Abstract
Manganese–zinc (MnZn) ferrite nanoparticles were synthesized via a hydrothermal method, and their structural and magnetic properties were studied. It was found that a low Fe content and the hydrothermal temperature play crucial roles in the synthesis of single phase MnZn ferrites. The hydrothermal temperature also affects the topography and the magnetic properties markedly. The best soft magnetic properties of M s = 45.5 emu/g and H c = 0.68 kA/m were obtained for the specimen with a Fe deficiency of 7% in the starting materials and hydrothermally synthesized at 180 °C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
As one of the most important soft-magnetic materials, spinel manganese–zinc (MnZn) ferrite is widely used as the core of power transformers and inductors due to their relatively high initial magnetic permeability, high saturation magnetization and low power loss [1]. Nanoparticles have some attractive performances, which promotes the extensive studies of many magnetic materials, in which including nanoscale MnZn ferrite [2, 3]. As a main chemical method to prepare nanomaterials, the sol–gel technique is currently often used to obtain MnZn nanoferrites, such as the studies from Peng [1], Lutsev [4], Ebrahimi [5], Choi [6] and so on. As is well-known, the nanoparticles obtained via a hydrothermal or solvothermal method are homogeneous and ultrafine. However, compared with the sol–gel method, the hydrothermal or solvothermal method is seldom used to synthesize MnZn ferrite possibly due to the difficulty of formation of single phase. Recently, Freire and Drofenik [7, 8] studied the superparamagnetic properties and the effect of solvent composition on the structural and magnetic properties of MnZn ferrite nanoparticles. Rozman [9] studied the sintering of nanosized MnZn ferrite powders. However, the synthesis of single-phase MnZn ferrite was not discussed in these reports. Recently, we have systematically studied the synthesis of MnZn nanoferrites obtained via a hydrothermal method, and studied the effects of hydrothermal temperatures and low Fe content in the starting materials on the phase formation and magnetic properties.
2 Experimental
2.1 Synthesis of MnZn nanoparticles
MnZn ferrites with a nominal composition (Mn0.4Zn0.6)Fe2O4 were synthesized via a hydrothermal method. Analytically pure nitrates (Mn(NO3)2, Fe(NO3)3, Zn(NO3)2 and NaOH) were used as the starting materials without further treatment. According to our previous study in spinel NiZnCu ferrites [10], excess Zn2+ ions of 5% in the starting materials benefit the synthesis of single-phase MnZn ferrites for a wet chemical method due to the loss of Zn2+ ions during the process of chemical reaction. A deficiency of Fe3+ ions (DF, 6 or 7%) in the starting materials was set to study the synthesis of single-phase MnZn ferrite. First, all the nitrates for preparing 4 mmol MnZn ferrites were dissolved in the 60 ml distilled water, and 1.37 g NaOH was dissolved in 15 ml distilled water. The NaOH solution was dropped into the mixed nitrates solution until the pH value is 11, and was simultaneously stirred vigorously by a magnetic stirrer for 10 min. Then, the aqueous solution and obtained precipitates were moved into a Teflon liner and hydrothermally reacted under different temperatures (T h , 180, 200 and 220 °C) for 6 h. Finally, the powders obtained were used as the specimens after washing by deionized water and absolute ethylalcohol for several times, respectively.
2.2 Characterization
The phase identification was carried out by using an X-ray diffractometer (XRD, Rigaku D/max-2550V/PC) with Cu Kα radiation and a Fourier transformed infrared spectrometer (FTIR, Nicolet 6700). The micrographs were obtained by using a field emission scanning electron microscope (FESEM, Zeiss Merlin Compact). The magnetic hysteresis loops (MHLs) were measured on a vibrating sample magnetometer (VSM, Lakeshore 7410) under a maximum external field H m = 1591 kA/m (~20,000 Oe).
3 Results and discussion
3.1 Phase composition and microstructure
In our previous experiments, it was found that Fe2O3 is the most common impurity in the specimens obtained via a hydrothermal process, which can be ascribed to the existence of amphoteric Zn(OH)2 in the precursor of hydrothermal reaction [10]. Therefore, it is necessary to use the starting materials with low Fe content to synthesize the single-phase MnZn ferrites in this study.
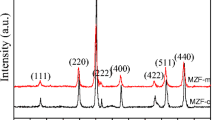
Figure 1 gives the XRD patterns of MnZn ferrites synthesized with the same DF (6%) but different T h . Seen from the figure, there is typical peak information from spinel structure (JCPDS no. 22-1012), which suggests the formation of spinel MnZn ferrites in the specimens. However, there are also impurity peaks from Fe2O3 (JCPDS no. 33-0664) in the figures. Moreover, seen from the relative intensity of peaks from Fe2O3, the content of Fe2O3 increases with the increase of temperature from 180 to 220 °C. Obviously, a higher temperature may have an adverse effect on the phase formation of MnZn ferrite during a hydrothermal process. In order to synthesize single-phase MnZn ferrite, the specimens with DF = 7% were synthesized at different T h , and the corresponding XRD patterns are shown in Fig. 2. Compared with the standard pattern of typical spinel structure (JCPDS no. 22-1012), no impurity peak can be found in Fig. 2a, which suggests a single-phase specimen of MnZn ferrite for T h = 180 °C and DF = 7%. However, there are still small impurity peaks from Fe2O3 in the XRD patterns of specimens with T h = 200 and 220 °C. Obviously, the low Fe content in starting materials and the hydrothermal temperature affect the phase formation of MnZn ferrites greatly during a hydrothermal process.
In order to confirm the formation of spinel structure, the FTIR spectra of specimens synthesized with DF = 7% were measured and given in Fig. 3. As is well-known, Fe3+ ion can occupy both the tetrahedral and the octahedral sites in a spinel structure. In Fig. 3, the characteristic band around 563 and 400 cm−1 can be attributed to the vibrations of Fe–O (Fe3+) bond in the tetrahedral and octahedral sites, respectively [11, 12]. It is also found that though there is impurity Fe2O3 in the specimens of T h = 200 and 220 °C, no obvious difference can be found for the FTIR spectra, suggesting a small amount of impurity in these specimens. The result of FTIR confirms the formation of MnZn ferrites further.
Figure 4 is the SEM images of MnZn ferrites synthesized with DF = 7%. It is found that all the specimens consist of ultrafine nanoparticles. Compared with some pervious reports [13, 14], our specimens exhibit a more uniform distribution of grains. Several particles’ size has been marked in Fig. 4c. Compared Fig. 4c with a and b, there is a marked increasing tendency of average grain size from less than 10 nm to more than 20 nm with the increase of T h from 180 to 220 °C. Obviously, the hydrothermal temperature has a significant effect on the topography of MnZn nanoferrites.
3.2 Magnetic properties
The magnetic hysteresis loops of MnZn ferrites synthesized with different DF and T h measured on a VSM are shown in Fig. 5, and the corresponding saturation magnetization (M s ) and coercivity (H c ) are listed in Table 1.
The H c of most specimens is lower than 0.8 kA/m (10 Oe), exhibiting a typical and fine soft magnetic property. However, the H c is decided not only by the intrinsic properties, such as the chemical composition and crystalline structure, but also by the extrinsic properties, such as the average crystallite size, morphology, stress, lattice defects and so on [15]. Seen from Table 1, the variation of H c is obviously irregular.
As is known, due to the surface effect of magnetic nanoparticles [16], the M s should decrease with the decrease of average grain size in MnZn ferrites. Seen from Fig. 4, the grain size of specimens increases obviously with the increase of T h . However, in Table 1, it can be found a marked decreasing tendency of M s with the increase of T h . Combined with the results of XRD, it is clear that the effect from the impurity on M s exceeds that from the surface effect. In addition, compared with the specimens synthesized with DF = 6%, the M s of the corresponding specimens with DF = 7% and the same T h is larger, which is also can be attributed to the effect of impurity. The specimens with DF = 7% have less impurity than that of the corresponding specimens with DF = 6%. In general, the specimen with DF = 7% and T h = 180 °C whose M s is 45.5 emu/g and H c is 0.68 kA/m (8.6 Oe) exhibits the best soft magnetic properties.
4 Conclusions
Single-phase MnZn ferrites were successfully synthesized via a hydrothermal method using starting materials with a low Fe content. It is found that both a low Fe content and the hydrothermal temperature play crucial roles in the formation of single phase MnZn ferrite, which consequently affects the topography and the magnetic properties markedly. When DF = 7% and T h = 180 °C, the obtained specimen exhibits the best soft magnetic properties (M s , 45.5 emu/g; H c , 0.68 kA/m).
References
Y.D. Peng, L.L. Chen, H.W. Ren, L.Y. Li, J.H. Yi, Q.L. Xia, J. Mater. Sci. 27, 587 (2016)
H.W. Cheng, J. Li, S. Wong, C.J. Zhong, Nanoscale 8, 19359 (2016)
M.J.N. Isfahani, M. Myndyk, D. Menzel, A. Feldhoff, J. Amighian, V. Šepelák, J. Magn. Magn. Mater. 321, 152 (2009)
L. Lutsev, V. Shutkevich, J. Phys. D 49, 505002 (2016)
S.A.S. Ebrahimi, S.M. Masoudpanah, H. Amiri, M. Yousefzadeh, Ceram. Int. 40, 6713 (2014)
W.O. Choi, W.H. Kwon, K.P. Chae, Y.B. Lee, J. Magn. 21, 40 (2016)
R.M. Freire, T.S. Ribeiro, I.F. Vasconcelos, J.C. Denardin, E.B. Barros, G. Mele, L. Carbone, S.E. Mazzetto, P.B.A. Fechine, J. Nanopart. Res. 15, 1616 (2013)
R.M. Freire, P.G.C. Freitas, T.S. Ribeiro, I.F. Vasconcelos, J.C. Denardin, G. Mele, L. Carbone, S.E. Mazzetto, P.B.A. Fechine, Microfluid. Nanofluid. 17, 233 (2014)
M. Rozman, M. Drofenik, J. Am. Ceram. Soc. 81, 1757 (1998)
A.L. Xia, H.L. Zhang, Curr. Appl. Phys. 10, 825 (2010)
R.H. Kadam, S.T. Alone, M.L. Mane, A.R. Biradar, S.E. Shirsath, J. Magn. Magn. Mater. 355, 70 (2014)
I. Szczygieł, K. Winiarska, A. Bieńko, K. Suracka, D. Gaworska-Koniarek, J. Alloys Compd. 604, 1 (2014)
L. Xiao, T. Zhou, J. Meng, Particuology 7, 491 (2009)
L. Nalbandian, A. Delimitis, V.T. Zaspalis, E.A. Deliyanni, D.N. Bakoyannakis, E.N. Peleka, Microporous Mesoporous Mater. 114, 465 (2008)
A.L. Xia, X.Z. Hu, D.K. Li, L. Chen, C.G. Jin, C.H. Zuo, S.B. Su, Electron Mater. Lett. 10, 423 (2014)
A.L. Xia, C.H. Zuo, L.J. Zhang, C.X. Cao, Y. Deng, W. Xu, M.F. Xie, S.L. Ran, C.G. Jin, X.G. Liu, J. Phys. D: Appl. Phys. 47, 415004 (2014)
Acknowledgements
This work was supported by the Provincial Training Programs of Innovation and Entrepreneurship for College Students (TPIECS) under Grant No. 201510360134.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, X., Sun, R., Luo, B. et al. Synthesis and magnetic properties of manganese–zinc ferrite nanoparticles obtained via a hydrothermal method. J Mater Sci: Mater Electron 28, 12268–12272 (2017). https://doi.org/10.1007/s10854-017-7043-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-017-7043-y