Abstract
Plasmonic solar cell efficiency strongly depends on parameters of Au/TiO2 nanoparticles (NPs) embedded system including Au sizes, distribution, aggregation, and dielectric environment—polymer encapsulated around Au/TiO2 NPs. This paper briefly outlines the technological developments for preparation of the nanoparticles Au/TiO2 solutions with different Au ratios, in presence of 200, 500 mg PVP and in absence of polyvinylpyrrolidone (PVP). After that from the obtained solutions, the Au/TiO2 thin films are prepared by spin coating method aiming to get different Au nanoparticles (Au NPs) sizes with uniform dispersion. The next step, the Au/TiO2 thin films samples have been annealed at different temperatures, the absorption spectra of the different Au/TiO2 NPs thin films are investigated using the UV–Vis spectroscopy techniques. The obtained experiment results show that the absorption spectra in the case of not thermal annealing is blue shift meanwhile in the case of thermal annealing, the absorption spectra are red shifted, enhanced depending on the annealing temperatures, Au sizes, ratios and PVP amounts. The enhancement and red shifts of absorption spectra for each case of Au/TiO2 NPs thin films have been discussed and explained by interaction between PVP and Au NPs features at different annealing temperatures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Recently the scientists have devoted many attempts for development of renewable energy sources, especially for development of new solar cell generation for application [1–6], among them the plasmonic structural solar cell is a promising new solar cell type with low cost and larger area [4–6]. The most important structure in the plasmonic solar cells is the integrated noble metal nanoparticle Au(Ag)/TiO2 (ZnO, Si…) system. This structure, so called the plasmonic nanostructure, can support the formation of the surface plasmons resonance in response to a photon flux, localizing electromagnetic energy close to their surfaces [5–8]. So far, the plasmonic solar cells are developed at the beginning stage that has still many research issues, including both theoretical and practical problems that must be overcome, among them to increase the efficiency of plasmonic solar sell is vital important task [4–8]. As known the efficiency of the plasmatic solar cell is determined by the characterizations of several main parameters: Au NPs, TiO2 NPs, the nanoparticle Au/TiO2 embedded system, environment material encapsulated around Au/TiO2 NPs in the form of matrix and so forth, these factors consist of many parameters: the sizes of nano particles (Au, TiO2), the weight ratio of Au to TiO2, the thickness of the single layer or multilayer of Au/TiO2, the arrangements of Au and TiO2 nano particles in integrated-matrix system, the capacity of the light absorption, scattering and local surface plasmon resonance (LSPR) capacities of the Au/TiO2 system. These parameters, however, depend on the technological conditions, thermal annealing, the structure of plasmonic solar cell as well as the substrate materials used [8–12]. In order to prepare Au/TiO2 solution, firstly one have to synthesize the suitable Au nanoparticles by a definite method then blend with commercial TiO2 NPs. As known the fabrication of Au NPs is of great interest not only for various nano electronic, biomedical applications but also for plasmonic solar cell [9–14]. For plasmonic solar cell application, it is very important requirement that Au NPs have to controllable size, Au percentage ratio, shape and uniform dispersion in volume. By using the method producing the core-shell nanoparticles of noble metal Au (Ag, Pt…) with polymers (PANI, PVP…) one can be controlled the Au size, ratios, distribution and clusters (aggregation) and dielectric environment which can strongly influence on the surface plasmon resonance behaviour of the GNPs [15–20]. By this reason several kinds of polymers have been investigated and produced by various synthesis approaches like hydrothermal synthesis, solvothermal synthesis, sol–gel method, emulsion polymerization, microemulsion polymerization, and so forth [11–16]. In order to optimize the characterizations of Au NPs and Au/TiO2 NPs system there are many attempts devoted for developing the different methods for optimizing fabrication of noble metal nanoparticles including Au NPs as well as the nanoparticles Au/TiO2 system using PVP as dispersant and encapsulating Au/TiO2 NPs to obtain stable metal nanoparticles (Au, Ag, CdS, Fe3O4 NPs) in a solid polymeric matrix [12–14, 16, 18–21]. So far there are many works outlined the different methods for production of Au/TiO2 system: many works have used chemical methods for Au NPs fabrication then mixed the solutions for producing Au/TiO2 solution, some other works dealt with the Au evaporation or sputtering onto the nano particle TiO2 thin film then thermal annealing [21–24]. In additional, many works also have been investigated the effects of polymeric ligands including, PANI, PVP polymers as dispersant and for encapsulated, stabilized nano metal particles (Au, Ag, Pt, Fe2O3…) with TiO2 nanoparticles for plasmonic solar cells [11, 14, 17–21]. The absorption spectra of various plasmonic structures (Au/TiO2, Ag/TiO2…), and of PVP-noble metals (Au, Ag, Pt. CdS…) have also been investigated and discussed, unfortunately there are still many problems concerning controllable capacity of Au sizes, ratios as well as Au distribution in volume of the Au/TiO2 in presence of PVP and in absence of PVP that must be discussed clearly [14–21]. There are also still a few results concerning the effects of thermal annealing at different temperatures, different Au sizes and ratios in presence of PVP on absorption spectra of Au/TiO2 thin films reported in detailed. Following our previously published works [23, 24], this paper outlines and discusses briefly some technological developments for preparation of the nanoparticles Au/TiO2 thin films in the presence of PVP used as dispersant and control nanoparticles Au sizes aiming for application in plasmonic solar cells. Using scanning electron microscopy (SEM), high resolution transmission electron microscope (HRTEM), energy dispersive X-ray spectroscopy (EDX) and UV–Vis spectroscopy techniques, the characterizations of the Au/TiO2 NPs solution and thin films are outlined and analysed. The effects of PVP, of thermal annealing up to high temperature 500 °C on absorption spectra of Au/TiO2 thin films are also investigated, discussed more in detailed.
2 Experimental procedures
2.1 The starting materials
Used in experiments are Chlorauric acid (HAuCl4·3H2O), sodium borohydride (NaBH4), polyvinylpyrrolidone (PVP K30 type), Titannium dioxide P25 powder and ethanol. These are commercial products, which imported from Aldrich Company. Polyvinylpyrrolydone (PVP) has its molecular weights (Mw) ranging from 2500 to about one million Carbon unit which is obtained by radical polymerization in solution. PVP of which chemical formula is PVP (C6H9NO)n, it is highly solved in water as well as in various organic solvents. The glass transition temperature of high molecular weight polymers (Mw = 1,000,000) is about 175 °C and falls to values of under 100 °C with decreasing molecular weight (Mw = 2500).
2.2 Au/TiO2 solution and thin film preparation
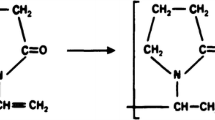
The Au/TiO2 solutions with different Au percentages are prepared as the following: we add 45 mg TiO2 (type of P25 powder) and 0.25 ml HAuCl4 into 25 ml H2O in presence of PVP (with 200 or 500 mg) and in absence of PVP. The obtained solutions were gently mixed by magnetic stirring at room temperature for 30 min so that the chemicals are well dispersed, after that an amount of 1 ml NaBH4—0.1 mM/ml is added with low or fast reaction rates into the above mentioned solution. The blended solution is continuously stirring for 20 min, finally we have Au(10 %)/TiO2 solution with the definite Au size NPs. The same procedures have been done for preparation of the Au(40 %)/TiO2 solution with the suitable calculated chemical amounts rates. The obtained solutions have grape purple colors. The reaction equation between HAuCl4 and NaBH4 in H2O solution follows the formula below:
Here it is worth to mention that TiO2 NPs are not taken part in the above chemical reaction. The final entire blended solutions were centrifuged for removing other excess chemicals and water to get the solid precipitated powder products of Au/TiO2 NPs complexes. The obtained solid powder Au/TiO2 NPs were added into 2 ml Ethanol (C2H5OH) to get the final Au/TiO2 NPs solutions using in preparation of Au/TiO2 NPs thin film by sol–gel method in later stage.
Here we would like to notice that in order to have different ratios or percentages of Au in Au/TiO2 we have to estimate theoretically the Au amounts based on Eq. (1) in comparison with amount of TiO2 added into solution. This means that we must choose amounts of HAuCl4 and NaBH4 so that the Au product in right hand of Eq. (1) reaching the expected ratios of 10–40 % in solution, respectively. Here, in this work we have prepared several kinds of Au/TiO2 NPs solutions to produce several kinds of Au/TiO2 NPs thin films with 10 % Au and 40 % Au ratios in presence of PVP and without PVP for experimental measurements of their characterizations. Tables 1 and 2 show the thin films samples produced on glass or glass/ITO substrates by spin-coating method with 3000 rpm for 30 s with different mass of PVP (200 and 500 mg), adding NaBH4 into chemical reaction in Eq. (1) with different reaction rates (low, fast) for controlling Au sizes, and thermal annealing at temperatures. The morphological and structural properties of Au/TiO2 for each kinds of solutions with PVP and without PVP have been investigated by, SEM, HRTEM on TECNAI G2-20 equipment and by UV–Vis spectroscopy on CARY 5000 equipment.
3 Results and discussions
3.1 The structural and morphological properties of Au/TiO2 solutions
As known, Au nanoparticle (Au NP) has the strong active surface level, so normal way, in order to prevent Au nanoparticles (Au NPs) being in Au/TiO2 solution from aggregating together one has to add a stabilizer polymer into Au solution to protect the Au NPs forming aggregations—clusters as well as to use for the surface stabilization. Surface stabilizer polymer used in this work is PVP because this kind of polymer is the excellent ligands as they surround the NPs with a substantial physical barrier that preventing the core NPs from coming into contact [11, 12, 14, 15]. As also known using the reducing agent—sodium borohydride (NaBH4) and HAuCl4 solution, the Au NPs sizes can be produced according to Eq. (1) with different sizes depending on the reaction rate and polymeric ligand [15–17]. The PVP encapsulated Au/TiO2 NPs can enhance the core separation to increase the hydrodynamic radius of the NPs. Thus, the role of PVP in the solution is involved preventing agglomeration of the gold nanoparticles together and to disperse uniformly the particles in the solution, and simultaneously increasing the viscosity of the solution [11, 12, 14–16].
Figure 1 shows HRTEM micrographs of Au/TiO2 NPs in solutions with different Au ratios (10, 40 %) in presence of PVP and without PVP. The Au sizes can control by the different reaction rates (slow or fast) of NaBH4. In case of solution without PVP the size of Au NPs sizes are not so uniform because there are no preventing agents limit growth process of nanoparticles (Fig. 1a) meanwhile in the case of solution in presence of PVP, the aggregation growth of Au NPs are limited due to PVP surrounded the Au NPs. The sizes of Au NPs depending mainly on the reaction rate of reducing agent (NaBH4) added into solution i.e depending on low or fast chemical reaction rate. Consequently, by adjusting reaction rate (slow or fast) of the reducing agent we controlled the different nanoparticles Au sizes and ratios in Au/TiO2 NPs being in solutions (see Fig. 1b, c, d). Here, it is worth to note that because Au NPs forming according to Eq. (1) are dispersed almost uniformly in whole volume of the initial prepared Au/TiO2 solution, the amount of Au NPs situated around TiO2 NPs is only a part of total Au amount being in solution, another considerable part of Au amount dispersed in solution outside from TiO2 NPs. By this reason the Au percentage in Au/TiO2 thin film is considerable less than that in Au/TiO2 solution.
HRTEM micrographs of: Au(40 %)/TiO2 in solutions in absence of PVP that produced Au NPs (dark color) with different sizes of 10–20 nm (a); Au(40 %)/TiO2 solutions in presence of PVP with fast reaction rate produced Au NPs (with dark color) with the rather uniform sizes of 8–10 nm (b); Au(10 %)/TiO2 solution in presence of PVP with low reaction rate produced the Au NPs (small dark color) with small size of about 2–3 nm (c); and Au(40 %)/TiO2 solution in presence of PVP with low reaction rate produced Au uniform sizes of about 7 nm (d)
Although PVP has many advantages as above mentioned but its drawback is the formation of separation membrane between Au and TiO2 NPs. If the contact layer (PVP) between Au and TiO2 NPs are not annealed, then a polymer PVP encapsulated around Au NPs as well as Au/TiO2 NPs will limit the direct carrier injections from Au NPs surface to TiO2 NPs. However, this problem can be solved when the Au/TiO2 thin films are annealed at temperature higher than the glass temperature of PVP is about 175 °C. In the case of annealing temperature higher than 175 °C, the Au NPs under reducing conditions can initiate the decomposition of the capping agent, PVP, below that of pure PVP. Under oxidizing conditions, both PVP/Au and PVP degrade to form amorphous carbon [12, 15, 28].
Figure 2a, b show HRTEM micrographs of Au/TiO2 NPs in presence of PVP. Here Au NPs are black circles (spheres) and TiO2 are in bright contrast. The Au NPs in presence of PVP are dispersed rather uniformly and their sizes are in the range of 3–5 nm. The PVP thickness surrounded Au/TiO2 NPs is about 8.3 nm. Figure 2c shows the selected area electron diffraction (SAED) patterns of Au/TiO2 system in the solution in presence of PVP. Figure 2d is HRTEM micrographs of plane-view projection of Au particles on anatase structural TiO2 surface, whereas the spacing between (111) planes of face center cubic (fcc) Gold is 0.235 nm while the spacing between (101) planes of anatase phase TiO2 is 0.345 nm. The measured results showed that both Au NPs and TiO2 NPs are nature crystalline. Our obtained results for nature crystalline for Au NP and TiO2 NP are almost as same as the results published in [24, 25]. Concerning the results of highly single crystalline for Au NPs formation, here we also think that the mechanism growth of highly single crystalline of Au NPs throughout the synthesis and appear to be formed by a diffusion-controlled Ostwald-ripening growth mechanism. The amounts of capping ligand and the reduction temperature have an important impact on nucleation and growth processes which directly influence the final sizes of the nanoparticles [26].
HRTEM micrographs of the PVP layer size of 8.3 nm surrounded the Au/TiO2 being in solution with Au average size of 3–4 nm (a); HRTEM magnification of Au/TiO2 NPs in solution with PVP (b); Diffraction picture of Au/TiO2 NPs in solution with Au (111), TiO2 (101, 200, 211) plans (c); HRTEM of Au/TiO2 NP pair with structural crystalline of Au (111) and the structural crystalline of TiO2 (101) planes (d)
3.2 The morphological features of Au/TiO2 spin-on samples and thin films
Figure 3 shows some Au/TiO2 thin films fabricated on glass substrates by spin-on method with 3000 rpm for 30 s for three times. These Au/TiO2 thin films samples produced from different Au/TiO2 solutions containing different Au sizes and ratios resulting by adding NaBH4 with fast or slow reaction rates into chemical reaction. Figure 3a, b are photos of Au(10 %)/TiO2 thin films containing 500 mg PVP and 200 mg PVP in 25 ml ethanol solution, respectively. Figure 3c is photo of Au(10 %)/TiO2 thin films without PVP and Fig. 3d is photo of Au(40 %)/TiO2 thin films containing 500 mg PVP in 25 ml ethanol solution. It can be seen from the photos that their colors are different and the surfaces of thin films containing PVP are more uniform in comparison with those of the films without PVP. Although the Au/TiO2 thin films samples were spin coated in the same conditions but due to different their viscosities of different solutions containing PVP, without PVP with different Au size and Ratios, so resultant the morphologies, surface situations and their thicknesses of thin films are also different. The thickness of Au/TiO2 thin films were measured by α-step equipment, the thickness value of the samples with PVP is about 1000 ± 200 nm, meanwhile the thickness of samples without PVP is about 1400 ± 400 nm.
Figure 4a, c show SEM micrographs of Au(10 %)/TiO2 thin films after annealing at 500 °C in absence of PVP (a), and in presence of PVP (b), respectively. We can also observed that the surface of the sample containing PVP is more uniform than that of the sample without PVP which has many holes. Figure 4c, d are EDX spectra of Au(10 %)/TiO2 in absence of PVP and in presence of 500 mg PVP in 25 ml athanol solution, respectively. The Au weight % values in two cases are different. Here it is worth to note that the amount of Au is 10 % of weight percentage according to theoritical calculation for chemical reaction based on Eq. (1) is not same as Au ratio being in the thin film measured by EDX. As mentioned in above, the percentages of Au in Au/TiO2 thin films are always less than those of Au weight percentages in the Au/TiO2 solutions due to partly the loss of Au NPs during preparation via many technological steps from Au/TiO2 solution to Au/TiO2 thin film.
3.3 Absorption spectra of the different Au/TiO2 NPs thin films samples
As known that the light scattering of Au NPs as well as PVP encapsulated Au/TiO2 thin films is special research interest today for a variety of applications for the energy conversation field where they are used to increase the efficiency of solar cells, the way these nanoparticles scatter light is of vital importance to the research [7–9, 12, 15, 20, 25–27].
Light scattering is, in general, the attenuation of a beam of light by Nanoparticles, either by absorption or scattering. The sum of these two parts is known as the extinction [8]. For nanoparticles with diameters well below the wavelength of light, a point dipole model well describes the absorption and scattering of light. The scattering and absorption cross-sections, C scat and C abs , respectively, are given by following expressions [8]:
where
Here V is the particle volume, ε p is the dielectric factor of the particle and ε m is the dielectric factor of the embedding medium. From Eqs. (2), (3) we can see that when ε p = −2ε m , the particle polarizability (α) in Eq. (3) will become very large. This is known as the surface plasmon resonance. This sometime is called the dipole plasmon resonance of the particle [8]. At the surface plasmon resonance the scattering cross-section can well exceed the geometrical cross section of the particle; consequently, the absorption coefficient vs. wavelength will be enhanced in certain range of wavelength depending on nanoparticles system being on the substrate. The experiment results showed that the enhanced absorption coefficient of Au/TiO2 spherical nanoparticles have dominated strongly in the range of 500–600 nm [8, 12, 22–27].
Figure 5 shows the measured absorption spectra of different Au/TiO2 NPs thin films prepared with different technological conditions. From Fig. 5a shows absorption spectra of three thin films samples not thermal annealing: sample without PVP, and samples containing 200 and 500 mg PVP. The peaks values of absorption spectra for Au/TiO2 thin film samples in presence of 200, 500 mg of PVP are about 536 nm, lower the that of sample without PVP (about 540 nm). It seems to be that somewhat blue shift is dominated caused by PVP encapsulated around Au/TiO2 NPs (as in Fig. 2a, b).
For the samples annealed at temperature 160 °C, the peak of absorption spectrum of Au/TiO2 sample without PVP is somewhat red shift, moved to 545 nm meanwhile the peaks values of absorption spectra for two samples containing 200 and 500 mg PVP are still located at 536 nm, not red shifted. When the annealing temperature increased to 350 °C, there are red shifts found in all of the absorption spectra both for sample without PVP and sample containing PVP, however these shifts are not the same (to 548, 551, 555 nm) due to the samples containing different amount of PVP. After being annealed for all samples at 500 °C, the peak positions of all Au/TiO2 NPs thin films are almost coincided around 568 nm.
Here we would like to note that The glass transition temperature of high molecular weight polymers is about 175 °C and falls to values of under 100 °C, so when the Au/TiO2 in presence of PVP are thermal annealed at 160 °C that is lower than 175 °C, the PVP structures are not yet changed so much, their peaks values of absorption spectra also is change very little to about 538 nm (Fig. 5b), but when thermal annealing at high temperatures for samples containing PVP, the red shifts are considerable larger. Based on the obtained results we can conclude that the effect PVP on the absorption spectra of the samples containing PVP is negligible when the samples are annealed at temperatures higher 500 °C.
Figure 6a shows the dependence of the absorption peak wavelengths on the annealing temperatures for Au/TiO2 sample without PVP and samples with different Au ratios. The absorption peaks values of the samples in presence of PVP are always lower than that of samples without PVP. Here we could state that the blue shifts are also dominated in the samples containing PVP that are thermal annealed up to 500 °C. The blue shift phenomenon in the noble metals (Au, Pt)/TiO2 in presence of PVP also reported by several authors mentioned in work [24] Authors stated that in the presence of PVP, the blue shift in the typical SPR band to 532 nm is ascribed to the quantum confinement effect. Such effect arises when Au NPs are covered with a thin film of a dielectric material such as polymeric layer which helps in congregating n-electrons of PVP on the Au surface.
Figure 6b shows the absorption peak wavelengths vs. annealing temperatures between two Au/TiO2 samples with same Au ratios but with different reaction rates of adding NaBH4 into solution: with low reaction rate produces small Au sizes (as in Fig. 1c, d) meanwhile with the fast reaction rate produced larger Au sizes (as in Fig. 1a). The absorption peaks values of samples with small size of Au NPs, for all thermal annealed cases, are always higher than those of the sample with larger Au size. These results are agreed with theoretical estimation.
From the results of absorption spectra in Figs. 5, 6 and 7, we observed that the absorption spectra are considerable strongly depending on amount of PVP encapsulated around Au/TiO2 NPs, Au sizes and Au ratios to TiO2 amounts and thermal annealing temperatures.
In the case of without thermal annealing, there is somewhat blue shift of absorption peaks for the Au/TiO2 thin film samples in presence of PVP in comparison with the Au/TiO2 thin film sample in absence of PVP. This result could be explained by PVP layer encapsulated around Au/TiO2 NPs that is ascribed to the quantum confinement effect. Such effect arises when Au NPs are covered with a thin film of a dielectric material such as polymeric PVP which helps in congregating n-electrons of PVP on the Au surface. This also influences on the surface plasmon resonance behaviour of the GNPs [16, 17].
In the case of thermal annealing, the absorption peaks of all Au/TiO2 thin films are shown to red shifts: the more higher annealing temperatures are, the more red shifts of absorption peaks are. The absorption spectra of thin films samples are also increased when Au ratios increased (from Au(10 %)/TiO2 to Au(40 %)/TiO2). The absorption spectra of Au/TiO2 NPs thin films with small Au sizes are also higher than those of Au/TiO2 thin films with larger Au sizes.
The obtained results can be explained mainly by effect of thermal annealing process on structures of Au/TiO2 thin films as well as on PVP by several reasons: (1) on one hand, thermal annealing in air for 30 min for thin films samples, this means that Au NPs were heated under reducing conditions, this initiates the decomposition of the PVP capping agent. We assume that at low annealing temperature below the glass transition temperature of 175 °C, the structure of PVP is still remaining characteristics of almost pure PVP. Under oxidizing conditions, both PVP encapsulated Au NPs and PVP degrade to form amorphous carbon, this strongly influence on red shifts of absorption spectra: (2) On another hand, when PVP gets adsorbed on the surface of the Au NPs during chemical reaction, it can help in altering the electronic properties of Au NPs by accumulating n-electrons of C=O moiety of PVP molecules on the Au surface in the Au-PVP nano size composite [25–28]. When the PVP percentage is increased then the activation energy will be lowered due to the predominance of ion conduction mechanism caused by PVP in the blend. The decrease in binding energies of the Au4f band in the presence of PVP molecules reveals interfacial interaction between the Au NP and PVP molecule [17, 21]. The above mentioned effects could be main reasons to influence the enhanced absorption spectra and red shifts in our results.
4 Conclusions
The different Au/TiO2 thin films based on Au/TiO2 solutions in presence of PVP and in absence of PVP containing different Au Ratio have been prepared. The different Au NPs with different controllable sizes have been synthesized by chemical method with slow and fast reaction rates of adding NaBH4 into chemical reaction. PVP was used as stabilizer and dispersant. The structural and morphological properties of Au/TiO2 solutions as well as of Au/TiO2 thin films are shown. In presence of PVP the Au NPs are dispersed rather uniformly in solutions with sizes are in range of from 3 nm to 30 nm. The Au NPs have natural crystalline. The spacing between (111) planes of face centered cubic (fcc) Gold is 0.235 nm while the spacing between (101) planes of anatase phase TiO2 is 0.345 nm. The absorption spectra of Au/TiO2 NPs thin films containing different Au ratios, in presence of PVP and in absence of PVP, thermal annealed at different temperatures which have been investigated more in detailed. Based on experiment measured results we can conclude that there are blue shifts of absorption peaks for the Au/TiO2 thin film samples in presence of PVP, this blue shift phenomenon appears until up to annealing temperature at 500 °C. The main effect of thermal annealing is caused to red shift at all annealing temperatures. The more increasing annealing temperatures are, the more red shifts of absorption peaks are. The absorption spectra of thin films samples are increased when Au ratios increased as well as when Au size decreased. The effects of PVP and temperatures annealing causing the enhancement, red shifted and widening peaks of absorption spectra of Au/TiO2 NPs thin films can be explained by interactions between PVP and Au NPs at definite technological condition. The high temperature thermal treatments for Au/TiO2 NPs thin films, both for samples without PVP and samples containing PVP, are very important processes. This process not only helps to disappear the separation effect of PVP between Au and TiO2 NPs but also to increase and widen the absorption spectra of the Au/TiO2 thin films. Using the technological process with suitable PVP amount, small sizes of Au NPs, uniformly dispersed in Au/TiO2 thin film, thermal annealing at high temperature for plasmonic solar cell preparation, the efficiency of plasmonic solar cell could be expected to increase somewhat. This task will be discussed in forthcoming paper.
References
B. O’Regan, M. Grätzel, Nature 335, 737 (1991)
M. Grätzel, Nature 414, 338 (2001)
T.M. Razykov, C.S. Ferekides, D. Morel, E. Stefanakos, H.S. Ullal, H.M. Upadhyaya, Sol. Energy 85(1580), 1580–1608 (2011)
Nozik A J, Quantum Dot Solar Cells, Center for Basic Sciences, National Renewable Energy Laboratory, 1617 Cole Boulevard, Golden, Colorado. 80401–3393, NREL/CP-590-3101. (2001)
A. Harry, Atwater and Albert Polman, Plasmonics for improved photovoltaic devices. Nat. Mater. 9, 205–214 (2010)
K.R. Catchpole, A. Polman, Plasmon. solar cells 16, 21793–21800 (2008)
V.H. Nguyen, B.H. Nguyen, Visible light responsive titania-based nanostructures for photocatalytic, photovoltaic photoelectrochemical and applications. Adv. Nat. Sci. Nanosci. Nanotechnol. 3, 023001 (2012)
S.A. Mair, Plasmonics-Fundamentals and Applications (Springer Science, New York, 2007). ISBN 0-387-33150-6
S. Mubeen, G. Hernandez-Sosa, D. Moses, J. Lee, M. Moskovits, Plasmonic photosensitization of a wide band gap semiconductor: converting plasmons to charge carriers. Nano Lett. 11, 5548–5552 (2011)
N.T.K. Thanh, L.A.W. Green, Functionalization of nanoparticles for biomedical applications. Nano Today 5, 213–230 (2010)
K. Santhosh Kumar, V.B. Kumar, P. Paik, Recent advancement in functional core-shell nanoparticles of polymers: synthesis, physical properties, and applications in medical biotechnology. J. Nanopart. 2013, 1–24 (2013). doi:10.1155/2013/672059
M. Behera, S. Ram, Spectroscopy-based study on the interaction between gold nanoparticle and poly (vinylpyrrolidone) molecules in a non-hydrocolloid. Int. Nano Lett. 3, 17 (2013)
H.N. Verma, P. Singh, R.M. Chavan, Gold nanoparticle: synthesis and characterization. Vet. World 7(2), 72–77 (2014). doi:10.14202/vetworld.2014.72-77
A. Pal, S. Shah, S. Devi, Synthesis of Au, Ag and Au-Ag alloy nanoparticles in aqueous polymer solution. Colloid Surf A 302, 51–57 (2007)
F. Haaf, A. Sanner, F. Straub, Polymers of N-vinylyrrolidone: synthesis, characterization and uses. Polym. J. 17(1), 143–152 (1985)
K. Vanherck, T. Verbiest, I. Vankelecom, Comparison of two synthesis routes to obtain gold nanoparticles in polyimide. J. Phys. Chem. C 116, 115–125 (2012)
A. Rawat, H.K. Mahavar, A. Tanwar, P.J. Singh, Study of electrical properties of polyvinylpyrrolidone/ polyacrylamide blend thin films. Bull. Mater. Sci. 37(2), 273–279 (2014). (C. Indian Academy of Sciences)
D. Malina, A. Sobczak-Kupiec, Z. Wzorek, Z. Kowalski, silver nanoparticles synthesis with different Concentrations of polyvinylpyrrolidone. Dig. J. Nanomater Biostruct. 7(4), 1527–1534 (2012)
H. Wang, X. Qiao, J. Chen, X. Wang, S. Ding, Mechanisms of PVP in the preparation of silver nanoparticles. Mater. Chem. Phys. 94, 449–453 (2005)
L. Saravanan, S. Diwakar, R. Mohankumar, A. Pandurangan, R. Jayavel, Synthesis, structural and optical properties of PVP encapsulated CdS nanoparticles. Nanomater. nanotechnol 1(2), 42–48 (2011)
N. Arsalani, H. Fattahi, M. Nazarpoor, Synthesis and characterization of PVP-functionalized super paramagnetic Fe3O4 nanoparticles as an MRI contrast agent. Expr. Polym. Lett. 4(6), 329–338 (2010). www.expresspolymlett.com. doi:10.3144/expresspolymlett.2010.42
H.M. Magnusson, K. Deppert, J.O. Malm, J.O. Bovin, L. Samuelson, Gold nanoparticles: production, reshaping, and thermal charging. J. Nanopart. Res. 1, 243–251 (1999)
K.A. Dao, T.T. Nguyen, T.M.H. Nguyen, D.T. Nguyen, Comparison of some morphological and absorption properties of the nanoparticles Au/TiO2 embedded films prepared by different technologies on the substrates for application in the plasmonic solar cell. Adv Nat Sci Nanosci Nanotechnol 6(1), 015018 (2015)
T.T. Nguyen, T.T. Nguyen, H.T. Pham, D.T. Nguyen, Van V. Le, K.A. Dao, The effects of Polyvinylpyrrolidone on the Au sizes, dispersion and enhancement of absorption spectra of the nanoparticles Au/TiO2 solutions for application in plasmonic solar cell. J. Mater. Sci. Mater. Electron. (2016). doi:10.1007/s10854-016-5263-1
M. Murawskaa, A. Skrzypczakb, M. Kozaka, Structure and morphology of gold nanoparticles in solution studied by TEM, SAXS and UV–Vis. Acta Phys. Polonica A 121, 888 (2012)
R. Sardar, J.S. Shumaker-Parry, Spectroscopic and microscopic investigation of gold nanoparticle formation: ligand and temperature effects on rate and particle size. J. Am. Chem. Soc. 133, 8179–8190 (2011)
K.L. Kelly, E. Coronado, L.L. Zhao, G.C. Schatz, The optical properties of metal nanoparticles: the influence of size, shape, and dielectric environment. J. Phys. Chem. B 107(3), 668–677 (2003)
Y. Borodko, S.E. Habas, M. Koebel, P. Yang, H. Frei, G.A. Somorjai, Probing the interaction of poly(vinylpyrrolidone) with platinum nanocrystals by UV-Raman and FTIR. J. Phys. Chem. B 110, 23052–23059 (2006)
Acknowledgements
The authors would like to express their gratitude to the NAFOSTED for financial funding the basic research project with code 103.02-2013.47 in period from 2014-2017. This work has been done in Energy materials and Devices Laboratory, IMS-VAST. Authors also express many thanks to the supports of Institute of Materials Science (IMS), Vietnam Academy of Science and Technology (VAST), and also thank to BSc Pham Van Dai for taken part in measurements of absorption spectra.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nguyen, T.T., Pham, H.T. & Dao, K.A. The effects of polyvinylpyrrolidone and thermal annealing on red shifts for absorption spectra of the nanoparticle Au/TiO2 thin film with different Au ratios. J Mater Sci: Mater Electron 28, 2075–2085 (2017). https://doi.org/10.1007/s10854-016-5769-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-5769-6