Abstract
Polymers/ZnO nanocomposites have been receiving great interest due to their wide range of applications in optical devices. So, there is an essential need to enhance their optical properties. In this work, nanocomposites of four types of polymer and ZnO nanoparticles have been prepared as flexiable foil by using the casting method. Poly (methyl methacrylate) (PMMA), poly (vinylidene fluoride) (PVDF), polyvinyl alcohol (PVA), and polystyrene (PS) are used as polymer matrix while different concentrations of ZnO nanoparticles are used as filler. The analysis of energy dispersive X-ray (EDX) is satisfied of the high purity of as-prepared samples. UV-visible transmittance spectra have shown low transmittance in UV region which is inversely proportional with the concentration of ZnO nanoparticles. Linear absorption coefficient (α) has shown the presence of absorption edges. The energy gap is calculated, it is noticed that the optical band gap of all nanocomposites are red shifted. The values of the energy gap for all samples decreased with the increase of the weight percentage of ZnO nanoparticles in nanocomposites. However, the decrement in nanocomposites samples is different.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Optical properties of nanocomposite have received much attention because they are different from individual polymers. They offer unexpected properties which greatly differ from conventional materials [1]. The optical properties of nanocomposites are certainly influenced by many factors such as size of filler, size distribution, degree of dispersion, and filler contents [2]. Zinc oxide (ZnO) is one of the most attractive semiconductors due to a unique combination of electrical and optical properties [3]. ZnO has a wide band gap (3.37 eV), which makes it an efficient UV absorber with the absorption edge of about 368 nm. The stability [4] of the electron–hole pairs is constant even at room temperature due to its large exiton binding energy (60 meV), which is much larger than the room temperature thermal energy (26 meV). Consequently, ZnO has become a promising material for optoelectronic devices of short wavelength,especially UV laser diode and light-emitting diodes.
The polymers used as the polymer matrix in this work are poly (methyl methacrylate) (PMMA), poly (vinylidene fluoride) (PVDF), polyvinyl alcohol (PVA), and polystyrene (PS). Polymethyl methacrylate(PMMA) [5, 6] has the unlimited care and significant attributes because of its unique properties such as rigidity, hard, high transparency in the visible region, low optical absorption, thermal capability, electrical performance, excellent mechanical properties, low cost,simple synthesis and low refractive index. It is a thermoplastic,so it can be designed into anything that we want. PMMA has a high energy gap, which is around (5.75–6 eV) [7]. PVDF has been the center of scientific interest. It is a semi crystalline polymer which has a special feature that distinguishes it from other polymers which is its uncommon polymorphism. It shows five crystalline phases named α, β, γ, δ, and ε [8]. In the recent years, many studies have focused on the crystalline structure and the crystalline phases produced during the preparation of PVDF film. Most of those studies are interested in the formation of β phase [8], and sometimes γ phase because they are very important to be used as a piezoelectric material due to its high piezoelectric activity. Despite few studies have been looking at the optical properties of PVDF, there is a clear lack of details in those studies. PVA has attractive properties like nontoxic, water soluble polymer,strong film forming ability,good charge storage capacity, high dielectric strength, and its optical and electrical properties depend on the type of doping filler. In addition to the important properties,it could function as a high performance polymer matrix or template to contain nanostructured materials [9, 10]. PS is an amorphous and optically clear thermoplastic material which is flexible as a thin films. It can be naturally transparent but can be colored with colorants. PS is used as the host matrix because of its ideal properties for investigating the optical properties of its nanocomposite [3].
The greatest problem [11, 12] during the preparation of polymer nanocomposites, is the homogenous and accomplishment of a uniform filler distribution inside the polymer matrix. Usually, the preparation method of nanocomposites and the interaction between the nanoparticles and the polymer matrix are considered as the main effect parameters on that dispersion, besides the nature of solvent that is used to dissolve the polymer [13]. The nanoparticles of material has a high surface energy, so it has a tendency to agglomerate or aggregate. This has been known from literature review which were referred to difficulty [11, 14, 15] of achievement a good distribution of inorganic nanoparticles in the polymer matrix. Different ways have been applied to avoid the agglomeration and aggregation [12, 14]. One of them is the preparation of the nanoparticles of material independently then, they are mixed with monomer solution and in situ polymerization is completed [16, 17]. The second one way is represented by the desperation of the nanoparticles and dissolving the polymer in the same suitable solvent separately, or in two different solvents which are soluble in each other. After that,the mixing of the two solutions together are done. This way requires the modification of nanoparticle surface [5, 17–19], which was done by different methods like using organic carboxylic acids as modifiers such as oleic acid [20]. In addition, the modification can be done when the nanoparticles were coated and modified by polymer [21, 22]. Furthermore, ultrasound treatment can be used to break the nanoparticles aggregates; however,the duration of sonication should be chosen carefully [22–24]. Lastly,the nanoparticles were precipitated in the polymer matrix, which are in the form of bulk polymer or monomer [15, 17, 25, 26]. In this method, the polymer matrix does not offer a suitable fluid for the nanoparticles to aggregate due to kinetic reasons. Previous literature has revealed that the sonication is very effective in breaking up ZnO aggregates. Hence ZnO nanoparticles cannot re-agglomerate again after sonication is stopped due to the entrapment in the polymer [5]. So during the preparation of nanocomposites in this work, ultrasound treatment was used with a specific duration time which was determined based on previous literature and lab experimental works to avoid agglomeration and aggregation.
The value and nature of the energy gap (Eg) depend on the linear absorption coefficient(α). To determine (Eg), one can use the equation [27, 28]:
where α is the linear absorption coefficient, ν is the frequency, h is Planck’s constant and Eg is the optical energy band gap between the valence band and the conduction band. A is a constant which depends on the transition probability, m is an index that describes the optical absorption process. Theoretically, it is equal to 2 for direct allowed, 2/3 for direct forbidden, 1/2 for indirect allowed and 1/3 for indirect forbidden transition [27, 29]. The value of m decides the nature of the energy gap or transition involved. Few literatures [30] revealed that determination of index (m) depends on the value of absorption coefficient. Another literature [31] indicated that estimation of (m) is obtained from the slope of the graph between log (α) and log (hυ). However, in general, the graph between (αhυ)m and photon energy (hυ) is first plotted, then the value of index m which gives the best linear graph is chosen [27].
By using Tauc’s plot, the value of the band gap (Eg) is calculated from the graph of (αhυ) 2 versus (hυ). The value of Eg will be given by extrapolating the linear portion of the curve to the hυ-axis.
In this work, polymer/ZnO nanocomposit as a flexiable foil have been successfully prepared by the casting method. Four different polymers; PMMA, PVDF, PVA, and PS are used as polymer matrix,in addition to different concentrations of ZnO nanoparticles are used as filler. The composition of nanocomposites is examined. Then, the energy band gap of samples are calculated. To the best of our knowledge, there was no report published to describe the preparation of polymer/ZnO nanocomposites as a flexiable foils for that purpose.
2 Method and materials
Four different types of polymers were used as polymer matrix; poly (methyl methacrylate) (PMMA), poly (vinylidene fluoride) (PVDF) polyvinyl alcohol (PVA), and polystyrene (PS). All of them were supplied by Sigma-Aldrich Zinc Oxide (ZnO) was purchased from Sigma-Aldrich. It was used as a filler in polymer/ZnO. The size of the nanoparticles was 50 < size < 100 nm.
2.1 Preparation of polymer/ZnO nanocomposites
Nanocomposites PMMA/ZnO were prepared in two steps; firstly, the PMMA solution was prepared by adding chloroform (CHCl3) to the (PMMA). Secondly, Zinc oxide (ZnO) with concentrations of (0, 1, 3, 5, 10 and 15 wt%) were added to the mixture of PMMA/chloroform. The procedures of preparation was maintained in our previous literature [32].
PVDF/ZnO nanocomposites were prepared using the same procedures applied for the preparation of PMMA/ZnO nanocomposites. PVDF was dissolved in DMF as solvent using a magnetic stirrer (angular velocity of 400 rpm and time duration of 2 hrs at 60 °C). Zinc oxide (ZnO) with concentrations(0, 1, 3, 5, 8 and 10 wt%) were added to the mixture of PVDF/DMF. Then a sonicator was used for 15 min to disperse the nanoparticles in the solution. After that, the solution was stirred at room temperature for 2 hrs by a magnetic stirrer (angular velocity 400 rpm) at room temperature to get a homogeneous solution.
PVA/ZnO nanocomposites was prepared by the same procedures used to prepare PMMA/ZnO nanocomposites. Firstly, PVA solution was prepared by adding distilled water (H2O) to the PVA. A magnetic stirrer (angular velocity of 400 rpm and duration time 2 hrs at 70 °C) was used to help it dissolve. Secondly, zinc oxide (ZnO) with concentrations of (0, 1, 3, 5, 10 and 15 wt%) were added to the mixture of PVA/water.
PS/ZnO nanocomposite was prepared by applying the same procedures used to prepare PMMA/ZnO nanocomposites. Firstly, PS was dissolved in toluene to prepare PS solution. Then it was stirred using a magnetic stirrer (angular velocity of 400 rpm and time duration of 2 hrs at 60 °C). Secondly, zinc oxide (ZnO) with concentrations of (0, 1, 3, 5, 10 and 15 wt%) were added to the mixture of PS/toluene. The same steps which used after adding ZnO nanoparticles to mixture of PMMA/Chloroform was followed when adding ZnO nanoparticles to PVA/water and PS/toluene.
Casting technique was used to prepare foils like pure polymer and like PMMA/ZnO, PVDF/ZnO, PVA/ZnO, and PS/ZnO nanocomposites. Pure PMMA solution with 0 wt% ZnO and PMMA/ZnO solution was casted uniformly on a glass petri dish. Whereas each one of three other pure polymer solutions and nanocomposite solutions were casted uniformly on a ceramic petri dish at room temperature. After few hours the film was pulled out easily as flexible foil. Next, the foil was kept at room temperature for one day for pure PMMA and PMMA/ZnO, and at 60 °C for one day for pure PVDF, PVDF/ZnO,pure PVA, PVA/ZnO,pure PS and PS/ZnO to solidify. Later, the foils like polymer/ZnO of four different types of polymer matrix were collected.
2.2 Characterization of the samples
The linear transmittance spectra values as well the reflectance values of the as-prepared samples were measured using UV-vis spectrophotometer (PerkinElmer instruments-Lambda 900 UV/VIS Spectrometer).
The chemical composition of the samples was evaluated via electron diffraction spectroscopy EDX, ZEISS, Merlin Compact which operated at an average distance of 8.5 mm and an acceleration voltage of 15 kV. Prior to the analysis, the sample was coated with a fine layer of platinum in order to reduce the charging effect.
3 Results and discussion
3.1 The compositional of nanocomposites materials
To further confirm the composition of the existence element in the sample, the energy dispersive X-ray (EDX) survey scan was carried on the as-prepared samples and shown in Fig. 1. The EDX of four different polymer/ZnO nanocomposites was shown in Fig. 1. Figure 1a shows the EDX spectra of PMMA/ZnO nanocomposites. There were three elements detected on the sample denoted as carbon (C), zinc (Zn) and oxygen (O) which emerged from the carbon chain in PMMA structure and the dopant source. Figure 1b shows the EDX spectra of PVDF/ZnO nanocomposites. Four elements are shown in this figure which are carbon and fluorine elements that represent the presence of PVDF structure, oxygen and zinc elements in the compound (ZnO). Figure 1c shows the EDX spectra of PVA/ZnO nanocomposites. Three elements are shown in this figure which are carbon element that represents PVA structure, the presence of oxygen element in the form of polymer (PVA) and the compound of ZnO, besides zinc element.
Figure 1d shows the EDX spectra of PS/ZnO nanocomposites. The three elements shown are carbon element which represents the presence of PS structure, oxygen, and zinc elements in the compound (ZnO). This result also suggests that the as-prepared samples used were in high-purity condition.
3.2 UV-vis transmittance spectra
Figure 2 shows the UV-visible transmittance spectra of pure PMMA, PVDF, PVA and PS in addition to the nanocomposites of PMMA/ZnO, PVDF/ZnO, PVA/ZnO and PS/ZnO as flexible foils with different concentrations of ZnO nanoparticles.
The pure PMMA showed high transparency in the visible region (approximately 90 %)but relatively lower in the UV region (around 80 %). Meanwhile pure PVA showed transparency around 85 % in the visible region and less than it in the UV region. Pure PS showed transparency approximately 80 % in the UV and visible regions. However pure PVDF showed the lowest transparency at UV and visible regions of the four nanocomposites, it was around 15 %. The effect of adding ZnO nanoparticles to four nanocomposites was clear; they have low transmittance in the UV region that decreases with the increasing content of ZnO in the nanocomposite. High concentration of ZnO nanoparticles showed the lowest transmittance in all four nanocomposites. These results are in accordance with Anžlovar et al. and Khan et al. [12, 33] for PMMA/ZnO, Indolia and Gaur [1] for PVDF/ZnO, Kumar et al. and Mallika et al. [34, 35] for PVA/ZnO, and Jeeju et al. [3] for PS/ZnO. The peak absorption of four nanocomposites was observed at the range of 370–377 nm which indicates the effect of polymer matrix. Although ZnO nanoparticles had lower absorption spectrum in the visible region, the transmittance of polymer/ZnO for this spectral region declined with the increasing of ZnO concentration due to the smoothness of the surface that increases the reflectivity and leads to low transmittance in the visible range.
3.3 Calculation of linear absorption coefficient (α)
The linear absorption coefficient (α) for as-prepared samples was calculated from [10]:
where d is the thickness of the sample, which was determined using a digital micrometre at different places in each film and an average was determined; they were around 70 μm for PMMA/ZnO, 30 µm for PVDF/ZnO,80-90 µm for PVA/ZnO, and 100–120 µm for PS/ZnO. T is the transmittance and R is the reflectance. The transmittance and the reflectance were measured by UV-vis spectroscopy (i.e., the reflectance was not obtained from the equation T + A+R = 1 because it measured as a separate parameter by using UV-vis spectroscopy itself that has the feature to measure values of R).
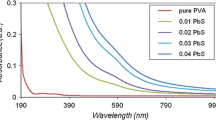
Figure 3a shows the linear absorption coefficient (α) spectra of PMMA/ZnO nanocomposites. They indicated a small deference in the values of α between the UV and visible regions. The values of α decreased with increasing wavelengths in all samples, however it seemed to be gradual. Figure 3b shows the linear absorption coefficient (α) spectra of PVDF/ZnO nanocomposites. They show decreasing value of (α) with increase the wavelength to visible region. Figure 3c, d show the linear absorption coefficient (α) spectra of PVA/ZnO and PS/ZnO nanocomposites, respectively. In both types of nanocomposites, the value of α in the UV region was higher than in the visible region.
Generally in all nanocomposites, the results showed that α increased with the increase of ZnO nanoparticles contained, especially in short wavelengths. This means that there is a high probability of the transition to be directly allowed to take place [36]. The presence of absorption edges was observed, which is an indication of a good degree of crystallinity of nanocomposite samples [28]. In addition, α depends on the wavelength of light that is actually being absorbed.
3.4 Calculation of energy gap (Eg)
Figure 4a demonstrates the relationship between (αhυ)2 and photon energy (hυ) of PMMA/ZnO nanocomposites. The optical energy gap of pure PMMA (0 wt% ZnO) is equal to 5.08 eV. It can be clearly seen that the values of the optical energy gap depend on the weight percentage of ZnO nanoparticles. There is a decrease in the optical energy gap of nanocomposites with the increase of ZnO weight compared to pure PMMA, which is shown in Fig. 5. The proportion of the energy gap with the weight percentage of ZnO nanoparticles is inversed. This is due to the increase in the shift of the valence and the conduction band. In addition, the enhancement of carrier–carrier interaction due to the high concentration of carrier in valence and conduction bands leads to a reduction in the bandgap [37]. Besides that, the presence of unsaturated defects caused an increase in the density of localized states in the bandgap and then decreased the optical energy gap [38]. The decrement of the energy gap is equal to 0.5 eV with higher weight percentage of ZnO (15 wt%).
Figure 4b shows the relationship between (αhυ)2 and photon energy (hυ) of PVDF/ZnO nanocomposites. The optical energy gap of pure PVDF (0 wt% ZnO) is equal to 5.88 eV. As in PMMA/ZnO nanocomposites, the energy band gap is directly proportional with the increase of the weight percentage of ZnO nanoparticles, but the decrement of the energy gap has a value with higher weight percentage of ZnO (15 wt%) in nanocomposites equal to 0.78 eV. These results correspond to the studies by Indolia and Gaur [1] and Bhunia et al. [39].
Figures 4c, d demonstrate the relationship between (αhυ)2 and photon energy (hυ) of PVA/ZnO and PS/ZnO nanocomposites,respectively. The optical energy gap of pure PVA and pure PS (0 wt% ZnO) is equal to 5.65 and 4.5 eV, respectively, while the values of the higher weight percentage of ZnO (15 wt%) in nanocomposites equal to 4.75 and 3.9 eV respectively.
It was noticed that the optical band gap of the samples were red shifted, and the comparison between all nanocomposites is shown in Fig. 5. The highest energy gap of pure polymer (0 wt% ZnO) is seen in pure PVDF as foil which equals to 5.88 eV, while the lowest energy gap is shown by pure PS as foil which equals to 4.5 eV. The highest decrement is shown by PVA/ZnO nanocomposites, while the lowest decrement is shown by PMMA/ZnO.
4 Conclusion
Flaxiable foils like polymer/ZnO nanocomposites,with different concentrations of ZnO nanoparticles and four differint types of polymers, were successfully prepared. The composition of the existence elements of the as-prepared samples was satisfied by EDX. All nanocomposites showed low transmittance in UV spectrum due to the behavior of ZnO nanoparticles. The linear absorption coefficient (α) of the samples were calculated. They depended on the wavelength of light that is actually being absorbed. The optical enrgy band gap of pure polymer has more value than polymer/ZnO nanocomposites for all differnt types of nanocomposites. The relation between the energy gap with the weight percentage of ZnO nanoparticles in nanocomposites is inversely proportional, also, the optical band gap of nanocomposites is red shifted. The amount of ZnO nanoparticles that is required to reduce the value of energy gap in nanocomposite had different effect depending on type of polymer matrix.
References
A.P. Indolia, M.S. Gaur, Optical properties of solution grown PVDF-ZnO nanocomposite thin films. J. Polym. Res. 20(1), 1–8 (2013)
N. Rajagopalan, A.S. Khanna, Effect of size and morphology on UV-blocking property of nanoZnO in epoxy coating. Int. J. Sci. Res. Publ. 3(4), 1–14 (2013)
P.P. Jeeju, S. Jayalekshmi, K. Chandrasekharan, P. Sudheesh, Size dependent nonlinear optical properties of spin coated zinc oxide-polystyrene nanocomposite films. Opt. Commun. 285(24), 5433–5439 (2012)
N.K. Hassan, M.R. Hashim, Structural and optical properties of ZnO thin film prepared by oxidation of Zn metal powders. Sains Malaysiana 42(2), 193–196 (2013)
A. Anžlovar, K. Kogej, Z. Crnjak Orel, M. Žigon, “Polyol mediated nano size zinc oxide and nanocomposites with poly(methyl methacrylate)”. Express Polym. Lett. 5(7), 604–619 (2011)
S.S. Chiad, “Dispersion Parameters of Copper Sulphate Doped PMMA”. Mater. Methods 7(1), 162–167 (2010)
V. Švorčík, O. Lyutakov, I. Huttel, Thickness dependence of refractive index and optical gap of PMMA layers prepared under electrical field. J. Mater. Sci.: Mater. Electron. 19(4), 363–367 (2008)
B. Ahmed, S.K. Raghuvanshi, N.P. Sharma, J.B.M. Krishna, M.A. Wahab, “1.25mev Gamma Irradiated Induced Physical and Chemical Changes in Poly Vinylidene Fluoride (PVDF) Polymer’’. Prog. Nanotechnol. Nanometerials 2(2), 42–46 (2013)
W. M. M. El.Sayed “Physical properties of ZnO/PVA nanocomposite films” in Sixth International Conference on Nano- Technology in Construction, Egyptian-Russian University, 2014
A. Hamdalla, T.A. Hanafy, A.E. Bekheet, “Influence of erbium ions on the optical and structural properties of polyvinyl alcohol”. J. Spectrosc. 2015, 1–7 (2015)
M. Agrawal, S. Gupta, N.E. Zafeiropoulos, U. Oertel, R. Häßler, M. Stamm, Nano-level mixing of ZnO into poly(Methyl methacrylate). Macromol. Chem. Phys. 211(17), 1925–1932 (2010)
M. Khan, M. Chen, C. Wei, J. Tao, N. Huang, Z. Qi, L. Li, Synthesis at the nanoscale of ZnO into poly(methyl methacrylate) and its characterization. Appl. Phys. A 117(3), 1085–1093 (2014)
C.H. Dan, Y.D. Kim, M. Lee, B.H. Min, “Effect of solvent on the properties of thermoplastic polyurethane/clay nanocomposites prepared by solution mixing”. J. Appl. Polym. Sci. 108, 2128–2138 (2008)
S. Li, M. Meng Lin, M.S. Toprak, D.K. Kim, M. Muhammed, Nanocomposites of polymer and inorganic nanoparticles for optical and magnetic applications. Nano Rev. 1, 1–19 (2010)
T. Kos, A. An, Z.C. Orel, PMMA-b-PMAA Diblock copolymer as a reactive polymeric surfactant for the functionalization of ZnO nanoparticles. Acta Chim. Slov. 61, 497–505 (2014)
E. Tang, G. Cheng, X. Pang, X. Ma, F. Xing, Synthesis of nano-ZnO/poly(methyl methacrylate) composite microsphere through emulsion polymerization and its UV-shielding property. Colloid Polym. Sci. 284(4), 422–428 (2006)
R.Y. Hong, J.Z. Qian, J.X. Cao, Synthesis and characterization of PMMA grafted ZnO nanoparticles. Powder Technol. 163(3), 160–168 (2006)
D.P. Liu, G.D. Li, Y. Su, J.S. Chen, Highly luminescent ZnO nanocrystals stabilized by ionic-liquid components. Angew. Chemie—Int. Ed. 45(44), 7370–7373 (2006)
V. Khrenov, M. Klapper, M. Koch, K. Müllen, Surface functionalized ZnO particles designed for the use in transparent nanocomposites. Macromol. Chem. Phys. 206(1), 95–101 (2005)
L. Zhu, H. Jiang, B. Wang, J. Wei, “Surface Modification for Nano-ZnO and Its Mechanism”. J. Inorg. Mater. 22(2), 219–222 (2007)
H.-M. Xiong, Photoluminescent ZnO nanoparticles modified by polymers. J. Mater. Chem. 20(21), 42514262 (2010)
M.H. Nia, M. Rezaei-tavirani, A.R. Nikoofar, H. Masoumi, R. Nasr, H. Hasanzadeh, M. Jadidi, M. Shadnush, Stabilizing and dispersing methods of TiO2 nanoparticles in biological studies. J. Paramed. Sci. 6(2), 96–105 (2015)
N. Mandzy, E. Grulke, T. Druffel, Breakage of TiO2 agglomerates in electrostatically stabilized aqueous dispersions. Powder Technol. 160(2), 121–126 (2005)
V. Sygouni, C.V. Chrysikopoulos, Characterization of TiO2 nanoparticle suspensions in aqueous solutions and TiO2 nanoparticle retention in water-saturated columns packed with glass beads. Chem. Eng. J. 262, 823–830 (2015)
A. Anžlovar, Z.C. Orel, M. Žigon, Sub micrometer and nano ZnO as filler in PMMA materials. Mater. Tehnol. 45(3), 269–274 (2011)
J.A. Paramo, Y.M. Strzhemechny, A. Anžlovar, M. Žigon, Z.C. Orel, Enhanced room temperature excitonic luminescence in ZnO/polymethyl methacrylate nanocomposites prepared by bulk polymerization. J. Appl. Phys. 108(2), 1–6 (2010)
K. Al-ammar, A. Hashim, M. Husaien, Synthesis and study of optical properties of (PMMA-CrCl2) composites. Chem. Mater. Eng. 1(3), 85–87 (2013)
A.M.A. Al-Hussam, S.A.-J. Jassim, Synthesis, structure, and optical properties of CdS thin films nanoparticles prepared by chemical bath technique. J. Assoc. Arab Univ. Basic Appl. Sci. 11(1), 27–31 (2012)
V.S. Sangawar, M.C. Golchha, Evolution of the optical properties of polystyrene thin films filled with zinc oxide nanoparticles. Int. J. Sci. Eng. Res. 4(6), 2700–2705 (2013)
F.A. Mustafa, “Optical properties of NaI doped polyvinyl alcohol films”. Phys. Sci. Res. Int. 1, 1–9 (2013)
D. Dorranian, Y. Golian, A. Hojabri, Investigation of nitrogen plasma effect on the nonlinear optical properties of PMMA. J. Theor. Appl. Phys. 6(1), 1–8 (2012)
H.M. Shanshool, M. Yahaya, W.M.M. Yunus, I.Y. Abdullah, Third order nonlinearity of PMMA/ZnO nanocomposites as foils. Opt. Quantum Electron. 48(1), 1–14 (2016)
A. Anžlovar, Z.C. Orel, K. Kogej, M. Žigon, “Polyol-mediated synthesis of zinc oxide nanorods and nanocomposites with poly(methyl methacrylate)”. J. Nanomater. 2012, 1–9 (2012)
N.B.R. Kumar, V. Crasta, B.M. Praveen, Advancement in microstructural, optical, and mechanical properties of PVA (Mowiol 10-98) doped by ZnO nanoparticles. Phys. Res. Int. 2014, 1–9 (2014)
A.N. Mallika, A.R. Reddy, K.V. Reddy, Annealing effects on the structural and optical properties of ZnO nanoparticles with PVA and CA as chelating agents. J. Adv. Ceram. 4(2), 123–129 (2015)
A.C. Nwanya, C. Chigbo, S.C. Ezugwu, R.U. Osuji, M. Malik, F.I. Ezema, “Transformation of cadmium hydroxide to cadmium oxide thin films synthesized by SILAR deposition process: role of varying deposition cycles”. J. Assoc. Arab Univ. Basic Appl. Sci. 20, 49–54 (2016).
L.M. Irimpan, “Spectral and nonlinear optical characterization of ZnO nanocomposites” Cochin University of Science and Technology. PhD Thesis. (2008)
M.A.R.H. El-Zahed, A. El-Korashy, Effect of heat treatment on some of the optical parameters of Cu9Ge11Te80 films. Vacuum 68, 19–27 (2003)
R. Bhunia, D. Ghosh, B. Ghosh, S. Hussain, R. Bhar, A.K. Pal, “Free-standing flexible nanocrystalline-ZnO-impregnated polyvinylidene fluoride composite thin films”. J. Compos. Mater. 49(25), 3089–3101 (2015)
Acknowledgments
The authors would like to acknowledge the contribution and the financial support by the Malaysian Ministry of Higher Education and Universiti Kebangsaan Malaysia under the research Grant (FRGS/1/2013/SG02/UKM/01/1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shanshool, H.M., Yahaya, M., Yunus, W.M.M. et al. Investigation of energy band gap in polymer/ZnO nanocomposites. J Mater Sci: Mater Electron 27, 9804–9811 (2016). https://doi.org/10.1007/s10854-016-5046-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-5046-8