Abstract
The effect of Ni and Li co-substitution on the structural and dielectric properties of barium titanate (BaTiO3) ceramics has been investigated. Ni and Li co-substituted barium titanate (BT) with general formula Ba(1−x)LixNix/2TiO3 where x = 0, 0.02, 0.04, and 0.08 is synthesized using microwave-assisted heating. This synthesis process extraordinarily reduced the processing time to 1 h, which includes heating and the dwell durations. The XRD investigation confirmed the formation of a single phase tetragonal structure with a space group of P4mm. The surface morphology of the sintered samples exhibits a high density and a homogeneous distribution of the grains. Impedance analyzer was used to measure the dielectric constant and loss tangent values at different frequencies as well as at various temperatures range. Ni and Li co-substituted BaTiO3 shows many interesting features, such as shifting of phase transition towards higher temperature, increasing in dielectric constant, decreasing in dielectric loss and increasing in diffuse phase transition with increased frequency. The activation energy of all samples was investigated using Arrhenius plots.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Barium titanate (BT) is an extensively studied and attractive ferroelectric material that is being used in a wide range of applications. The perovskite ABO3 structure of BT provides ample choice for substitution or co-substitution at A site with +1, +2, +3 cations or B site with +3, +4, +5 cations respectively [1–4], this, in turn, affects the crystal structure of barium titanate (BT) and through a combination of processing conditions gives rise to material properties useful in modern electronics for fabrication of multilayer ceramics Capacitors MLCC, Micro-Electro Mechanical Systems (MEMS) and piezoelectric devices [5–8]. At room temperature, barium titanate has tetragonal phase structure, which changes to cubic phase above Curie temperature [9]. The difference in ionic radius between A and B cations in BaTiO3 leads to distortion in the crystal structure, by doping Ni2+ and Li+. The possible applications of Ni-doped barium titanate materials in the bulk form are still being explored over a wide range of compositions, controlled ion substitutions, material preparation and sintering conditions. It has been reported that by Jana A et al. in [10] dielectric permittivity increased from 150 to 400 in Ni-doped barium. Carlos Pecharroman et al. have reported a high dielectric constant of 80,000 in BaTiO3–Ni ceramic–metal composites due to the percolation phenomenon [11]. The microwave sintering has been shown to affect grain structure by preserving the uniformity of microstructure and is energy saving [12–15]. Till now there are many reports related to the densification of barium titanate BT based materials by using microwave heating [16–19]. Saleem et al. reported that the dense microstructure, uniform grain size, and high dielectric permittivity could be induced by using microwave sintering. However, there is no report on Ni and Li co-substituted BaTiO3 sintered through microwave heating. In the present paper, microwave sintered Ba(1−x)LixNix/2TiO3 composites were synthesized and characterized.
2 Experimental
Polycrystalline ceramic pellets of Ba(1−x)LixNix/2TiO3 were synthesized in a multi-mode microwave sintering furnace (Enerzi Microwave systems). The starting precursors were BaCO3, NiO, Li2CO3 (Sigma-Aldrich, 99.99 % purity) and TiO2 powders (Sigma-Aldrich, 99.98 % purity. The precursors were heated at 200 °C for 3 h to remove moisture. They were weighed and milled for 4 h in acetone. After drying, the mixture was calcined in Microwave Sintering System at 1000 °C and the dwell time was 20 min, the heating and cooling rates were 50 °C/min. The calcined powder was again ball milled under same conditions and then dried. The powder was mixed with 1 wt- % (PVA) as a binder and compacted under 100 Mpa into disc-shaped pellets with a diameter of 10 mm and finally sintered at 1200 °C for 40 min duration using Microwave Sintering System with heating and cooling rate of 50 °C/min. The experimental density was measured using Archimedes principle [20] and then compared to the theoretical density determined from X-ray measurements. The porosity value for both samples is obtained using the following formula.
The morphological structure was characterized by using Carl Zeiss, Ultra 55 Field emission scanning electron microscopy (FE-SEM). Silver paste was applied on both Polish sides of the pellet, and dielectric properties were determined using an Impedance Analyzer, Agilent E4292A in the frequency range of 10 2Hz to 10 7Hz.
3 Result and discussion
3.1 Structural analysis
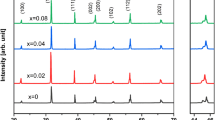
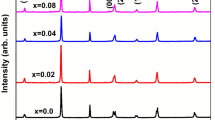
The crystallinity and purity of the prepared samples were examined by powder X-ray method. X-ray Diffraction of sintered pellets of Ba(1−x)LixNix/2TiO3 ceramics is shown in Fig. 1. It is observed that the co-substituted sample shows similar XRD patterns as that of pure BaTiO3 which exhibits the splitting of diffraction peaks at 2θ \(\approx\)45° corresponds to (200) and (002) planes of tetragonal phase with space group P4mm standard JCPDS file no 5-0626 of perovskite BaTiO3. A trace amount of nickel oxide and lithium oxide were not observed as impurities in Ni and Li co-substituted BTO. This confirmed that the Ni and Li incorporated in BaTiO3 lattice successively without apparent of any secondary phase. Rietveld refinement analysis has been carried out to estimate the structural parameters. The best fit is observed in Fig. 2, by using BTO phase with a tetragonal structure of P4mm space group. The lattice parameters and crystal structure have been evaluated by the Rietveld refinement methods were recorded in Table 1. The corresponding peaks of the crystal planes (111) are selected and fitted using Gaussian fitting as shown in Fig. 3 and from this fitting the FWHM has been calculated and by using Scherrer formula [21], the average crystallite sizes are calculated and reported in Table. It is observed that the peak is shifted towards higher 2θ angle with an increase of Ni and Li, which confirmed by the decrease of lattice parameter and unit cell volume as shown in Table 1. This decreasing could be due to the substitution of smaller radii of Ni2+ (0.69 Å) and Li+(0.76 Å) ions to the higher radii Ba2+(1.42 Å) (Table 2).
The morphology of the powder samples of Li and Ni-co- substituted BaTiO3 powders calcined at 1000 °C are shown in Fig. 4. The powders show a nonuniform formation of grain sizes those ranges from 50 to 200 nm. The abnormal grain growth is observed where the larger grains have 400 nm. The picture of Ba(1−x)LixNix/2TiO3 pellets before and after sintering are shown in Fig. 5. It was observed that the dimensions of the pellets were decreased after sintering temperature. The microstructures of Ba(1−x)LixNix/2TiO3 ceramic sintered at 1200 °C using MWSS are depicted in Fig. 6. It can be seen that the image of pure sample consists of different grains separated by pores, this indicates to that sample have a lower density. As Ni and Li co-substituted, BaTiO3, a uniform and dense microstructure is observed with an increase in average grain size which could be due to grain boundary diffusion mechanism. Also, the addition of Li significantly reduces the sintering temperature of BaTiO3-based ceramics, which leads to great assists in the densification of BTO-based ceramics because of the production of liquid phase due to the low melting point of Li-containing compounds. A similar enhancement in densification was reported in Li-doped BNT-based ceramics [22]. The uniformity of microstructure with microwave sintering because of the fact that the microwave heating is a volumetric heating [23]. The average grain sizes of samples are calculated using linear intercept method [24]. The average grain size and relative densities of all samples are reported in Table 1.
3.2 Dielectric analysis
The dielectric constant and dielectric loss of Ba(1−x)LixNix/2TiO3 ceramic sintered at 1200 °C using MWSS as a function of frequency measured at room temperature and frequency range of 102 to 107 Hz is shown in Fig. 7. The dielectric permittivity of Ba(1−x)LixNix/2TiO3 ceramics is high at lower frequencies and decreases rapidly with increase frequency. The higher value of the dielectric permittivity in the present samples could be attributed due to the increase of ion jump orientation, space charge polarization, and rotational polarization, which take place mainly in the interfaces. Also, the higher value of dielectric constant at a lower frequency results in the existence of space charge polarization due to inhomogeneous dielectric structure [25, 26]. From the figure, it is clear that the dielectric constant of Ba(1−x)LixNix/2TiO3 ceramic decreases with an increase of frequency which confirm a strong dielectric dispersion in the study range of frequency. This decreasing can be explained on the base of dipolar relaxation due to Maxwell- Wagner mechanism, in which the dipoles at a lower frequency can easily follow the applied field, this leads to enhance the value of dielectric constant at this region. At higher frequency region, due to the difficulty of the dipole to follow the fast alternation of an applied field, the value of dielectric constant decreased [25, 26]. Also, the higher value of dielectric constant is due to all contribution of different types of polarization such as electronic, ionic, atomic and interfacial polarizations, etc. However, the dielectric constant occurs due to the electronic polarization at higher frequencies. As the frequency increased, the contribution of ionic and orientation polarization come down, and this leads to inhibited of dielectric constant [27]. Also, it can be observed that, the dielectric constant increases with an increase of Ni and Li concentrations. This increment could be due to dense microstructure and large grain size as shown in SEM images in Fig. 6. Jieyu Chen reported that the large grain size results in enhancement of polarization as well as dielectric constant [28]. The appearance of dielectric loss at a lower frequency is due to a space charge polarization, i.e., accumulation of free charges at the interface between the electrode and sample [29]. As the frequency increased, the electric field periodic reversal becomes very fast; this leads to resist the occurrence of charge diffusion in the direction of applied electric field. Therefore, the accumulation of the charges will decrease resulting in the decrease of loss value with the increase in frequency as shown in Fig. 7.
The variation of dielectric constant and dielectric loss of the Ba(1−x)LixNix/2TiO3 ceramic sintered at 1200 °C using MWSS as a function of temperature measured at a selected frequencies is shown in Fig. 8. It is observed that the dielectric constant gradually increases with an increase in temperature up to the transition temperature (Tc) and then it is decreased. The region around the dielectric peak of the co-substituted samples is broadened with comparing to the peak of pure. The broadening behavior is one of the most important characteristics of disordered perovskite structure with the diffuse phase transition. This broadening is considered to result in compositional fluctuations [30]. The position of the peak of phase transition was shifted with co-substituted towards higher temperature side. The shift in phase transition could be attributed to the large grain size as exhibited in SEM images [31]. Hiroshima et al. suggested that the internal stress developed in the constrained grains at the phase temperature, which leads to shifting the Tc to higher temperature side with increased grain size [32]. This behavior is considered helpful for improving high- temperature capacitance stability of the dielectrics [33]. Also from Fig. 8 it can be observed that the dielectric peaks of co-substitution samples become broads as comparing to pure BTO sample which confirms the formation of diffuse phase transition. The value of dielectric constant increases with an increase of Ni and Li co-substitution, this increment could be due to high dense microstructure [33]. Interestingly, it is to be noted that the dielectric loss of Ni and Li co-substituted BTO based ceramics is very low comparing to the earlier reports of microwave sintered BTO ceramics. Han et al., reported that the microwave sintered of BTO ceramics shows high loss tangent (tanδ = 6.901) [34]. V. Reddy et al. reported that the loss tangent of microwave sintered BTO ceramics is >0.1 [35]. However, the loss tangent in the present work is <0.1 as shown in Fig. 9 which is related to densification of the samples.
To investigate the effect of Ni and Li addition on diffuse phase transition BaTiO3 ceramic, a modified Curie–Weiss law has been used. Obviously, the degree of diffusive phase transition changes as the addition of Ni and Li increased, which can reflect by diffuseness parameter γ [36].
where C and γ are constant, ɛmax and Tm are the maximum dielectric constant and the corresponding temperature of maximum dielectric constant at Curie temperature (Tmax). The diffuseness parameter γ gives the information about the nature of the transition Fig. 10. For normal Curie–Weiss low γ = 1, whereas for complete diffuse phase transition γ = 2. The plots of \(\ln \left( {\frac{1}{\varepsilon } - \frac{1}{{\varepsilon_{\hbox{max} } }}} \right)\) as a function of \(\ln \left( {T - T_{\hbox{max} } } \right)\) at different frequencies namely 1, 10, 100 kHz, 1 and 2 MHz for Ba(1−x)LixNix/2TiO3 ceramic are shown in Fig. 11. The values of diffuseness parameter γ were measured from the slope of fitting curves using Eq. (2). It is observed that the γ value of samples is decreased with the increasing of Ni and Li concentrations. It is may be due to the increase of grain size [37] with an increase of Ni and Li content ref SEM images. Clearly the deviation from Curie-Weiss law can be noticed in Fig. 10. This deviation is also a measure of degree of diffuseness which is defined as the difference between the deviation temperature (Tdiv) and temperature of maximum dielectric constant (Tm) and the values are reported in Table 2.
For more confirmation the diffusion with Ni and Li addition, the normalized dielectric constant is plotted aginst the normalized temperature [38] as shown in Fig. 12, which leads to the same conclusion that the diffusivity is increased with increase in frequency. In literature, the occurrence of this diffuseness is explained regarding variation in local composition giving rise to distinct micro-regions, each of which has slightly different Tc for its phase transition [39, 40].
3.3 Activation Energy study
From the real and imaginary part of dielectric permittivity the AC conductivity has been obtained [41]. The activation energy of Ba(1−x)LixNix/2TiO3 ceramics sintered at 1200 °C is estimated at five different frequencies using the formula [42]
where \(E_{a}\) is the activation energy for conduction, T is the temperature, kB is Boltzmann constant and \(\sigma_{o}\) is a pre-exponential factor. The Arrhenius plots of the temperature dependence of AC conductivity for Ba(1−x)LixNix/2TiO3 ceramics is shown in Fig. 13. The activation energy was obtained by using the linear least- squares fitting of AC conductivity data to Eq. 3 and reported in Table 3. It can be noted that the activation energy of all sintered samples is decreased with an increase of frequency. Such decreasing can be explained by the rise of externally applied field enhances the charge carriers jumping in between the localized states. Therefore, the activation energy reduced with an increase in frequency [42].
Arrhenius plots of temperature dependence of AC conductivity for Ba(1-x)LixNix/2TiO3 ceramic sintered at 1200 °C using MWSS, and measured at different frequencies (symbols: experimental data, solid line: fits to Eq. (3))
4 Conclusion
Polycrystalline sample of Ba(1−x)LixNix/2TiO3 were synthesized using microwave-assisted heating. The processing time was reduced significantly to 1 h compared to conventional method. No change in phase structure was observed with Ni and Li co-substituted BaTiO3 ceramic, however, a decrease in tetragonality and grain size was observed with co-substitution. SEM images exhibited high density and homogeneous distribution of grains. Impedance analyzer was used to measure the dielectric constant and loss tangent values at different frequencies as well as at different temperatures. Shifting of phase transition towards higher temperature was observed with Ni and Li co-substitution BaTiO3 ceramics. The co-substitution sample shows high dielectric constant with a low loss tangent at room temperature and 1 kHz. Also, the present work shows a low loss as comparing to earlier reports with microwave sintering. The dielectric loss <0.03 over a temperature range of 50–200 °C, these properties make it an ideal material for MLCC applications. The activation energy of all samples was investigated using Arrhenius plots.
References
F. Jona, G. Shirane, Ferroelectric Crystal (Dover Publication, New York, 1993)
P. Goel, K.L. Yadav, A.R. James, J. Phys. D Appl. Phys. 37, 3174 (2004)
P. Goel, K.L. Yadav, J. Mater. Sci. 42, 3928 (2007)
M.A. Mohiddon, K.L. Yadav, J. Sol-Gel. Sci. Technol. 49, 88 (2009)
W.D. Maison, R. Kleeberg, R.B. Heimann, S. Phanichphant, J. Eur. Ceram. Soc. 23, 127 (2003)
J.S. Obhi, A. Patel, Integr. Ferroelectric. 5, 155 (1994)
J.F. Scott, D. Galt, J.C. Price, J.A. Beall, R.H. Ono, C.A. Paz de Araujo, L.D. McMillan, Integr. Ferroelctric. 6, 189 (1995)
M.A. Mohiddon, P. Goel, K.L. Yadav, M. Kumar, P.K. Yadav, Indian J. Eng. Mater. Sci. 14, 64 (2007)
B. Jaffe, W.R. Cook, H. Jaff, Piezoelectric Ceramics, vol. 3 (Academic Press, New York, 1971), pp. 53–56
A. Jana, T.K. Kundu, Mater. Lett. 61, 1544 (2007)
C. Pecharromán, F. Esteban-Betegón, J.F. Bartolomé, S. López-Esteban, J.S. Moya, Adv. Mater. 13, 1541–1544 (2001)
D.L. Johnson, J. Am. Ceram. Soc. 74, 849–850 (1991)
B. Meng, J.H. Booske, R.F. Cooper, S.A. Freeman, Mater. Res. Soc. Symp. Proc. 347, 467–472 (1994)
J.D. Katz, Annul. Rev. Mater. Sci. 22, 153–170 (1992)
C.Y. Fang, C.A. Randal, M.T. Lanagan, D.K. Agrawal, J. Electroceram. 22, 125–130 (2009)
C.S. Chen, C.C. Chou, W.C. Yang, I.N. Lin, J. Electroceram. 13, 573–577 (2004)
H. Kishi, Y. Mizuno, H. Chazono, Jpn. J. Appl. Phys. 43, 1–15 (2003)
D.L. Johnson, J. Am. Ceram. Soc. 74, 849–850 (1991)
F. Selmi, F. Guerin, X.P. Yu, V.K. Varadan, V.V. Varadan, S. Komarneni, Mater. Lett. 12, 424–428 (1992)
Bin Cui, Yu. Pengfei, Jing Tian, Zhuguo Chang, Preparation and characterization of Co-doped BaTiO3 nanosized powders and ceramics. Mater. Sci. and Eng. B 133, 205–208 (2006)
Leroy Alexander, Harold P. Klug, Determination of Crystallite Size with the X Ray Spectrometer. J. App. Phys. 21, 137 (1950)
D. Lin, K.W. Kwok, H.L.W. Chan, Structure and electrical properties of Bi0.5Na0.5TiO3–BaTiO3–Bi0.5Li0.5TiO3 lead-free piezoelectric ceramics. Solid State Ion. 178, 1930–1937 (2008)
D.K. Agrawal, Curr. Opin. Solid State Mater. Sci. 3, 480–486 (1998)
Halle Abrams, Grain size measurement by the intercept method. Metallography 4(1), 59–78 (1971)
B.K. Bammannavar, L.R. Naik, B.K. Chougule, Studies on dielectric and magnetic properties of (x) Ni0.2Co0.8Fe2O4 +(1−x)barium lead zirconate titanate magnetoelectric composites. J. Appl. Phys. 104, 064123 (2008)
T. Kanata, T. Yoshikawa, K. Kubota, Grain-size effects on dielectric phase transition of BaTiO3 ceramics. Solid State Commun. 62, 765 (1987)
P. Kumar, M. Kar, Effect of structural transition on magnetic and optical properties of Ca and Ti co-substituted BiFeO3 ceramics. J. Alloys Compd. 584, 566 (2014)
J. Chen, Z. Tang, Y. Bai, S. Zhao, Multiferroic and magnetoelectric properties of BiFeO3/Bi4Ti3O12 bilayer composite films. J. Alloys Compd. (2016). doi:10.1016/j.jallcom.2016.03.119
Y. Wang, W. Rao, M. Wang, G. Li, Y. Li, J. Gao, W. Zhou, J. Yu, Dielectric, ferromagnetic and ferroelectric properties of the (1−x)Ba0.8Sr0.2TiO3–xCoFe2O4 multiferroic particulate ceramic composites. J. Mater. Sci.: Mater. Electron. 23, 1064–1071 (2012)
P. Goel, K.L. Yadav, J. Mater. Sci. 42, 3928 (2007)
H.J. Hwang, T. Nagai, T. Ohji, M. Sando, M. Toriyama, K. Niihara, Curie temperature anomaly in lead Zirconate Titanate/Silver composites. J. Am. Ceram. Soc. 81, 709–712 (1998)
T. Hiroshima, K. Tanaka, T. Kimura, Effects of microstructure and composition on the Curie temperature of lead barium niobate solid solutions. J. Am. Ceram. Soc. 79, 3235–3242 (1996)
M.S. Alkathy, R. Gayam, K.C. James Raju, Effect of sintering temperature on structural and dielectric properties of Bi and Li co-substituted barium titanate ceramic. Ceram. Int. (2016). doi:10.1016/j.ceramint.2016.06.194
H. Han, D. Ghosh, J.L. Jones, J.C. Nino, Colossal permittivity in microwave sintered barium titanate and effect of annealing on dielectric properties. J. Am. Ceram. Soc. 96, 485–490 (2012)
V.R. Reddy, S.K. Upadhyay, A. Gupta, A.M. Awasthi, S. Hussain, Enhanced dielectric and ferroelectric properties of BaTiO3 ceramics prepared by microwave assisted radiant hybrid sintering. Ceramics Int. 40, 8333–8339 (2014)
K. Uchino, S. Nomura, Ferroelectrics 44, 55 (1982)
X.G. Tang, J. Wang, X.X. Wang, H.L.W. Chan, Solid State Commun. 131, 163–168 (2004)
D.D. Gulwade, S.M. Bobade, A.R. Kulkarni, P. Gopalan, J. Appl. Phys. 97, 074106 (2005)
M.A. Mohiddon, K.L. Yadav, J. Phys. D Appl. Phys. 41, 225406 (2008)
M.A. Mohiddon, R. Kumar, P. Goel, K.L. Yadav, IEEE Trans Dielectric Insul Mater 14, 204 (2007)
M. Hashim, S. Kumar, S.E. Shirsath, E.M. Mohammed, H. Chung, R. Kumar, Studies on the activation energy from the ac conductivitymeasurements of rubber ferrite composites containing manganese zinc ferrite. Physica B 407, 4097–4103 (2012)
N.A. Hegab, M.A. Afifi, H.E. Atyia, A.S. Farid, J. Alloys Compd. 477, 925 (2009)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alkathy, M.S., Gayam, R. & James Raju, K.C. Effect of nickel and lithium co-substituted barium titanate ceramics on structural and dielectric properties. J Mater Sci: Mater Electron 28, 1684–1694 (2017). https://doi.org/10.1007/s10854-016-5714-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-5714-8