone:TEOS:TMSPM). The colorless gel obtained was dried at 75 °C and then was dissolved in benzene alcohol solvent so that homogeneous solution resulted.
Povidone-SiO2 nano-composite solution was deposited on the p-type silicon substrate by spin-coating technique as follows: a single crystal Si (p) with (100) surface orientation was cut into 1 × 1 cm2 pieces. Both acetone and alcohol were used to clean the Si (p) wafers. They were then immersed in ultrasonic cleaner containing alcohol for 20 min. The obtained povidone-SiO2 nano-composite solution was deposited on the Si (p) substrates by spin-coating process at 7000 rpm for 10 min. Four films were deposited in similar way and were annealed at 150, 200 and 240 °C for 1 h.
3 Results and discussion
3.1 Thermal analyze
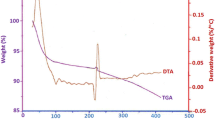
TG analyze was done using STA 504 TGA with a heating rate of 10 °C min–1 in an air atmosphere. Figure 1 shows TGA curve of prepared povidone-SiO2 nano-composite in the temperature range of 0 to 900 °C. This curve can be divided into five zones. In the zone I (0–75 °C), significant weight loss occurs with a sharp slope which is because of desorbed water and residual solvents [25]. In the zone II (75–220 °C), no major event is occurred. In zone III (220 to about 400 °C), about 5% of nano-composite material weight is lost. It is probably due to decomposition organic phase [25]. In the zone IV (400 to about 700 °C), nano-composite material weight is a constant (45% of total nano-composite material) which corresponds to its sustainable weight. It could be associated with silicon [25].The weight of nano-composite material gives raise from around 700 °C. It could originate from oxidation of an element of nano-composite [26].
3.2 Interactions studies
The influence of annealing temperatures on the interactions of nano-composite material was studied by a Bruker Tensor 27 FTIR spectrometer. Figure 2 displays FTIR spectra of pure povidone and povidone-SiO2 nano-composite was not annealed and was annealed at temperatures of 150, 200 and 240 °C. Table 1 summarizes the obtained peaks characteristics. Accordingly, the number and characteristics of peaks of nanocomposite material annealed at various temperatures are same but are dissimilar to pure polymer. It is because interactions formed between povidone and SiO2 nano-particles by sol–gel process [12, 25]. In the spectrum of nano-composite material was not annealed (Fig. 2b), a broad band at 3453 cm−1 is associated with various vibrations of O–H groups [9, 12, 25]. Double peak at 1081 cm−1 can be related to overlap of O–Si–C and Si–O–Si signals [12, 25]. The other peaks at 460, 566, 800, 962 and 1646 cm−1 can be assigned to Si–O–Si [27], N–C=O [6], Si–O–Si [27, 28], Si–OH [25] and C=O [25, 29] bonds respectively.
Figure 2c shows that Si–O–Si/O–Si–C bond position is shifted by 9.64 cm−1 to lower wavenumber with respect to that at Fig. 2b. This large shift could be due to the formation of new chemical interactions by annealing temperature at 150 °C [25, 29]. The shift to a lower wavenumber could be attributed that the some bonds of Si–C are broken and the new bonds of Si–O are formed. In addition to, O–H bond position is shifted by 3.47 cm−1 to higher wavenumber. The other bonds are also shifted by about 1 cm−1 to higher wavenumbers. The small shifts in FTIR spectra are probably related to the physical interactions [25, 30].
In the spectrum of nano-composite material annealed at 200 °C (Fig. 2d), wavenumber of O–H, C=O and Si–O–Si/O–Si–C peaks are shifted by −6.17, 8.31 and 7.97, respectively. The large shifts in wavenumber of O–H and C=O bonds can be attributed to the formation of hydrogen bonds between oxygen of C=O group and hydrogen of nano-composite material content [25, 30].
The position of O–H, C=O and Si–O–Si/O–Si–C peaks in the spectrum of nano-composite material annealed at 240 °C (Fig. 2e) are shifted by 1.62, 8.84 and 2.6 cm−1 to lower wavenumbers with respect to those in the spectrum of nano-composite material annealed at 200 °C. The other Si–O–Si signals are also shifted of 4.17 cm−1 and 1.76 cm−1 to higher wavenumbers.
In general can be said, the stronger chemical interactions occurred on the Si–O–Si/Si–O–C bonds at 150 °C, as these bonds have the highest wavenumber shift. And When annealing temperature increased (200 and 240 °C), the strong chemical interactions also occurred on the C=O bonds. It is well known that Si–O–Si bonds are associated with covalent interactions between organic and inorganic phases [16, 29], confirming the formation of cross-liked nano-composite network [1, 3, 10]. So it seems that the annealing temperature of 150 °C is suitable for formation of reactions between two phases.
3.3 Thin films characterization
The identification of constituent elements and the calculation of the concentration of them were done by Energy dispersive spectroscopy (EDS). Figure 3a–d illustrates EDS spectra of povidone-SiO2 films cross-section annealed at various temperatures on the Si (p) substrate. The weight ratio (wt%) of the elements and effective factors for determination concentration of elements consisting the relative intensity of spectral lines (K-ratio) and correction factor of each element (ZAF) are classified in Table 2. The ZAF correction factor includes the effects of atomic number (Z), the X-ray absorption within sample (A) and fluorescence within the sample (F) [13]. Figure 3a–d displays peaks related to carbon, oxygen, nitrogen and silicon elements. According to Table 2, wt% of carbon (from 40.5 to 63.06%), oxygen (from 4.1 to 6.59%) nitrogen (from 0.1 to 9.41%) increases and wt% of silicon (from 49 to 20.95%) decreases by increase in annealing temperature up to 200 °C. However at 240 °C, wt% of carbon, oxygen and nitrogen significantly decreases so that wt% of film structure is dominated by silicon (99%). It is probably due to decomposition of organic phase from film structure as TGA curve shows weight loss from 220 °C.
EDS mapping images of silicon and carbon elements of cross-section of povidone-SiO2 films not annealed and annealed at temperatures of 150, 200 and 240 °C are depicted in Fig. 4a–d. The uniform distributions of both silicon and carbon elements in nano-composite structure are evident in povidone-SiO2 nano-composite films not annealed and annealed at 150 and 200 °C. It is confirmed that organic and inorganic particles are homogeneously dispersed in nanocomposite structure. However the structure of nano-composite film aggregates at 200 °C. It is might be because of the interactions organic and inorganic phases separately which confirms FTIR results. The EDS mapping image of the film annealed at highest temperature (240 °C), displays extremely significantly high dispersion of silicon elements. It suggests that nano-composite structure degraded at 240 °C.
3.4 Electrical studies
The capacitance–Voltage measurements were done at various frequencies of 0.12, 1, 100 and 500 KHZ (at room temperature) using Keithy K361 parameter analyzer on the metal-insulator-semiconductor (MIS) structure. An Au metal, povidone-SiO2 nanocomposite and Si (p) were used as a gate, insulator and semiconductor films, respectively. Fabricated MIS capacitors based on insulating povidone-SiO2 films not annealed and annealed at 150, 200 and 240 °C were called MIS capacitor A, B, C and D. The average capacitance of samples obtained in voltage range of −5 to 5 V is compared in Fig. 5. When frequency is increased, the average capacitance of all samples is decreased (from about 10−8 F at 0.12 KHZ to about 10−12 F at 1, 100 and 500 KHZ). It is probably due to electrical dipoles to follow the changes in an applied electric field at low frequencies which are not too fast to affect the rotation and orientation of dipoles [13, 28]. The electric dipoles orientation leads to the polarization which influences on electrical capacitance [13, 28].
As annealing temperature of the nano-composite films increase up to 200 °C, the capacitance of samples increase and then decrease in sample D (at all frequencies). It could be attributed to the increased organic phase of insolating povidone-SiO2 film, as EDS analyze shows (see Fig. 3a–d). Because the present of polar groups (like C=O and C–H) raise the electrical capacitance [25, 28].
Insolation characteristic of nano-composite films was studied by I–V measurements on the MIS structure at room temperature. The Au metal as a gate, source and drain electrodes, povidone-SiO2 nano-composite as an insulator and silicon (p) with (100) surface orientation as a semiconductor were applied. The source and drain electrodes were patterned on Si (p) films by standard photolithography. The gold contacts were thermally evaporated using shadow mask on top-contact structure. Fabricated FET based on insulating povidone-SiO2 films not annealed and annealed at 150, 200 and 240 °C were called FET A, B, C and D. Fig. 6 shows semi-log I–V curves of fabricated FETs in gate voltage of −5 to 5 V. The lowest leakage current density belongs to sample B (4.25 × 10−8 at −5 V). It can be related to the strongest interactions between organic and inorganic particles of dielectric nano-composite material annealed at 150 °C (according to FTIR results). Because cross-linked dielectric material decrease gate leakage current [22,23,24,25]. The larger thickness of the dielectric film could also lead to decrees in leakage current [31,32,33]. This value is lower than many high-K oxides and pure organic dielectric films [3, 7,8,9]. The leakage current density of samples C and D increase. It is known that the nano-structure of interface between dielectric and semiconductor films influences on the charge transport in the FET channel [12, 24, 25]. Discontinues dielectric nano-composite film annealed at 200 °C (according to Fig. 4c) could trap the mobile charges at semiconductor-insulator interface. These trapped charges can be leaked to gate film through gaps and defects formed in dielectric film structure [34]. The degradation of nano-composite structure and the concomitant desorption of organic phase in dielectric film annealed at 240 °C (according to EDS results) can also increase leakage current [12, 24, 25].
4 Conclusions
In this study, the presence of organic particles and cross-linking agent prevented the aggregation of SiO2 nanoparticles. The leakage current of FET based on povidone-SiO2 gate dielectric reduced compared to that based on SiO2 gate dielectric. The dielectric nano-composite film structure was affected by various annealing temperatures. The annealing temperature of 150 °C was suitable for the interactions between organic and inorganic phases. At 200 °C, the structure of nao-composite dielectric film aggregated. At highest annealing temperature of 240 °C, the structure of nao-composite dielectric film degraded. The reduced leakage current of povidone-SiO2 dielectric film annealed at 150 °C is a key point for gate dielectric replacing SiO2 in electronic devices.
References
A. Bahari, M. Roeinfard, A. Ramzannezhad, J. Mater. Sci. 27, 9363 (2016)
A. Bahari, D. Shajari, S. Khodadad, Int. J. Electrochem. Sci. 8, 9338 (2013)
A. Bahari, R. Gholipur, J. Electron. Mater. 42, 12 (2013)
S.K. Ray, R. Mahapatra, S. Maikap. J. Mater. Sci. 17, 689 (2006)
A. Bahari, A. Qhavami, J. Mater. Sci. 27, 5934 (2016)
H.-W. Lu, J.-G. Hwu, Appl. Phys. A 115, 837 (2014)
J.P. Xu, C.X. Li, C.L. Chan, P.T. Lai, Appl. Phys. A 99, 177 (2010)
A. Srivastava, R.K. Nahar, C.K. Sarkar, W.P. Singh, Y. Malhotra, Microelectron. Reliab. 51, 751 (2011)
L.Q. Khor, K.Y. Cheong, J. Mater. Sci. 24, 2646 (2013)
J. Li, W. Shi, L. Shu, J. Yu, J. Mater. Sci. 26, 8601 (2015)
X. Li, W. Shi, X. Yu, J. Yu, J. Mater. Sci. 26, 7948 (2015)
M. Shahbazi, A. Bahari, S. Ghasemi. Synth. Met. 221, 332 (2016)
M. Shahbazi, A. Bahari, S. Ghasemi, Organ. Electron. 32, 100 (2016)
B. Soltani, M. Babaeipour, A. Bahari, J. Mater. Sci. 28, 4378 (2017)
A. Bahari, M. Babaeipour, B. Soltani. J. Mater. Sci. 27, 2131 (2016)
A. Hashemi, A. Bahari, S. Ghasemi. Appl. Surf. Sci. 416, 234 (2017)
Hayati A., A. Bahari, JNS 4, 9 (2014)
M. Roodbari-Shahmiri, R. Gholipur, A. Bahari, J. NanoMater. Biostruct. 7, 1509 (2012)
D. Dastan, A. Banpurkar, J. Mater. Sci. 28, 3851 (2017)
A. Bera, A.J. Pal, J. Phys. Chem. C 120, 19011 (2016)
S.K. Saha, A. Bera, A.J. Pal, ACS Appl. Mater. Interfaces 7, 886 (2015)
J.J. Wang, W.-H. Lee, J. Chung-Ho, T. Shiang-Hu, J. Mater. Sci. 20, 355 (2009)
J.C-C. Wu, S. Ray, M. Gizdavic-Nikolaidis, J. Jin, R.P. Cooney, Synth. Met. 217, 202 (2016)
Nawaz A., M.S. Meruvia, D.L. Tarange, S.P. Gopinathan, A. Kumar, A. Kumar, H. Bhunia, A.J. Pal, I.A. Hummelgen, Organ. Electron. 38, 89 (2016)
M.D. Morales-Acosta, C.G. Alvarado-Beitra, M.A. Quevedo-Lopez, B.E. Gnade, J. Non-Cryst. Solids 326, 124 (2013)
K. Chrissafis, K.M. Paraskevopoulos, G.Z. Papageorgiou, D.N. Bikiaris, J. Appl Polym. Sci. 110, 1739 (2008)
C.N. Hsiao, K.S. Huang, J. Appl. Polym. Sci. 96, 1936 (2005)
M.D. Morales-Acosta, M.A. Quevedo-Lopez, J. Sol-Gel Sci. Technol. 58, 218 (2011)
B. Gao, Z. Wang, Q. Liu, R. Du, Colloids Surf. B 79, 446 (2010)
K. Chrissafis, K.M. Paraskevopoulos, G.Z. Papageoriou, D.N. Bikiaris, J. Appl. Polym. Sci. 110, 1739 (2008)
S. Faraji, T. Hashimoto, M.L. Turner, L.A. Majewski, Organ. Electron. 17, 178 (2015)
L.S. Cardoso, J.C. Stefanelo, R.M. Faria, Synth. Met. 220, 286 (2016)
Y. Ran, L.Z. Dong, H.Y. Feng, C.S. Bo, T. Feng, H.Y. Bing, L.X. Jun, Sci. China 57, 1142 (2014)
X. Wu, F. Fei, Z. Chen, W. Su, Z. Cui, Compos. Sci. Technol. 94, 117 (2014)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hashemi, A., Bahari, A. & Ghasemi, S. Reduction the leakage current through povidone-SiO2 nano-composite as a promising gate dielectric of FETs. J Mater Sci: Mater Electron 28, 13313–13319 (2017). https://doi.org/10.1007/s10854-017-7167-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-017-7167-0