Abstract
CuO thin films prepared by spray pyrolysis method has wide range of applications. CuO thin films were coated on glass substrate at various temperatures such as 250, 350, 400, and 500 °C by spray pyrolysis. Thin films prepared were characterized by various techniques such as X-ray diffraction, SEM, UV–visible spectroscopy, PL and Hall measurements. XRD diffraction patterns reveal that the films are polycrystalline in nature exhibiting monoclinic structure. The prominent peak corresponds to (200) plane and the corresponding calculated crystallite size varies from 4 to 7 nm. SEM images exhibit grains of uniform size. It is found that the optical absorbance decreases with the increase in temperature and the optical energy band gaps vary from 2.22 to 2.75 eV for various substrate temperatures. Electrical studies done using Hall measurement system show that the copper oxide films are p-type semiconductors with carrier concentration in the order of 1016 cm−3. The electrical resistivity of the copper oxide films obtained was in the range 3.689 × 10−3 to 8.538 × 10−3 Ω cm. Hence, the CuO film is suitable for solar cell applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Transparent conducting oxides (TCOs) gain momentum in semiconductor technology due to their low cost and high performance [1]. In research studies done earlier using spray pyrolysis, CuO thin films were obtained with fixed or varying molar concentrations of solution at fixed or varying temperatures and significant changes in optical and morphological properties were observed [1, 2]. Transparent conducting oxides for instance, pure copper oxide, indium oxide, zinc oxide, tin oxide and cadmium oxide are broadly studied because of their utilization in optoelectronic device technology. To know about their chemical and physical properties, to use them for various applications, it is essential to characterize them. The obtained experimental results indicate the suitability of this material as transparent and conducting window materials in thin film solar cells and gas sensor devices [2, 3]. In particular, CuO based TCOs are of great attention due to their metal like charge transport behaviour with an incredibly large carrier mobility. Metal oxide thin films have received great attention in the recent days for their special characteristics in optical properties for solar cell fabrication. Moreover, CuO is considered as a material in capacitive type gas sensor for sensing and detecting a large number of gases. CuO films are used in the fabrication of optoelectronic devices such as solar PV cells, photo transistors, photo diodes and transparent electrodes in flat-panel displays, IR detectors anti-reflection coatings and solar thermal applications as well. Structural and optical properties combined with the surface morphology, for the films deposited on glass substrates at variable temperatures and spray times were examined [4, 5]. These copper oxides have been grown by reactive ion-sputtering, oxidation of evaporated copper metal, and metal organic chemical vapour deposition and spray pyrolysis. Further, it is a suitable candidate for a window layer and hetero junction solar cells [6]. As the sensitivity and electrical conductivity of the CuO films are consistent, they are used as gas sensors as well.
The materials offering high performance with the ease of preparation at lower cost for fabrication of thin films is always essential for today’s technology. Non stoichiometric copper oxide thin films usually exhibit low resistivity due to native defects of oxygen vacancies and copper interstitials. Therefore, the resistivity and conductivity of the films can be improved by a controlling various temperature of these native defects. It is controlling from order to control the optical and electrical properties of CuO thin films, the type and properties of copper oxide ions play a crucial role. Bulk CuO has an energy bandgap of 2.7 eV which offers high optical transparency in the short wavelength range of the visible spectra. The microstructural and physical properties of metal oxide can be modified by introducing changes into the procedure of its chemical synthesis. Especially for the application of CuO as gas sensors porous microstructure of the materials with controlled pore size is preferred [7]. It is found that when CuO films are thick ~350 nm it provides improved electrical conductivity and change its optical energy bandgap for lower temperatures up to 450 K and becomes temperature independent at higher temperatures [8].
CuO films have been deposited by all conventional deposition methods, which include thermal oxidation [9, 10]; electrodeposition [11]; dip coating [12]; chemical vapor deposition [13]; plasma evaporation [14]; and reactive sputtering [15] techniques. The CuO films have also been grown typically prepared by thermal oxidation of copper substrate [16, 17]. Attempts were made to reduce the grains radius from micrometer dimensions to nanometer dimensions. The preparation of nanocrystalline CuO thin films improves the performance of solar cell application devices. Nanotechnology therefore, helps in forming more preferred material for solar applications [18]. In the present work, we have prepared CuO films by spray pyrolysis method and investigated the properties using a various characterization tools. In order to enhance the conductivity. We have chosen the material at various temperatures ranging from 250 to 500 °C and systematically studied CuO thin films using the various structural, optical and electrical characterization techniques. As transparent conducting window materials in thin film solar cells and gas sensor device. Today the use of solar energy is strongly related to the conversion device efficiency and its cost. Integrated solutions for solving these two problems represent a subject of involving advances in cost–benefit analysis and simple fabrication method that was chosen in this work.
2 Experimental details
Copper acetate was dissolved in distilled water at room temperature and water was used as solvent to synthesize CuO thin films using spray pyrolysis technique. The copper Acetate solution (CH3COO)2Cu·H2O) was sprayed onto the cleaned at 0.1 M concentration and glass substrates heated at four different Temperatures, namely 250, 350, 400, and 500 °C. Well cleaned glass substrates were placed on the heater and a distance of 18 cm was maintained between the tip of the nozzle and surface of the substrate. A constant flow rate, 3 ml/min has been chosen for all films which would be sprayed on substrates kept at temperature of namely 250, 350, 400, and 500 °C. Before supplying the compressed air, the heater was allowed to heat the substrate to attain the required temperature. When compressed air along with the precursor solution was passed through the nozzle at constant pressure, finely formed aerosol descended to reach the reactor zone where the film was deposited of the heated substrate. The chemical reactions that take place on the heated substrate to produce CuO films may be given as follows [19]:
X-ray diffraction analysis was performed using X ray diffractometer (Shimadzu X-600) in order to study the crystal structure of the spray pyrolysed thin films. The surface morphology was recorded using scanning electron microscope (SEM, JOEL 2-JSM 6000). Optical transmission spectra of all the deposited films were obtained using UV–VIS spectrophotometer (UV Vis NIR Jasco V-670). The electrical studies were carried out using Hall measurement system (D.O.No. SR/S2/CMP-35/2004).
3 Results and discussion
3.1 X-ray diffraction analysis
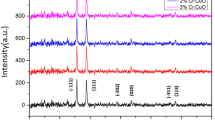
The spray pyrolysed thin films were smooth, well adherent and visually transparent. Figure 1 shows the X-ray diffraction patterns of CuO thin films deposited at 250, 350, 400, and 500 °C substrate temperatures. The good agreement between experimental data and JCPDS data card No. 89-2838 confirms the formation of monoclinic crystal structure. For temperatures 250, 350, 400, and 500 °C film, the peaks appearing in the diffraction spectrum at 2θ values of 38.67 and 35.63 °C indexed to (111), (200) are allowed planes, respectively indicating the polycrystalline nature of the CuO thin film. As the substrate temperature increases, the intensity of (111) plane increase evidently up to 500 °C and then decreases for higher substrate temperatures but intensity of (200) plane increases with substrate temperature, exhibiting crystal reorientation effect [19]. The determined lattice constant for the dominant peak of CuO is found to be a = 5.23–7.09 nm with increase in substrate temperature.
The crystallite size is estimated using the Scherrer formula.
where D is the crystallite size, λ is the wavelength, β is the full width at half maximum of diffraction peak measured in radians and θ is the Bragg’s angle of diffraction. The variation in crystallite size for (111), (200) plane of CuO thin films deposited at various substrate temperatures is shown in Table 1.
The dislocation density (δ) was calculated using the relation,
From the calculation it is observed that ‘δ’ decreases with increasing annealing temperature which implies decrease in lattice imperfection due to increase in crystallite size.
The micro strain (ε) was calculated from the relation,
The strain gets lower for increasing temperature gives the best crystalline structure. The microstrain and dislocation density for the CuO films were obtained from full width at half maximum which can be expressed as linear combination of contributes from the particle size, D and strain ε given above. The dislocation density (δ) and the microstrain (ε) of CuO thin films with different deposition potentials. Dislocation density value is low so defect is very less. Microstrain and dislocation density are increasing with increase in deposition potential.
3.2 Morphological studies
Figure 2a–d shows that the top and cross sectional view (top-right) of (SEM) scanning electron microscopy images of Copper Oxide thin films. This surface morphology of the sample of copper thin film at temperatures 250, 350, 400 and 500 °C films sample show smooth coating of copper on the substrate with dense uniform tiny grains.
The film had a metallic brown color of Cu. From the SEM images, the thicknesses of all the samples are measured and the values range from 445 to 779 nm (Table 1). It is observed that as the temperature varies, the thickness of the samples is also found to increase which is a consistent with the reported results [19]. The surface of the sample turned black but with a hue of reddish brown underneath, which indicates partial oxidation of the film. This may be result of high stress on the film that can lead to a peeling off from this substrate.
3.3 EDAX analysis
Figure 3 shows the (EDAX) Energy Dispersive X-ray analysis is one of these various versatile techniques have been used for determining that chemical composition of unknown material, by identifying the peaks in an EDAX spectrum which is that unique to an atom and therefore corresponds to a single element. Figure 3 shows the EDAX spectrum of CuO thin films. The Ka radiation shown in figure indicates that all the electronic transition from ranges is L-shell to K-shell. Energy Dispersive X-ray analysis are spectrum proves that a synthesize samples are composed of an Cu and O elements by the representation of different copper and oxygen peaks. So, that the atomic percentage of Cu and O is 54.12/45.88 (Table 2) for 0.1 molar concentration. The elemental composition analysis shows that the surface of the samples was rich in copper for molar concentration 0.1 [20].
3.4 Optical studies
3.4.1 Absorbance and bandgap
The values of absorbance wavelength spectra were recorded graphically for the CuO film at various temperatures to obtain information of optical properties wavelength of the copper oxide thin films. These absorbance spectra of the copper oxide films obtained in the range between the 200 and 900 nm is shown in Fig. 4 These absorbance values slightly increase in the range of 250–800 nm and then rabidly increases revealing the modification of the glass substrate and temperature [21]. Besides, as can be seen in Fig. 4 shows the optical absorbance varies with respect to the temperature value. The effect of solution temperature on the absorbance of the copper oxide films may be due to the variations associated with film thickness, structural properties, surface smoothness and defect density, etc. From (XRD) X-ray diffraction analyses, we have determined that the films consist of CuO monoclinic phase. On the other hand, CuO crystalline is a monoclinic structural and it has a biaxial and optically anisotropic. The double refraction is a polycrystalline films brings that the light scatterings. Hence the reduced absorbance in higher solution temperatures prepared film is a due to that light scatterings from the CuO thin films. Also, the decrease of absorbance values can also be interpreted with inferences from the (XRD) X-ray diffraction spectra. The absorbance spectrum shows the surface plasmon’s resonance indicating that crystallite sizes are in the nanometer ranges. It is observed that absorbance decreases with the wavelength from 60 to 50%. The values and maximum range of absorbance for CuO thin film deposited at concentration 0.1. Since copper oxide thin films are some p-type materials, and their absorption property is important. Also, all films have direct band gaps, and this is a property is suitable for the photovoltaic applications. These optical parameters such as absorption coefficient and band gap measurement are determined from the optical absorption measurements.
The value of absorption coefficient for strong absorption co-efficient region of thin films are calculated using that following Eq. (5),
where α is the absorption coefficient in cm−1, t is that thickness off the films, A is that absorbance and T is that transmittance. The nature of transition is determined using the following Eq. (6) [21],
where α is the absorption coefficient in cm−1, hϑ is the photon energy in eV, Eg is an energy gap in that Ev and K is constant which is then related to effective masses of associated with the valence and conduction band. The values of ‘n’ determine that types of transition and present in the materials. In this case n = ½ indicate that the transition involved in the material is direct allowed. A plot of hϑ versus (αhϑ)1/2 for CuO thin films obtained at solution 0.1 concentration of values shown in Fig. 5 shows the extrapolation of linear portion of the graph to X-axis [energy (hv) axis] gives the bandgap values of present in the material. Mainly bandgap values of the material obtained in the present work is found be 2.39, 2.41 and 2.49, 2.55 eV for solution concentration of 0.1 respectively. This is close agreement with the value reported earlier for CuO thin films [21]. It is found that there are significant variations in the optical band edge, it will be due to a thickness of the thin films.
3.4.2 Refractive extinction coefficient
These value of a refractive index are (n) and extinction coefficient of (k) are determined using the following Eqs. (8) and (9). The value of real and imaginary dielectric constants (ε1 and ε2 are evaluated using the following Eqs. (10) and (11):
where n is the refractive index of the material, k is the extinction coefficient, ε1 and ε2 are real and imaginary dielectric constants, R is reflectance (%) and λ is the wavelength in nm. The refractive index and extinction coefficient values are presented at various temperature values is as shown in Fig. 6 shows the maximum value of refractive index is observed at 2.49, 3.49,4.00,5.00 respectively [21].
This refractive index variation may be a due to that film thickness. The same variation of an observed in the extinction coefficient of the CuO thin films as shown in Fig. 6 In this case lower value is observed at 0.085 for CuO thin film prepared at different temperatures.
The complex dielectric constant is known to be fundamental intrinsic material property. The real part of dielectric constant is associated with the property of slowing down of the speed of light in the material. The maximum value of real part of dielectric constant is observed at the film prepared at different temperatures and it is due to the higher refractive index value. The film prepared at solution many temperatures value of imaginary part of the dielectric constant value may be associated with low extinction coefficient value. The real part of the dielectric constant relates to the dispersion, while the imaginary part of dissipative rate in the wave of the medium. The increased of optical conductivity of CuO optical conductivity at high photon energies is due to the high absorbance of copper oxide thin films and also may be due to the electron excited by photon energy.
3.4.3 Photoluminescence
Figure 7 represents three emission bands at 514, 530 and 551 nm respectively, along green and blue emission are exhibited in CuO thin film [22] when excited with 260 nm. The peak 551 nm are more intense than 514 and 540 nm peaks. The central factor of this discussion is the nature of the characterized samples. It must be pointed out that the CuO samples are used to study the PL behavior is usually synthesized with some distinct methods. Furthermore, the relative peak intensities of each peak depend also corresponding radiative combination efficiency. Thus PL spectra of the CuO samples demonstrate that the fabricated material has an enough quality to be used for the research of semiconductor devices.
3.5 Electrical studies
Hall effect measurements were carried out different temperature (CuO) copper oxide thin films. The (RH) hall co-efficient values are calculated for the different temperature based CuO films using the relation RH = 1/ne where e is the electronic charge. The (µ) mobility of the carriers is calculated using the relation µ = σ/ne.
Figure 8 shows that variation of mobility and carrier concentration in CuO thin films with different temperature concentration. It is observed that these carrier concentration goes higher as that dopant concentration increases. Mobility dropped continuously from a high value of 60–10 cm2/vs. When the temperature changed from 250 to 500 °C which might be due to greater ionized. Impurity scattering. From the electrical conductivity and the hall measurement data, it is evident that increase in the carrier concentration up to 3.00 × 1016 cm−3. To improve the conductivity, the carrier concentration needs to be a higher value of this can also be that achieved by heavy temperatures. This in turn might degrade the transparency of the mobility which may lead to a more charge carrier scattering from the ionized impurities. CuO thin film is a P-type semiconductor and shows the good electrical conductivity are even without any temperatures. The oxygen vacancies and Cu interstitials are responsible for high electrical conductivity in thin films. First, the conductivities on the rise with increase in temperature variation.
Figure 9, increase in observed electrical conductivity due to can be explained are as follows: the concentration of free charge carriers in CuO becomes higher with copper oxide valence electron more when compared to copper [23]. We may consider that copper atom or it occupies the interstitial sites. In both cases the copper results in higher conductivity. On the contrary, copper oxide crystallite size structure which may be result in a greater absorption of a free carrier to diminish the conductivity in copper oxide different thin film substrate temperature.
4 Conclusion
Thin film substrate of CuO have been deposited successfully onto glass substrates using chemical spray pyrolysis method, technique at 250, 350, 400 and 500 °C respectively. X-ray diffraction analysis revealed that the prepared films were polycrystalline in nature with monoclinic structure with preferential orientation along (111) plane. Also the microstructural and parameters such as that crystallite size and micro strain, dislocation density were estimated. From cross sectional SEM (Scanning electron microscopy) images, higher concentration at thicker films was evident. (EDAX) Energy dispersive X-ray analysis revealed with films that CuO elements are good substrate at specific temperature for coating using spray pyrolysis technique. The optical absorbance measurements indicate that the deposited film indirect bandgap value of 2.22, 2.36 and 2.55, 2.75 Ev for 250, 350, 400 and 500 °C respectively. Photoluminescence (PL) spectra of the CuO samples demonstrate that the produced material has enough quality to be used in the research of semiconductor devices. From Hall effect measurements it is found that the charge carrier concentration and mobility also increases. Therefore, the CuO system with monoclinic structure may be an attractive choice for solar cell applications and good quality films using spray pyrolysis techniques can be fabricated.
References
I. Akyuz, S. Kose, F. Atay, V. Bilgin, Mater. Sci. Semicond. Process. 13, 109–114 (2010)
S.S. Roy, A.H. Bhuiyan, J. Podder, Sens. Transducers, 18, 21–27 (2015)
T. Mahalingam, V. Dhanasekaran, G. Ravi, S. Lee, J.P. Chauhan-Jo Lim, J. Optoelectron. Adv. Mater. 252, 1327–1332 (2010)
J. Charles, Lawrence, S. Rajbharath, Int. J. Adv. Sci. Res. Dev. 20, 28–33 (2015)
H.H. Afify, S.E. Demian, M.A. Helal, F.A. Mahmoud, Indian J. Pure Appl. Phys. 37, 379–383 (1999)
R. Kumaravel, S. Menaka, S.R.M. Snega, K. Ramamurthi, K. Jeganathan, Mater. Chem. Phys. 122, 444–452 (2010)
R. Kumaravel, S. Bhuvaneswari, K. Ramamurthi, V. Krishna Kumar, Appl. Phys. A 5(109), 579 (2012)
M. Muhibbullah, M.O. Hakim, M.G.M. Choudhury, Thin solid Films 423, 103–107 (2003)
I. Singh, R.K. Bedi, Appl. Surf. Sci. 257, 7592–7599 (2011)
Y.S. Chaudhary, A. Agrawal, R. Shrivastu, V.R. Satsangi, S. Dass, Int. J. hydrog. Energy 29, 131–134 (2004)
K.R. Nemade, S.A. Waghuley, Int. J. Mater. Sci. Eng. 2, 63–66 (2014)
T. Kosugi, S. Kaneko, J. Ceram. Sci. 81, 3117–3124 (1998)
Y.S. Gong, C. Lee, C.K. Yang, J. Appl. Phys. 77, 5422–5425 (1995)
L.S. Huanga, S.G. Yanga, T. Lia, B.X. Gua, Y.W. Dua, Y.N. Lub, S.Z. Shi, J. Cryst. Growth 260, 130–135 (2004)
E.R. Kari, K.S. Brown, Choi, Chem. Commun. 3, 3311–3313 (2006)
N. Serin, T. Serin, S. Horzum, Y. Celik, Semicond. Sci. Technol. 20(5), 398 (2005)
T. Maruyama, Jpn. J. Appl. Phys. 37, 4099–4102 (1998)
K. Santra, C.K. Sarkar, M.K. Mukherjee, B. Ghosh, Thin Solid Films 213, 226–229 (1992)
S.J. Helen, S. Devadason, T. Mahalingam, J. Mater. Sci.: Mater. Electron. 27, 4426–4432 (2016)
C. Ravi Dhas, D. Alexander, A. Jennifer Christy, K. Jeyadheepan, A. Moses Ezhil Raj, C. Sanjeevi Raja, Asian J. Appl. Sci. 7(8), 671–684 (2014)
V. Ramya, K. Neyvasagam, R. Chandramohan, S. Valanarasu, A. Milton Franklin Benial, J. Mater. Sci.: Mater. Sci. Electron. 28, 8489–8496 (2015)
P.B. Ahirrao, S.R. Gosavi, D.R. Patil, M.S. Shinde, R.S. Patil, Arch. Appl. Sci. Res. 3(2), 288–291 (2011)
M.T.S. Nair, L. Guerrero, O.L. Arenas, P.K. Nair, Appl. Surf. Sci. 150, 143–151 (1999)
Acknowledgements
The authors are grateful to Karunya University for providing all the necessary help for fabrication and characterization of samples. The authors are thankful to Dr. R. Ramesh Babu, Assistant Professor, School of Physics, Bharathidasan University, Tiruchirappalli-24, for extending the Hall measurement facilities established under the DST Grant (D.O. No. SR/S2/CMP-35/2004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muthukrishnan, S., Subramaniam, V., Mahalingam, T. et al. Improved properties of spray pyrolysed CuO nanocrystalline thin films. J Mater Sci: Mater Electron 28, 4211–4218 (2017). https://doi.org/10.1007/s10854-016-6043-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-6043-7