Abstract
In this work, the dielectric and magnetic properties of (Co, Ni) co-doped SnO2 nanoparticles were studied using ac impedance spectroscopy and magnetic properties measurement system or quantum design superconducting quantum interference device. Results showed that dielectric constant (ε r ), dielectric loss (ε″), and ac electrical conductivity (σ AC ) are strongly frequency dependent. A decrease in frequency was accompanied with an increase in ε r and ε″ values, whereas, a decrease in the dielectric constant was observed with the increase of Ni co-doping concentration. It was found that the dielectric constant and dielectric loss values decrease, whilst AC electrical conductivity increases with increase in co-doping concentration. Magnetization measurements revealed that the Ni co-doped SnO2 samples exhibits room temperature ferromagnetism. The results illustrate that (Co, Ni) co-doped SnO2 nanoparticles have an excellent dielectric, magnetic properties, and high electrical conductivity than those of co-doped samples reported previously, indicating that these (Co, Ni) co-doped SnO2 materials can be suitable for the purpose of high frequency device and spintronic applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Transition metal (TM) doped with SnO2 has been proposed as one of the most promising candidates for dilute magnetic semiconductor (DMS) oxide materials with Curie temperature (T c ) above room temperature, 300 K [1, 2]. It is believed that the simultaneous presence of two kinds of dopants [3], especially multi-valence ions [4], could tailor the occupancy and position of the Fermi energy of the oxide materials and its magnetic response. Thus, co-doping SnO2 simultaneously with two TM ions might be a promising route to achieve and enhance room temperature ferromagnetism (RTFM). Some previous works on SnO2 prepared with co-doped TM exhibited the expected increase in ferromagnetism (FM) [5–7], while other studies have showed reduction of ferromagnetic (FM) behavior [8, 9]. Thus, it is evident that the actual role and effect of co-doped TM ions in SnO2 are not conclusive and in fact very conflicting. Therefore, more studies are required in order to improve our comprehension on the origin of the magnetism in these interesting oxide nanocompounds. However, recent experimental reports indicate ferromagnetism in n-type semiconductor oxides such as Ti1−x Co x O2 [10] and Sn1−x Co x O2 [8]. It was investigated that Co doped SnO2 thin films prepared by pulsed-laser deposition method exhibited strong ferromagnetic behavior at room temperature with a giant magnetic moment of 7.5 ± 0.5 µ B /Co [8]. In their research, Aragon et al. [11].observed RTFM in Sn1−x Ni x O2 nanoparticles prepared by a polymer precursor with Ni content x = 0.03, but with a low coercive field (Hc) of 40 Oe and saturation magnetization (Ms) of ∼1.2 × 10−3 emu/g. It is reported that the co-doping of Fe and Co into rutile-type TiO2 enhances the saturation magnetization compared with that of the single doping situation [12]. In previous report, saturation magnetization was enhanced by doping Fe-doped SnO2 with Sb5+ [13], which suggests that the increase in the number of electron carriers by co-doping induces ferromagnetism. Thus, it is evident that the actual role and effect of co-doped TM ions in SnO2 are not clear and in fact very conflicting. Therefore, more studies are required in order to improve our comprehension on the origin of the magnetism in these interesting compounds. For these reasons, we are interested in the co-doping effect to diluted ferromagnetism, dielectric and electric response in co-doped SnO2, and we report the ferromagnetic behavior of dilute Co and Ni co-doped SnO2 nanoparticles.
2 Experimental detail
The (Co, Ni) co-doped SnO2 nanoparticle samples with Ni contents of 1, 2, 3, 4 and 5 %, and Co content (fixed) of 5 % were synthesized by a chemical co-precipitation method. First 2.5 g SnCl2·2H2O (0.22 M) was dissolved in 50-ml distilled water and then 30-ml aqueous ammonia solution (2 M) was added to the pervious solution with constant stirring at a pH value between 1 to 10 [14]. The resulting white precipitates were collected by centrifugation and then washed in distilled water. The washed precipitate was dried at 60 °C for 24 h in a box furnace. Finally, the synthesized SnO2 nanopowder was annealed at 600 °C for 2 h. In order to get (Co, Ni) co-doped SnO2 samples, CoCl2 (0.05 M) and NiCl2·6H2O were added at various concentration (x = 0.01 M, 0.02 M, 0.03 M, 0.04 M and 0.05 M, respectively) to the SnCl2·2H2O solution, and allowed to react. The samples were characterized by X-ray diffractometer (PAN analytical) using CuK α radiation. The GSAS suite of Rietveld programs were used to find the lattice parameters. Energy Dispersive X-ray Spectrometer (EDXS) was used to determine the crystal composition, and stoichiometric (Co, Ni) co-doped SnO2 was confirmed. The dielectric properties and conductivity as a function of frequency were measured in centered Gold electrode pellet with a typical Impedance analyzer (Agilent 4292) in the frequency range of 40 Hz–15 MHz. DC magnetization measurements were carried out using a quantum design superconducting quantum interference device (SQUID) in the temperature range of 2–300 K.
3 Results and discussion
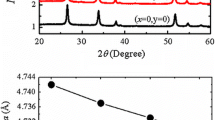
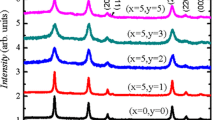
Figure 1a shows the room temperature X-ray diffraction (XRD) of (Co, Ni) co-doped SnO2 nanoparticle samples with Ni of 1, 2, 3, 4 and 5 % concentrations, and Co (fixed) 5 %. These patterns have been compared with standard XRD data. All the XRD peaks can be indexed to a tetragonal structure (space group P42/mnm) of SnO2. Figure 1a reveals that, there is no change in the tetragonal structure of SnO2 after (Co, Ni) co-doping. No extra peaks were found in the XRD pattern indicating the formation of single phase in Sn0.95−xCo0.05NixO2. A small shift in the position of main peak (110) to the higher side of 2θ value is observed for doped samples as shown in Fig. 1b. This observation is similar to the case in transition metal (Mn, Cu, and Ni) doped SnO2 previously studied [15–17]. This shift is mainly due to the smaller radius of Co2+ and Ni2+ compared to that of Sn4+. This result indicates that the doped Co2+ and Ni2+ atoms successfully substitute the Sn4+ atoms. The particle sizes of the samples have been calculated using Scherrer’s formula and are found to be in the range of 11–14 nm. Since the difference in radii between Sn4+ (0.069 nm) and Co2+ (0.060 nm), Ni2+ (0.062 nm) is considerable, significant changes in the lattice constants are expected for Ni co-doped samples. The calculated lattice parameters a = b and c are compared with standard values. With the increase of Ni concentration, the lattice parameters a, b and c are found to decrease. For example (from Fig. 1c, d) the lattice parameters decrease from a = 4.7360(6) Å and c = 3.1925(9) Å, for Ni content = 0 %, to a = 4.7166(4) Å and c = 3.1663(5) Å respectively, for Ni doping level of 5 %.The self-generated elemental composition (wt%) details are presented in Fig. 2. It is clear from the EDAX spectrum that Sn and O are the only main elemental species in pure SnO2 sample whilst, in addition, Co and Ni peaks were observed in (Co, Ni) SnO2 doped samples. Moreover, the weight percentages of the doped transition metal elements were found close to the nominal concentration used during the calculations and sample preparation.
Figure 3 shows the measurements of dielectric constant as a function of frequency at room temperature for the undoped and (Co, Ni) co-doped SnO2 (Ni = 1, 2, 3, 4 and 5 %) samples. In order to measure the capacitance, dielectric loss and AC conductivity as a function of frequency at room temperature, we used the circular shaped pellets with 9 min diameter prepared by pressing the powder. From the measured capacitance, the ε r can be calculated by the following mathematical equation:
where C is the capacitance (F), ε 0 is the free space dielectric constant value (8.854 × 10−12 F/m), d is the height of the cylinder, A is the capacitor area (m2) and ε r is the relative dielectric constant of the samples. It is obvious that ε r decreases with increase in frequency and Ni doping in our system.
The decrease of dielectric constant with increase in frequency is a normal behavior for oxide nano-materials [6, 18]. This increase in ε r at low frequencies can be attributed to both the deformational (electronic and ionic) and relaxation (orientational and space charge) polarization [6, 19]. The deformational polarization depends on the displacement of electrons and ions but the relaxation polarization depends on the orientational or interfacial effects. The increase in applied field leads to a reduction in orientational polarization, as a result, the molecular dipoles require much time to change their orientation with applied field. This effect reduces the value of ε r with increase in frequency. The decrease in ε r with Ni co-doping is due to more and more defects (positive, negative or neutral) or oxygen vacancies which are randomly created (at the surface) resulting in disorder in the system. As a result of their recombination, the number of dipole decreases which leads to a decrease in the orientational polarization [20]. The decrease in orientational polarization results in the decrease in ε r .
Figure 4 shows the frequency dependence of the imaginary part (ε″) of dielectric constant, i.e., the dielectric loss, at room temperature for (Co, Ni) co-doped SnO2 (Ni = 1, 2, 3, 4 and 5 %) nanoparticles. It can be observed from the figure that ε″ decreases with increasing frequency and Ni doping. For all samples, same behavior (dispersion at low frequency and frequency independent nature at high frequency) is observed. The reduction of ε″ at low frequencies is due to the migration of ions in the material. Similarly the dielectric loss at low and intermediate frequencies is due to the ionic hopping, conduction losses caused by ion migration. In addition ionic polarization losses can also be a vital factor for this behavior. However, at high frequencies, ion vibrations may be the only reason of ε″ for the current nanoparticles.
Figure 5 shows the frequency dependency of AC electrical conductivity, σ AC of (Co, Ni) co-doped SnO2 nanoparticles. As shown, AC conductivity (σac) increases with increase in frequency. It is clear from the equation σ AC = ε 0 ε″ω (where σ AC is the AC electrical conductivity, and ε 0 is the dielectric constant of the free space, ε″ is the imaginary part of dielectric constant and ω = 2πf, f is the frequency) depends upon the dielectric loss. Consequently, dielectric loss decreases with increase in frequency and, in turn, σ AC also increases. This result also corroborates with literature, where it is suggested that the increase in σ AC with increase in frequency is attributed to the series resistance effect [21, 22].
Two possible mechanisms can attribute to the increase in σ; one is the electric energy associated with the high AC frequency which can effectively promote the jumping of electric charge between the nano-size particles and the other is due to the enhanced dielectric relaxation of the polarization of SnO2 nanoparticles in high frequency region [23].
Figure 6 shows the magnetization, M of the Sn0.95−xNixCo0.05O2 (x = 1, 2, 3 and 5 %) samples measured as a function of temperature T at a constant field H = 1000 Oe. The ferromagnetic phase transition is characterized by a sudden increase in M at temperatures above 450 K and then tends to saturate at low temperatures. This is a typical process of the ferromagnetic phase transition. The magnetization of the samples clearly show that a transition from paramagnetic to ferromagnetic state occurs at temperatures near 434, 602, 531 and 482 K for the Ni contents of 1, 2, 3, and 5 %. The transition temperatures of these samples increase sharply below Curie temperature (Tc). It is obvious that the Tc of the samples increases with increase in Ni co-doping content up to 2 % and afterward the Tc decreased. These Tcs are high enough for the purpose of spintronic applications at room temperature.
Figure 7a shows the field dependence of the magnetization, i.e., M(H) curves at room temperature for the (Co, Ni) co-doped SnO2 (Ni = 0,1, 2, 3, 4 and 5 %) samples. The pure SnO2 sample exhibit a diamagnetism behavior at room temperature (here its data is not shown). The various Ni co-doped Sn0.95Co0.05O2 samples, the hysteresis observed in their M(H) curves indicate a ferromagnetic order emerging in the samples. The saturation magnetization (Mr) reach 0.101 memu/g for the Sn0.95Co0.05O2 sample, and reach a maximum for the 2 % Ni co-doping sample then decreases in the 3–5 % Nico-doping sample. Figure 7(b) shows the enlarged hysteresis loop near zero magnetic field, from which a coercive field (Hc) of 35 Oe and a remanent magnetization (Mr) of 0.11 memu/g for a 1 % Ni doping sample are obtained. The highest remanent magnetization (Mr) of 0.304 memu/g with coercive field (Hc) of 74 Oe was observed in the 2 % Ni co-doped SnO2 sample. The observed remanent magnetization (Mr) (in 2 % Ni co-doping) is higher than that reported in Ref. [24]. Furthermore, the lowest Mr of 0.036 memu/g with coercive field (Hc) of 28 Oe was observed for the 5 % Ni doped sample. A ferromagnetic response at low Co co-doping concentration was also reported in (Fe, Co) co-doped SnO2 by Nomura et al. [25].
In our system, ferromagnetic behavior was observed even at high Ni co-doping, but the remanent magnetization decreased at higher Ni co-doping (5 %). In addition, the creation of addition charge carriers and oxygen vacancies due to (Co, Ni) co-doping may cause RTFM [26]. However, higher Ni doping may lead to the expansion of SnO2 lattice and the structure disorder, suppressing its ferromagnetism. The simultaneous presence of Co and Ni greatly improves the ferromagnetic signal of SnO2 nanoparticles in comparison to situations when there is the presence of only one of these cations. Simultaneous doping of two ions increased the coercivity and saturation magnetization, and also eliminated the formation of impurity phases. The presence of Ni2+ ions in the co-doped samples in the ferromagnetic state with indication of magnetic coupling between them suggests that the ferromagnetism in this material is related to the magnetic moment of the dopant ions. The presence of both Co2+ and Ni2+ ions in the (Co, Ni) co-doped samples provide a powerful charge reservoir to facilitate stronger ferromagnetism in these samples. More studies to investigate possible charge transfer between these 3d ions and the presence of other valence states of Co and Ni are necessary to fully confirm the role of charge transfer ferromagnetism in these samples and this will be investigated in future.
4 Conclusions
The study on the effect of (Co, Ni) co-doping on SnO2 nanoparticles’ electrical, dielectric, and magnetic properties were carried out. All the (Co, Ni) co-doping samples have a tetragonal structure. It is found that with the increase of Ni doping concentration the dielectric constant and dielectric loss values decrease, but electrical conductivity increases. With the increase of frequency and Ni content, the dielectric constant and dielectric loss decreases, while electrical conductivity increases. The results indicate that the limited Ni co-doping can introduce the ferromagnetism in the SnO2 nanoparticles which are higher than those for co-doped samples previously reported.
References
G.A. Prinz, Magnetoelectronics. Science 282, 1660 (1998)
W. Prellier, A. Fouchet, B. Mercey, Oxide-diluted magnetic semiconductors: a review of the experimental status. J. Phys. Condens. Matter. 15, R1583 (2003)
N.N. Lathiotakis, A.N. Andriotis, M. Menon, Codoping: a possible pathway for inducing ferromagnetism in ZnO. Phys. Rev. B 78, 193311 (2008)
J.M.D. Coey, K. Wongsaprom, J. Alaria, M. Venkatesan, Charge-transfer ferromagnetism in oxide nanoparticles. J. Phys. D Appl. Phys. 41, 134012 (2008)
R. Khan, S. Fashu, Y. Zaman, Magnetic and dielectric properties of (Co, Zn) co-doped SnO2 diluted magnetic semiconducting nanoparticles. J. Mater. Sci. Mater. Electron. (2016). doi:10.1007/s10854-016-4517-2
R. Khan, Zulfiqar, Y. Zaman, Effect of annealing on structural, dielectric, transport and magnetic properties of (Zn, Co) co-doped SnO2 nanoparticles. J. Mater. Sci. Mater. Electron. 27, 4003–4010 (2016)
K. Nomura, J. Okabayashi, K. Okamura, Y. Yamada, Magnetic properties of Fe and Co codoped SnO2 prepared by sol–gel method. J. Appl. Phys. 110, 083901 (2011)
S.B. Ogale, R.J. Choudhary, J.P. Buban, S.E. Lofland, S.R. Shinde, S.N. Kale, V.N. Kulkarni, J. Higgins, C. Lanci, J.R. Simpson, N.D. Browning, S. Das Sarma, H.D. Drew, R.L. Greene, T. Venkatesan, High temperature ferromagnetism with a giant magnetic moment in transparent Co-doped SnO2−δ . Phys. Rev. Lett. 91, 077205 (2003)
J.M.D. Coey, A.P. Douvalis, C.B. Fitzgerald, M. Venkatesan, Ferromagnetism in Fe-doped SnO2 thin films. Appl. Phys. Lett. 84, 1332 (2004)
J.D. Bryan, S.M. Heald, S.A. Chambers, D.R. Gamelin, Strong room-temperature ferromagnetism in Co2+-doped TiO2 made from colloidal nanocrystals. J. Am. Chem. Soc. 126, 11640 (2004)
F.H. Aragón, J.A.H. Coaquira, P. Hidalgo, S.L.M. Brito, D. Gouvea, R.H.R. Castro, Structural and magnetic properties of pure and nickel doped SnO2 nanoparticles. J. Phys. Condens. Matter. 22, 496003 (2010)
D.H. Kim, S.I. Woo, S.H. Moon, H.D. Kim, B.Y. Kim, J.H. Cho, Y.G. Joh, E.C. Kim, Effect of Co/Fe co-doping in TiO2 rutile prepared by solid state reaction. Solid State Commun. 136, 554 (2005)
K. Nomura, C.A. Barrero, K. Kuwano, Y. Yamada, T. Saito, E. Kuzmann, 57Fe Mössbauer study of sol–gel synthesized Sn1−x−yFexSbyO2−δ powders. Hyperfine Interact. 191, 25 (2009)
R. Adnan, N.A. Razana, I.A. Rahman, M.A. Farrukh, Synthesis and characterization of high surface area tin oxide nanoparticles via the sol–gel method as a catalyst for the hydrogenation of styrene. J. Chin. Chem. Soc. 57, 222 (2010)
Y.H. Xiao, S.H. Ge, L. Xi, Y. Zuo, X.Y. Zhou, L. Zhang, G. Wang, X.F. Han, Z.C. Wen, Structure, optical, and magnetic properties of rutile Sn1−x Mn x O2 thin films. Appl. Surf. Sci. 255, 7981 (2009)
P.I. Archer, P.V. Radovanovic, S.M. Heald, D.R. Gamelin, Low-temperature activation and deactivation of high-Curie-temperature ferromagnetism in a new diluted magnetic semiconductor: Ni2+-doped SnO2. J. Am. Chem. Soc. 127, 14479 (2005)
Y.F. Li, R. Deng, Y.F. Tian, B. Yao, T. Wu, Role of donor–acceptor complexes and impurity band in stabilizing ferromagnetic order in Cu-doped SnO2 thin films. Appl. Phys. Lett. 100, 172402 (2012)
X.S. Fang, C.H. Ye, L.D. Zhang, T. Xie, Twinning mediated growth of Al2O3 nanobelts and their enhanced dielectric responses. Adv. Mater. 17, 1661–1665 (2005)
J.G. Han, Z.Y. Zhu, S. Ray, A.K. Azad, W.L. Zhang, M.X. He, S.H. Li, Y.P. Zhao, Optical and dielectric properties of ZnO tetrapod structures at terahertz frequencies. Appl. Phys. Lett. 89, 031107 (2006)
F. Gu, S.F. Wang, M.K. Lü, G.J. Zhou, D. Xu, D.R. Yuan, Photoluminescence properties of SnO2 nanoparticles synthesized by sol–gel method. J. Phys. Chem. B 108, 8119–8123 (2004)
P.S. Szu, Y.C. Lin, AC impedance studies of copper doped silica glass. Phys. Chem. Mater. 82, 295–300 (2003)
O. Pakma, N. Serinl, T. Serin, S. Altında, Influence of frequency and bias voltage on dielectric properties and electrical conductivity of Al/TiO2/p-Si/p+ (MOS) structures. J. Phys. D Appl. Phys. 41, 215103 (2008)
C.H. Ho, C.D. Liu, C.H. Hsieh, K.H. Hsieh, S.N. Lee, High dielectric constant polyaniline/poly(acrylic acid) composites prepared by in situ polymerization. Synth. Metals 158, 630–637 (2008)
H. Zhang, D. Wang, V. Hu, X. Kang, H. Liu, Synthesis and magnetic properties of Sn1−x Co x O2 nanostructures and their application in gas sensing. Sens. Actuators B 184, 288–294 (2013)
K. Nomura, J. Okabayashi, K. Okamura, Y. Yamada, Magnetic properties of Fe and Co codoped SnO2 prepared by sol–gel method. J. Appl. Phys. 110, 083901 (2011)
J. Hays, A. Punnoose, R. Baldner, M.H. Engelhard, J. Peloquin, K.M. Reddy, Relationship between the structural and magnetic properties of Co-doped SnO2 nanoparticles. Phys. Rev. B 72, 075203 (2005)
Acknowledgments
Rajwali Khan and Zulfiqar would like thankful to the Department of Physics, Islamia College Peshawar China; Abdul Wali Khan University, Mardan, Pakistan, and Harare Institute of Technology, Harare, Zimbabwe for providing some experimental facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khan, R., Zulfiqar, Fashu, S. et al. Effects of Ni co-doping concentrations on dielectric and magnetic properties of (Co, Ni) co-doped SnO2 nanoparticles. J Mater Sci: Mater Electron 27, 7725–7730 (2016). https://doi.org/10.1007/s10854-016-4759-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-4759-z