Abstract
Ni–Co/ZrO2 nanocomposite coatings were fabricated in a modified Watt’s bath by using high frequency pulse electrodeposition and the effects of pulse parameters such as frequency and duty cycle on the microstructure and properties were investigated. The surface morphology, phase structure, and microhardness of the Ni–Co/ZrO2 nanocomposite coatings were characterized by scanning electron microscopy with energy dispersive spectroscopy, X-ray diffraction and Vickers’ microhardness tester. The corrosion behaviour of the nanocomposites was evaluated by electrochemical impedance spectroscopy in the 3.5 wt% NaCl solution. The results revealed that increasing frequency and duty cycle resulted in a change of morphology from rough and porous structure to compact and homogeneous structure and reduced the ratio of relative intensity I(200)/I(111) of the Ni–Co/ZrO2 nanocomposites by intervening the adsorption–desorption of interfacial inhibitors at the cathode/solution interface. Furthermore, the effects of frequency and duty cycle on the microhardness of Ni–Co/ZrO2 nanocomposites should be associated with the ZrO2 nanoparticles according to dispersion strengthening from Orowan mechanism. It has been found that the corrosion resistance of the nanocomposites in 3.5 wt% NaCl solution depended on the incorporation of ZrO2 nanoparticles and the phase structure of Ni–Co/ZrO2 nanocomposites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Metallic composites, especially those based on nickel and cobalt, have captured considerable attention for the past several years owing to their excellent mechanical properties, such as hardness, corrosion and wear resistance, and high temperature inertness [1, 2]. Electrolytic deposition is a widely common method for producing those composites in which second-phase particles such as WC [3, 4], SiO2 [5, 6], Al2O3 [7, 8], SiC [9, 10], Cr2O3 [11], TiO2 [12, 13] and Si3N4 [14], are introduced in the plating solution and embedded into metal matrix. It has been established that improvement of mechanical behaviour of composite coatings depends on the distribution and content of the particles in coatings which are significantly influenced by electrodeposition conditions and electrolyte composition [10, 12, 15, 16]. Bakhit et al. [17] reported that Ni–Co/SiC nanocomposite coatings showed higher microhardness than the microcomposite ones and that the Ni–Co/SiC nanocomposite coatings with largest content of SiC nanoparticles (8.1 vol%) exhibited the best corrosion resistance in 3.5 % NaCl solution [18].
Pulse current electrodeposition (PCE) has emerged as a promising technique in fabricating multifunctional nanocomposite materials. Compared to the conventional direct current electrodeposition (DCE), the characterization of PCE requires three critical parameters, i.e. pulse-on time (T on ), pulse-off time (T off ) and peak current (I P ) [19]. In practice, the relationships between these variables are as follows:
It has been reported that pulse electrodepositing parameters have remarkable effect on the properties of coatings. For example, Lajevardi et al. [20] have investigated the electrodeposition of Ni–TiO2 nanocomposites and the results revealed that the enhancement of TiO2 particles was associated with the reduction of duty cycle. Yuan et al. [21] pointed out that increasing T on and I P not only resulted in the grain refinement but also changed the crystal orientation of nickel coatings. Gyftou et al. [22] studied the fabrication of pure Ni and composite Ni/SiC deposits in DCE and PCE methods. They showed that the hardness of Ni and Ni/SiC deposits depended on the pulse frequency and duty cycle and higher hardness was achieved at lower pulse current frequency and lower duty cycle.
Although a large number of reports have focused on the fabrication of various composites, the mechanical and corrosion behaviour of high frequency (above 10 kHz) pulse electrodeposited Ni–Co/ZrO2 nanocomposite coatings have rarely been reported in recent years. In this work, the Ni–Co/ZrO2 nanocomposite coatings were prepared by high frequency pulse electrodeposition and the effects of electrodeposition parameters on microstructure, microhardness and corrosion resistance were investigated. The morphology and phase structure of the coatings were characterized by scanning electron microscopy (SEM) and X-ray diffraction (XRD). The microhardness was measured by Vickers hardness tester. The corrosion resistance of the coatings was evaluated by electrochemical impedance spectroscopy (EIS) in the 3.5 wt% NaCl solution.
2 Experiment
Ni–Co/ZrO2 nanocomposite coatings with a thickness of at least 40 μm were electrolytically deposited on 1Cr18Ni9Ti stainless steel from a modified Watts bath by employing a self-made high-frequency pulse power supply. The composition of Ni–Co/ZrO2 nanocomposite bath and the experimental parameters of electrodeposition were given in Table 1. All reagents were analytical grade. To ensure a better dispersion of ZrO2 nanoparticles, sodium dodecyl sulfate (SDS) was added into the solution as a dispersant. A pure nickel plate (70 mm × 60 mm × 4 mm) was used as the anode, and the cathode was the 1Cr18Ni9Ti stainless steel substrate (20 mm × 20 mm × 1 mm). The parallel electrodes were vertically placed with a distance between cathode and anode of 3 cm in a 500 mL electrolytic cell. The substrate was mechanically polished with sandpaper, ultrasonically deoiled with acetone and cleaned in distilled water, finally activated in 5 vol% H2SO4 for 30 s at room temperature. Before the electrodeposition process, the plating solution was magnetically stirred for 4 h so that all electrolyte compositions had been fully dissolved. The pH of the solution was adjusted at 5.0 by using H2SO4 and NaOH. During the deposition, the bath was stirred with an agitation rate of 400 rpm and the temperature was maintained at 45 °C. The obtained coatings were ultrasonically cleaned in deionized water for 15 min so as to remove the loosely absorbed ZrO2 nanoparticles from the surface of the coatings.
The morphologies, phase structure, microhardness of Ni–Co/ZrO2 nanocomposite coatings were evaluated by a scanning electron microscopy (SEM, BCPCAS-4800), an X-ray diffraction (XRD, D8 ADVANCE BRUKER) with a Cu-Kα radiation, a Vickers’ microhardness instrument (VTD512, Woway Technology Co. Ltd., Beijing), respectively. The X-ray energy dispersive spectrometer (EDS) coupled to the SEM was adopted to analyze the elements of the coatings. The microhardness test was taken on the polished cross sections of the coatings mounted in resin by applying a load of 50 g and an indentation time of 15 s. Five measurements at different areas were performed for each sample and the corresponding final values were averaged in such a way to ensure the reliability and reproducibility of the results.
Electrochemical impedance spectroscopy (EIS) measurements were conducted in a standard three-electrode system, where the Ni–Co/ZrO2 nanocomposite coating was used as the working electrode, a saturated calomel electrode (SCE) as the reference electrode and a platinum sheet as the auxiliary electrode. The EIS tests were carried out in the 3.5 wt% NaCl solution at room temperature (25 °C) by using an electrochemical instrument (CHI750D) in a frequency range from 0.01 Hz to 100 kHz with 5 mV sinuous signal amplitude. Prior to EIS measurements, the samples were immersed in the 3.5 wt% NaCl solution for about 40 min to attain steady-state corrosion potential.
3 Results and discussion
3.1 Effects of frequency and duty cycle on the surface morphology
Figure 1 shows the surface morphologies of the Ni–Co/ZrO2 nanocomposites fabricated with frequencies as (a) 20, (b) 60, (c) 100, and (d) 140 kHz. The EDS elemental analysis of all samples is listed in Table 2. As seen in Fig. 1, increasing the frequency of pulse current from 20 to 140 kHz leads to a structural change from rough and porous structures (Fig. 1a, b) to smooth and compact structures (Fig. 1c, d). Comparing Fig. 1c, d, it is easy to find that Ni–Co/ZrO2 nanocomposites produced by 100 kHz possess more nanoparticles than those produced by 140 kHz. Additionally, Table 2 shows that the Zr element content of Ni–Co/ZrO2 nanocomposites increases with the rise of the frequency. However, the amount of Zr element decreases with further increasing the frequency up to 140 kHz. This phenomenon can be explained by the fact that when the frequency of pulse current is increased, the thickness of diffusion layer gets smaller. The thinner diffusion layer can reduce the deletion of electrolyte adjacent to the cathode, which has beneficial influence on the compact and homogeneous structure and the amount of ZrO2 particles. But too much high frequency decreases the time of one pulse cycle, which reduces the opportunity of ZrO2 particles and metal electrodeposition.
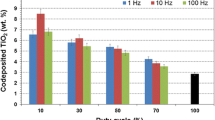
Figure 2 presents the surface morphologies of the Ni–Co/ZrO2 nanocomposites produced under different duty cycle conditions. As can be seen from Fig. 2, duty cycle variation has a significant effect on the structure of Ni–Co/ZrO2 nanocomposites. The result indicates that increasing duty cycle in some extent can reduce the porosity of Ni–Co/ZrO2 nanocomposites. The element quantity analysis reveals that with the duty cycle increasing from 20 to 40 %, the Zr element content in Ni–Co/ZrO2 nanocomposites increases from 0.46 to 8.62 wt%. When the duty cycle of pulse current is increased to 50 %, the value of Zr element content sharply declines to 2.78 wt%. These should be attributed to the followings. When duty cycle is increased, the pulse-on time is prolonged. When other conditions are kept constant, the increase of pulse-on time indicates the decline of the portion of time in which the double layer is charging, resulting in larger Faradaic current and greater cathode overpotential. It has been reported in previous paper [21] that the nucleation rate of new crystal increases with the increase of overpotential. Consequently, increasing duty cycle in some extent achieves the refinement of grain size and thus improves the quality of deposits. Furthermore, increasing duty cycle can promote the second absorption in the Guglielmi’s model [23], which enhances the ZrO2 nanoparticles in Ni–Co/ZrO2 nanocomposites. The decrease of ZrO2 particles content at the duty cycle of 50 % could be explained by concentration polarization.
3.2 Effects of frequency and duty cycle on phase structure
Figures 3 and 4 reveal the X-ray diffraction patterns the Ni–Co/ZrO2 nanocomposites deposited at different frequencies and duty cycles. The XRD spectra confirm that all Ni–Co/ZrO2 nanocomposites are composed of Ni–Co solid solution with the face centered cubic (FCC) structure. Since the content of ZrO2 nanoparticles is low, the ZrO2 peaks are not remarkable in the patterns. The ratio of relative intensity I(200)/I(111) of Ni–Co/ZrO2 nanocomposites are depicted in Table 3. As can be seen in these figures, the observed peaks at 2theta angles of approximately 44.8° and 52.1° correspond to (111) and (200) planes respectively. It is seen that the intensities of Ni–Co/ZrO2 nanocomposites diffraction peaks have been significantly influenced by the frequency and duty cycle of pulse current applied. The ratio of relative intensity I(200)/I(111) of the Ni–Co/ZrO2 nanocomposites which were deposited by low frequencies (below 100 kHz) are much higher than those deposited by high frequencies. Moreover, the ratio of relative intensity I(200)/I(111) of the Ni–Co/ZrO2 nanocomposites increase with decreasing the duty cycle. These results are closely related to the adsorption–desorption of interfacial inhibitors including H+ and Ni(OH)2 at the cathode/solution interface. It is interesting to note that, as Kollia et al. [24] stated, the worsening of the [100] texture results from the presence of the colloidal nickel hydroxide which are formed on the pulse on time and desorbed on the pulse off time. Higher duty cycles above 40 % (i.e. longer pulse on time) and higher frequencies above 100 kHz (i.e. shorter pulse off time) can reduce the ratio of relative intensity I(200)/I(111) of the Ni–Co/ZrO2 nanocomposites. Additionally, the nanoparticles can modify the [100] texture by influencing the adsorption–desorption of H+ [22], resulting in a decline of the ratio of relative intensity I(200)/I(111) of the Ni–Co/ZrO2 nanocomposites.
3.3 Effects of frequency and duty cycle on microhardness
Figures 5 and 6 demonstrate the influence of frequency and duty cycle on the microhardness of Ni–Co/ZrO2 nanocomposite coatings, respectively. It is shown in Fig. 5 that increasing the frequency to 100 kHz results in an increase in the microhardness up to 611 Hv and that increasing the frequency beyond 100 kHz gives rise in a decline in the microhardness. As the duty cycle increases, the microhardness initially increases from 375 to 611 Hv but then decreases down to 516 Hv, as shown in Fig. 6. The microhardness enhancement of Ni–Co/ZrO2 nanocomposite coatings should be attributed to the introduction of ZrO2 particles. According to Orowan mechanism, the Ni–Co alloy matrix carries the load and the incorporated ZrO2 nanoparticles hinder the dislocation mobility. As the embedded ZrO2 nanoparticles increase, the pinning sites of dislocation in Ni–Co/ZrO2 nanocomposite coatings increase, thereby reducing the deformation of the coatings. The result is similar to earlier reports by Yang et al. [10] for Ni–Co–SiC composites, Laszczyńska et al. [25] for Ni–Mo–ZrO2 coatings and Surender et al. [26] for Ni–WC system.
3.4 Effects of frequency and duty cycle on corrosion resistance
Figures 7 and 8 display the Nyquist plots of Ni–Co/ZrO2 nanocomposite coatings fabricated at various frequencies and duty cycles in 3.5 wt% NaCl solution. These diagrams of all samples are characterized by a single semicircle, the diameter of which is closely related to the charge transfer resistance [27]. It is seen from Fig. 7 that the diameters of the semicircles of Ni–Co/ZrO2 nanocomposite coatings increase with pulse frequency up to 100 kHz, then decrease with pulse frequency increasing from 100 to 140 kHz. According to Fig. 8, the Ni–Co/ZrO2 nanocomposite coating prepared at duty cycle of 40 % exhibits the biggest semicircle, indicating the highest anti-corrosion ability. In order to analyse the corrosion behaviour of these coatings in 3.5 wt% NaCl solution, the electrochemical equivalent circuit model shown in Fig. 9 is applied to simulate the Nyquist impedance plots. In Fig. 9, Rp is the charge transfer resistance, which can be evaluated the anti-corrosion ability. CPE and Rs represent the double layer capacitor and the resistance of corrosion medium (3.5 wt% NaCl solution), respectively. The Rp value of each samples are listed in Table 4. It could be founded that the Ni–Co/ZrO2 nanocomposite coatings produced with the frequency of 100 kHz and the duty cycle of 40 % exhibits the largest charge transfer resistance, indicating the best corrosion resistance. These phenomena could be attributed to the following factors. Firstly, the incorporation of ZrO2 nanoparticles can reduce the metallic matrix area exposed to 3.5 wt% NaCl solution. Furthermore, ZrO2 in nanoscales could occupy defects in Ni–Co/ZrO2 nanocomposite to form more compact structure, working as physical barrier to reduce defect corrosion. This result is in accord with earlier observations for Ni–SiO2 [28]. Secondly, the (111) plane has higher packings density than the (200) plane. Ramanauskas et al. [29] reported that lower corrosion rate of Zn–Ni alloy has been associated with the planes which has higher packings density. The ratio of relative intensity I(200)/I(111) of the Ni–Co/ZrO2 nanocomposites produced by higher frequencies (100 and 140 kHz) is lower than those produced by lower frequencies (20 and 60 kHz), which is in agreement with the change of corrosion resistance shown in Table 4. The Ni–Co/ZrO2 nanocomposites produced by 40 % duty cycle with the lowest ratio of relative intensity I(200)/I(111) show the best corrosion resistance in 3.5 wt% NaCl solution.
4 Conclusions
The study reveals the effect of electrodeposition condition such as frequency and duty cycle of pulse current on the microstructure and properties of Ni–Co/ZrO2 nanocomposite coatings by high frequency pulse electrodeposition. When the frequency is increased, the surface morphologies of the Ni–Co/ZrO2 nanocomposites change from rough and porous structures to smooth and compact structures. The increasing duty cycle reduces the porosity of Ni–Co/ZrO2 nanocomposites. Moreover, increasing frequency and duty cycle can reduce the ratio of relative intensity I(200)/I(111) of the Ni–Co/ZrO2 nanocomposites by intervening the adsorption–desorption of interfacial inhibitors at the cathode/solution interface. The influence of the frequency and duty cycle on the microhardness of Ni–Co/ZrO2 nanocomposites should be primarily attributed to the ZrO2 nanoparticles in deposits which provide the pinning sites of dislocation and reduce the deformation of the coatings. The Ni–Co/ZrO2 nanocomposites with highest corrosion resistance are fabricated under the condition of frequency of 100 kHz and duty cycle of 40 %. The corrosion behaviour of the nanocomposites in 3.5 wt% NaCl solution was studied based on the incorporation of ZrO2 nanoparticles and the phase structure of Ni–Co/ZrO2 nanocomposites.
References
C. Shi, L. Wang, L. Wang, J. Mater. Sci. Mater. Electron. 26, 7096 (2015)
Y. Min, T. Wang, Y. Chen, Y. Zhang, J. Mater. Sci. Mater. Electron. 22, 1404 (2011)
S. Mohajeri, A. Dolati, S. Rezagholibeiki, Mater. Chem. Phys. 129, 746 (2011)
S. Harsha, D.K. Dwivedi, A. Agrawal, Surf. Coat. Technol. 201, 5766 (2007)
H.S. Maharana, S. Lakra, S. Pal, A. Basu, J. Mater. Eng. Perform. 25, 327 (2015)
T.J. Tuaweri, G.D. Wilcox, Surf. Coat. Technol. 200, 5921 (2006)
M.H. Allahyarzadeh, M. Aliofkhazraei, A.R.S. Rouhaghdam, V. Torabinejad, J. Alloys Compd. 666, 217 (2016)
L.M. Chang, H.F. Guo, M.Z. An, Mater. Lett. 62, 3313 (2008)
Y.H. Xu, C. Li, Integr. Ferroelectr. 127, 71 (2011)
Y. Yang, Y.F. Cheng, Surf. Coat. Technol. 216, 282 (2013)
N. Malatji, A.P.I. Popoola, O.S.I. Fayomi, C.A. Loto, Int. J. Adv. Manuf. Technol. 82, 1335 (2015)
S. Mohajeri, A. Dolati, M. Ghorbani, Surf. Coat. Technol. 262, 173 (2015)
L. Benea, E. Danaila, J.-P. Celis, Mater. Sci. Eng. A 610, 106 (2014)
L. Shi, C.F. Sun, F. Zhou, W.M. Liu, Mater. Sci. Eng. A 397, 190 (2005)
M.R. Vaezi, S.K. Sadrnezhaad, L. Nikzad, Colloids Surf. A Physicochem. Eng. Asp. 315, 176 (2008)
P. Baghery, M. Farzam, A.B. Mousavi, M. Hosseini, Surf. Coat. Technol. 204, 3804 (2010)
B. Bakhit, A. Akbari, Surf. Coat. Technol. 206, 4964 (2012)
B. Bakhit, A. Akbari, F. Nasirpouri, M.G. Hosseini, Appl. Surf. Sci. 307, 351 (2014)
M.S. Chandrasekar, M. Pushpavanam, Electrochim. Acta 53, 3313 (2008)
S.A. Lajevardi, T. Shahrabi, Appl. Surf. Sci. 256, 6775 (2010)
Y. Xuetao, W. Yu, S. Dongbai, Y. Hongying, Surf. Coat. Technol. 202, 1895 (2008)
P. Gyftou, E.A. Pavlatou, N. Spyrellis, Appl. Surf. Sci. 254, 5910 (2008)
N. Guglielmi, J. Electrochem. Soc. 119, 1009 (1972)
C. Kollia, N. Spyrellis, J. Appl. Electrochem. 20, 8 (1990)
A. Laszczyńska, J. Winiarski, B. Szczygieł, I. Szczygieł, Appl. Surf. Sci. 369, 224 (2016)
M. Surender, B. Basu, R. Balasubramaniam, Tribol. Int. 37, 743 (2004)
A.C. Ciubotariu, L. Benea, M. Lakatos-Varsanyi, V. Dragan, Electrochim. Acta 53, 4557 (2008)
R. Li, Y. Hou, J. Liang, Appl. Surf. Sci. 367, 449 (2016)
R. Ramanauskas, P. Quintana, L. Maldonado, R. Porn, M.A. Pech-Canul, Surf. Coat. Technol. 92, 16 (1997)
Acknowledgments
This work is supported by National High Technology Research and Development Program 863 (2012AA112001, 2012AA112002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiang, Y., Xu, Y., Wang, M. et al. Effects of pulse plating parameters on the microstructure and properties of high frequency pulse electrodeposited Ni–Co/ZrO2 nanocomposite coatings. J Mater Sci: Mater Electron 28, 610–616 (2017). https://doi.org/10.1007/s10854-016-5565-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-5565-3