Abstract
Poly(3,4-ethylenedioxythiophene); poly(styrenesulphonate) (PEDOT-PSS) is one of the most successful polymer for many of the electronic applications. It can be easily dispersed in water and few polar organic solvents. It is well known that the PEDOT-PSS dispersed in aqueous solution has very low conductivity which hinders its application as a transparent electronic material. In this paper we report the conductivity enhancement and dielectric parameters of PEDOT-PSS transparent films prepared using DMSO as an organic solvent. The structural properties of prepared films were investigated by SEM, UV–visible spectroscopy and FTIR spectroscopy. The SEM studies on these films indicate the formation of granular islands in the composite films. The FTIR spectra of DMSO in PEDOT-PSS indicate the shifting of characteristics peaks at higher wave number with the decrease in peak intensity in comparison to the pristine PEDOT-PSS. The UV–visible studies show the sharp absorptions around 500–700 nm at NIR range for the PEDOT-PSS doped with DMSO. It is observed that the addition of DMSO does the conformational change in the PEDOT-PSS from coil structure to linear structure. The change in morphology due to addition of DMSO brings feasibility in charge transportation through PEDOT backbone of polymer chain resulting in conductivity enhancement and low dielectric permeability and tangent loss in the composite thin films. It is observed that, the addition of DMSO increases the conductivity of pristine PEDOT-PSS by three orders of magnitude. Therefore, these DMSO doped PEDOT-PSS can be used in many optoelectronic applications as a transparent and flexible conducting polymer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Poly(3,4-ethylenedioxythiophene); poly(styrenesulphonate) which is known as (PEDOT-PSS) has attracted considerable attention of researchers for its technological importance due its stability and transparency [1–7]. Even though indium tin oxide (ITO) is the most widely used conducting electrode in optoelectronics, its cost and mechanical brittleness hinders its usability as a flexible electrode [8, 9]. Hence developing newer transparent conductive materials that can replace ITO is the demand of present day technology. Conductive polymeric materials have highly attracted attention over the past several years mainly due to their potential advantage of low-cost, large area, light weight and vacuum free fabrication. Conductive polymeric materials not only replace lots of metal and inorganic conducting materials but also become the critical key in the modern industry and advanced technology [10–14]. Most of the conducting polymers have poor processability compared to PEDOT-PSS, which can be easily processed in aqueous media by dispersing in water or some polar organic solvents [2, 15] but unfortunately the conductivity of PEDOT-PSS is many orders lower than that of ITO. This issue has been resolved to some extent by a technique called secondary doping in PEDOT-PSS [16–20], thus increasing the conductivity by few orders of magnitude. Even though good number of publications are available to address the issue to enhance the conductivity of PEDOT-PSS, yet it could not meet the expectations of industry requirements. In the previous studies is been reported that PEDOT-PSS doped with secondary dopants such as polyhydroxy alcohol play a significant role in changing the morphology and transport properties of pristine PEDOT-PSS [5, 21–26]. Therefore is it is very essential from industry perspective to understand the effect of secondary dopants on physio-chemical properties of pristine PEDOT-PSS [15, 24, 26–30].
In this work we report the preparation of PEDOT-PSS based organic thin film obtained by using secondary dopants such as dimethyl sulfoxide (DMSO). We have made an attempt to understand the effect of DMSO loadings in different concentrations on the structure and properties of pristine PEDOT-PSS especially with respect its transport properties. This paper deals in detail with the morphological changes in pristine PEDOT-PSS films due to secondary doping of DMSO through SEM, UV–Vis and FTIR spectroscopy. The transport properties in these doped films were studied through temperature and frequency dependent conductivities (DC & AC conductivities) as well as dielectric and impedance measurements. It has been observed through our studies that the conformational changes that takes place in the polymer (PEDOT-PSS) back bone chain due to secondary doping of DMSO is mainly responsible for the change in conductivities of pristine PEDOT-PSS. In this work we report the conductivity enhancement of pristine PEDOT-PSS by three orders of magnitude due to secondary doping of DMSO. Hence due to their transparency, ease of fabrication and high conductivity, these DMSO doped PEDOT-PSS organic thin films may find extensive application in optoelectronic devices.
2 Experimental details
The commercially available aqueous dispersion of PEDOT-PSS (Baytron-P) and dimethyl sulfoxide (DMSO) were purchased from Sigma Aldrich (India). The PEDOT-PSS organic thin films were prepared by secondary doping of DMSO with pristine PEDOT-PSS. The doped PEDOT-PSS thin films were prepared by using four different weight ratio such as 5, 10, 15 and 20 wt% of dopant in PEDOT-PSS through the spin coating technique. The DC conductivity of pristine and doped PEDOT-PSS thin films were carried out by four probe method (Keithley 2410 Source meter) in the temperature range 20–200 °C. The AC conductivity and dielectric behavior of pristine and doped PEDOT-PSS thin films were carried by two probe method in the frequency range 100–2 MHz at different temperatures using LCR impedance analyzer (model Wayne Kerr 6500B).
The prepared samples were characterized by FTIR Spectra for structural analysis using FTIR spectrometer (Thermo-Nicolet 6700). The surface morphology was studied using scanning electron microscope (Model Zeiss Ultra 60). The absorption spectra of the samples in the UV and visible region were recorded using UV–Vis spectrometer (Analytikjena SPECORD S-600). The thickness of film is determined using surface profillometer (Nanosystem NV-E1000) and was found to be about 110 nm.
2.1 Preparation of organic thin films doped with DMSO
The solvent dimethly sufoxide (DMSO) which was purchased from Sigma Aldrich (99.9 %) was purified prior to use and added to the PEDOT-PSS solution directly with four different weight percentage of total solution such as 5, 10, 15 and 20 wt%, and then doped PEDOT-PSS solution was stirred for 2 h at room temperature using magnetic stirrer to obtain the homogeneous dispersion. The solution of PEDOT-PSS and DMSO is pre filtered through a whatman filter paper pores size 0.45-µm before preparation of thin films. The glass substrates for characterization and ITO coated glass of surface resistance 100 Ω/sq for electrical measurements with an area 2 cm × 2 cm were pre cleaned with acetone, isopropyl alcohol and de-ionized water and finally the glass substrates were dried in vacuum oven for 20 min. The doped PEDOT-PSS solution was coated by using Spin Coating unit (Spin NXG-P1), the spin coating was carried out at a rotation rate of 1000 rpm for 60 s. The doped PEDOT-PSS thin films were annealed at 150 °C for 30 min in a vacuum oven in order to remove water content or moisture from the film.
3 Results and discussion
3.1 Scanning electron microscopy
Figure 1a, b shows the SEM micrographs of pristine PEDOT-PSS and PEDOT-PSS doped with DMSO. The SEM micrograph of pristine PEDOT-PSS as shown in Fig. 1a, is smooth and homogeneous with an average grain size of 25 nm as calculated by liner intercept method. The micrograph also reveals that, the grains are separated by marginal distances with enough binding energy to interconnect between the neighboring grains. The addition of DMSO to PEDOT-PSS leads to the formation of micro grains that (Fig. 1b) are well interconnected, the grains are highly clustered, spherical in shape and are inter linked to each other, these highly clustered grains in polymer matrix plays a significant role in charge transfer mechanism in the system. The increased concentration of DMSO in PEDOT-PSS leads to formation of more aggregated grains with conducting islands that can facilitate the charge transfer in the polymer backbone.
3.2 FTIR analysis
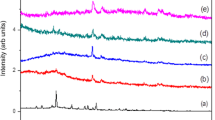
Figure 2 shows the FTIR spectra of pristine PEDOT-PSS and PEDOT-PSS doped with DMSO. The FTIR spectrum of pristine PEDOT-PSS shows characteristics peaks at the stretching frequency of 3700 cm−1 due to O–H stretching, 3500 cm−1 due to C–H stretching, 2900 cm−1 due to C=O and 2500 cm−1 due to C–N stretching, the peak corresponding to stretching frequency 1899 cm−1 is due to C=S bond stretching mode of the Sulfoxide groups. The composite sample shows characteristics absorption band at 2861 cm−1 due to intrinsic vibration of sulfoxide ion which confirm the formation of PEDOT-PSS doped DMSO nano composites. The addition of DMSO in PEDOT-PSS results in the increases of the intensity of peaks maxima as well as slight shift in the characteristics peaks to higher wave number. This clearly indicates the formation of composite between PEDOT-PSS and DMSO due to weak Vanderwaal’s interaction.
3.3 UV–visible spectrum
Figure 3 shows the UV–Vis spectra of pristine PEDOT-PSS and PEDOT-PSS doped with DMSO. The UV–Vis spectrum for both pristine PEDOT-PSS and PEDOT-PSS doped with DMSO exhibits the similar trend with relatively sharp absorptions in the UV at 500–700 nm and an intense, broad absorption at NIR range. The higher energy transition can be assigned to π–π* transitions in the polymer backbone. The band starting at 800 nm had been described as the free carrier tail. There is a substantial increase in the intensity of UV–Vis spectra of doped PEDOT-PSS in comparison to pristine PEDOT-PSS which indicates the effect of dopant on the characteristics features of pristine PEDOT-PSS.
4 Transport properties
4.1 DC conductivity
Figure 4 represents the variation of DC conductivity of pristine PEDOT-PSS and PEDOT-PSS doped with DMSO. The conductivity of pure PEDOT-PSS and PEDOT-PSS doped with DMSO exhibit linear 2 step variation with small increment in the temperature range 20–80 °C, between 80 and 120 °C the conductivity shows a rapid increase. This behavior may be attributed to thermally assist hopping conduction of charge carriers between favorable sites. The secondary doping of DMSO into PEDOT-PSS enhances the conductivity of doped films by three orders of magnitude when compared to pristine PEDOT-PSS. This change in conductivity is significantly due to the morphological changes of the thin films in the polymer backbone where the coil like structure changes into a linear structure which required less activation energy as shown in the Table 1. These conformational changes in polymer backbone facilitate the hopping of charge carriers leading to enhanced conductivity. The addition of DMSO into PEDOT-PSS leads formation of bond between conducting PEDOT phase and neutral PSS phase, thereby reducing the phase segregation between PEDOT and PSS, this in turn reduces the activation energy and charge carries can easily migrate between conducting sites in polymer backbone. It is observed through our studies (Fig. 4) that, the enhancement of conductivity of pristine PEDOT-PSS depends on the concentration of secondary dopants. Among the different composite films prepared, 20 wt% composite shows maximum conductivity with better chemical stability.
4.2 AC conductivity
Figure 5 represents the variation of AC conductivity for pure PEDOT-PSS and PEDOT-PSS doped with DMSO. All the samples exhibit similar behavior for frequency dependence of AC conductivity. The conductivity of pure PEDOT-PSS and doped PEDOT-PSS with DMSO increases with increasing frequencies, obey universal power law. At low frequencies the conductivity of all the samples is almost constant which is independent of frequency and at higher frequencies the conductivity shows strong frequency dependence, the increase in conductivity may be attributed due to the favorable hopping conduction at higher frequencies. From the plots 5 it can be observed that the addition of DMSO effectively increases the AC conductivity by 3–4 orders of magnitude as seen in case of DC conductivity. Among the different samples prepared, composite with 20 wt% of DMSO in PEDOT-PSS shows higher conductivity of the order 132.12 s/cm at 106 Hz. This may be attributed due to the dipole polarization effects at the grain boundaries. The addition of DMSO in the PEDOT-PSS leads to formation of more segregated grains that depends on the concentration of DMSO. These grains can cause the dipole polarization effects which facilitate the migration of charge carriers.
4.3 Temperature dependence of AC conductivity
Figure 6a–e shows temperature dependence of AC conductivity of pristine PEDOT-PSS and DMSO doped PEDOT-PSS for various weight percentage (5, 10, 15 and 20 wt%), it is observed from all the plots that the frequency assisted conductivity varies with respect to temperature. The conductivity increases with increase in temperature for the all the samples. This is attributed due to the thermally assisted hopping of charge carriers under the influence of applied frequency. At higher temperatures the polymer backbone chains opens up forming a linear structure and applied frequency facilitates the dipole polarization leading to better conducting pathways.
4.4 Dielectric parameters
Figure 7 shows the variation of real part of dielectric constant with frequency for pristine PEDOT-PSS and DMSO doped PEDOT-PSS nanocomposites films. The value of dielectric constant decreases with increasing frequency for all composites. Among all the composites, pure PEDOT-PSS shows higher dielectric constant and for PEDOT-PSS doped DMSO at various weight percentage (5, 10, 15 and 20 wt%) the permittivity value decreases with increasing concentration of DMSO, among all the composites, 20 wt% show least value of dielectric constant. The decrease in dielectric constant with applied frequency for all the samples can be attributed due to the interfacial and electrode polarization effects at the grain boundaries.
Figure 8 shows the variation of imaginary part of dielectric constant with frequency for PEDOT-PSS and DMSO doped PEDOT-PSS films with different concentration of DMSO. Imaginary dielectric constant shows dielectric losses characterized by relaxation frequency for all the samples. The imaginary dielectric constant gradually decreases with frequency and suddenly drops at a frequency of 106 Hz. The difference in the dielectric relaxation time with changing concentration is due to the electric charge displaced inside the polymer matrix.
Figure 9 shows the variation of dielectric tangent loss with frequency for PEDOT-PSS and DMSO doped PEDOT-PSS with different concentrations of DMSO at room temperature. The tangent loss of pure PEDOT-PSS and doped PEDOT-PSS gradually decreases with the increasing frequency, this gradual decrease of tangent loss in composites shows the non-existence of dipolar relaxations. Among the prepared composites 20 wt% shows more tangent loss, as the weight percentage increase in PEDOT-PSS composites the tangent loss also increases.
The variation of real part of electric modulus (M’) as a function of frequency for pristine PEDOT-PSS and DMSO doped PEDOT-PSS thin films is shown in Fig. 10. The real part of electric modulus increases with increase in frequency up to 106 Hz due to the formation of auxiliary magnetic field around the DMSO domain. In comparison to other compositions, pure PEDOT-PSS shows the lowest electric modulus due to small dielectric loss and lower bulk resistance as compared to other composites.
Figure 11 shows the variation of quality factor (Q) as a function of frequency for pure and doped PEDOT-PSS films with varying concentration of DMSO. It is observed that Q value changes with applied frequency as well as with the increasing dopant concentrations. The quality factor value for all the samples is observed to be almost constant up to frequency of 103 Hz. However, after 103 Hz frequency, it gradually increases and reaches maximum value between 105 and 106 Hz. All composites exhibit a smaller value of due to over damping which reflects the charge carriers does not oscillate at all. Among all prepared composites 20 wt% shows high Q value followed by other composites and pure PEDOT-PSS.
The cole–cole plots (Fig. 12) indicate a decrease in electrical resistance with increasing dopant concentration as well as with the applied frequency. It is observed that the impedance value decreases with increasing frequency as well as the variation of impedance depend on the dopant concentration in PEDOT-PSS doped DMSO composites. The response of real and imaginary parts of impedance can be associated with three different mechanisms that occur in multiphase materials, association of charges, dielectric dispersion of different dipole rotation and the presence of both dielectric dispersion as well as charge carrier union with in measured frequency range. From the cole–cole plots is observed that the effective resistance for doped PEDOT-PSS decreases in comparison to pure PEDOT-PSS and the composite with 20 wt% concentration of DMSO in PEDOT-PSS shows lowest resistance. The impedance value depends on grain and bulk resistance in complex LCR circuit as shown in inset figure. The addition of DMSO in PEDOT-PSS matrix decreases grain and bulk resistance of the polymer matrix in complex LCR circuit. It can also be ascertained to the fact that, series resistance and geometrical capacitance decrease results in increase of bulk conductivity of PEDOT-PSS doped DMSO thin films. The formation of maxima and minima in the cole–cole plots for all the samples indicates the clear distribution of relaxation time which is mainly due to the hopping and tunneling effects of charge carriers at various frequencies.
5 Conclusion
The organic thin films of PEDOT-PSS were prepared by secondary doping of DMSO with varying concentrations of DMSO in PEDOT-PSS. The films were characterized by SEM, FTIR and UV–Vis spectroscopy. The effect of DMSO addition on the physio-chemical features of PEDOT-PSS films were understood through these characterization techniques. The temperature and frequency dependent conductivity studies reveal the fact that the addition of DMSO into PEDOT-PSS matrix significantly enhances the conductivity of pristine PEDOT-PSS. The DMSO addition acts as a bond between conducting PEDOT phase and neutral PSS phases (in PEDOT-PSS) and reduces the phase segregation. Hence the rate of charge carrier transfer between favorable sites increases, leading to change in activation energies that enhances conductivities in doped PEDOT-PSS. Among all the composite films prepared, the sample with 20 wt% concentration of DMSO in PEDOT-PSS shows the maximum conductivity. The detailed studies on dielectric and impedance response of pristine PEDOT-PSS and DMSO doped PEDOT-PSS reveals the fact that, the addition of DMSO in PEDOT-PSS matrix has a strong influence on material properties. Through this study we have made an attempt to understand the role of DMSO doping on structure and properties of pristine PEDOT-PSS films. Due to ease of fabrication, transparency, flexibility and high values of conductivity these DMSO doped PEDOT-PSS organic thin films may be of great scientific importance especially in the fabrication of optoelectronic devices.
References
G. Heywang, F. Jonas, Adv. Mater. 4, 116–118 (1992)
B.L. Geoenendal, F. Jonas, D. Freitag, H. Pielartzik, J.R. Reynolds, Adv. Mater. 12, 481–494 (2000)
M. Ruppert, U. Ziener, K. Landfester, Colloid Polym. Sci 289, 1321–1328 (2011)
W.J. Hong, Y.X. Xu, G.W. Lu, C. Li, G.Q. Shi, Electrochem. Commun. 10, 1555–1558 (2008)
Y. Xia, K. Sun, J. Ouyang, Adv. Mater. 24, 2436–2440 (2012)
J.A. Arter, D.K. Taggart, T.M. McIntire, R.M. Penner, G.A. Weiss, Nano Lett. 10, 4858–4862 (2010)
S.M. Richardson-Burns, J.L. Hendricks, D.C. Martin, J. Neural Eng. 4, L6–L13 (2007)
O. Inganäs, Nat. Photonics 5, 201–208 (2011)
A. Chipman, Nature 449, 131–136 (2007)
A.M. Nardes, M. Kemerink, R.A.J. Janssen, J.A.M. Bastiaansen, N.M.M. Kiggen, B.M.W. Langeveld, A.J.J.M. van Breemen, M.M. de Kok, Adv. Mater. 19, 1196–1200 (2007)
P.K. Choudhury, D. Bagchi, C.S.S. Sangeeth, R. Menon, J. Mater. Chem. 21, 1607 (2011)
C.S.S. Sangeeth, M. Jaiswal, R. Menon, J. Appl. Phys. 105, 063713 (2009)
C. Winder, N.S. Sariciftci, J. Mater. Chem. 14, 1077–1086 (2004)
Y. Du, S.Z. Shen, K.F. Cai, P.S. Casey, Prog. Polym. Sci. 37, 820–841 (2012)
R.M. Howden, E.D. McVay, K.K. Gleason, J. Mater. Chem. A 1, 1334–1338 (2013)
F.L. Xue, Y. Su, K. Varahramyan, IEEE Trans. Electron Devices 52, 1982–1987 (2005)
J.A. Lim, J.H. Cho, Y.D. Park, D.H. Kim, M. Hwang, K. Cho, Appl. Phys. Lett. 88, 082102 (2006)
F.X. Jiang, J.K. Xu, B.Y. Lu, Y. Xie, R.J. Huang, L.F. Li, Chin. Phys. Lett. 25, 2202–2205 (2008)
C.S.S. Sangeeth, M. Jaiswal, R. Menon, J. Phys. Condens. Matter 21, 072–101 (2009)
C. Gravalidis, A. Laskarakis, S. Logothetidis, Eur. Phys. J. Appl. Phys. 46, 12505 (2009)
Y. Zhou, H. Cheun, S. Choi, J.W.J. Potscavage, C. Fuentes-Hernandez, B. Kippelen, Appl. Phys. Lett. 97, 153304 (2010)
M. Reyes-Reyes, I. Cruz-Cruz, R. Lopez-Sandoval, J. Phys. Chem. C 114, 20220–20224 (2010)
C. Badre, L. Marquant, A.M. Alsayed, L.A. Hough, Adv. Funct. Mater. 22, 2723–2727 (2012)
Y.J. Xia, H.M. Zhang, J.Y. Ouyang, J. Mater. Chem. 20, 9740–9747 (2010)
Y.J. Xia, J.Y. Ouyang, Org. Electron. 11, 1129–1135 (2010)
Y.J. Xia, J.Y. Ouyang, ACS Appl. Mater. Interfaces 2, 474–483 (2010)
Y.H. Kim, C. Sachse, M.L. Machala, C. May, L. Müller-Meskamp, K. Leo, Adv. Funct. Mater. 21, 1076 (2011)
Y. Xia, K. Sun, J. Ouyang, Energy Environ. Sci. 5, 5325 (2012)
D. Alemu, H.Y. Wei, K.C. Ho, C.W. Chu, Energy Environ. Sci. 5, 9662 (2012)
D.J. Lipomi, J.A. Lee, M. Vosgueritchian, B.C.K. Tee, J.A. Bolander, Z. Bao, Chem. Mater. 24, 373 (2012)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pasha, A., Roy, A.S., Murugendrappa, M.V. et al. Conductivity and dielectric properties of PEDOT-PSS doped DMSO nano composite thin films. J Mater Sci: Mater Electron 27, 8332–8339 (2016). https://doi.org/10.1007/s10854-016-4842-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-4842-5