Abstract
In this present study, we have reported the synthesis of ZnMn2O4, transitional metal oxide by sol–gel method. The structural and morphological properties are confirmed using various characterization techniques namely XRD, FT-IR and SEM with EDX. Dielectric studies of ZnMn2O4 are measured at the frequency varying from 50 Hz to 5 MHz for the temperature range of 303–573 K. The temperature dependent electrical parameters like impedance and modulus exhibit a strong correlation with the grains, grain boundaries and space charge effects in the synthesized material. Diffusion of oxygen vacancies in the dipoles and defects in the material due to oxygen vacancy complexes are investigated by the activation energy obtained from Arrhenius plot. It was found that the relaxation process was dominated by the hopping mechanism between the Mn3+ and Mn4+. Nyquist plot of impedance was attributed to the existence of space charge interface, grain boundary and grain conduction mechanism of the material.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nanocrystalline ZnMn2O4 is an important class of mixed transitional metal oxide with the general formula AB2O4. The physical and chemical properties of the transitional metal oxides exhibit a variety of interesting facts due to the accommodation of different cations with one or more oxidation states [1, 2]. Due to this, ZnMn2O4 have attracted a great attention to their electronic, optoelectronic, mechanical, electrochemical and semiconducting properties resulting in the prospective applications owing to the field of sensors, specific memory devices, NTC thermistor, lithium ion batteries as an anode and so on [2–5]. Spinel ZnMn2O4 with its unique electrical property accounts to potential application as negative temperature co-efficient (NTC) thermistor. In general, it has been investigated as a technologically significant functional material due to its semiconducting nature and its applications in the microelectronics as temperature sensors, power sensing terminators, fire detectors for practical devices [6–8]. However in ZnMn2O4, Zn-site exhibit single oxidation state of Zn2+ whereas Mn-site exhibit Mn3+ and Mn4+, which attributes to the formation of oxygen vacancy in the material with the variation of temperature and the relaxation process is related to the oxygen vacancy. Depending upon the temperature variation relaxation process exhibits single-ionized, doubly ionized, diffusion of oxygen in complexes, defects formed of oxygen and cation vacancy in the material [9, 10].
Zinc magnetite (ZnMn2O4) has been synthesized by various methods namely solid state reaction [11] hydrothermal method [2, 3], sol–gel method [1], spin coating [12]. To obtain a homogenous chemical composition, high Crystallinity, uniform particle size and morphology, sol–gel method using microwave assisted sintering technique was used in order to reduce the agglomeration of the material.
In this study, ZnMn2O4 is synthesized by sol–gel method via glycine as a chelating agent. The properties of the materials are strongly affected by the particle size and morphology. Hence, synthesis of the material in nano range has attracted the researchers deliberately [3, 7]. From literature, spinel ZnMn2O4 functional material is analysed for its thermal properties, magnetic properties, optical studies, photoelectrical properties, electrochemical studies for various applications [2–5, 13]. However until date, there were no reports to the best of author’s knowledge to understand in-depth analysis of dielectric, impedance and electric modulus on ZnMn2O4. The electrical properties for the synthesized ZnMn2O4 as a function of frequency varying from 50 Hz to 5 MHz at different temperatures have been measured. In particular, the contribution of grain, grain boundary and interfacial effects at different temperatures and relaxation process are analyzed. The formation of thermal motion of ions due to the oxygen vacancy in the material corresponds to the hopping mechanism in the Mn-site, i.e., Mn3+ and Mn4+ during temperature variation results in the dielectric relaxations in the material.
2 Experimental
ZnMn2O4 microspheres were synthesized by sol–gel method using glycine as a chelating agent. The stoichiometric ratio of zinc acetate dihydrate [(CH3COO)2Zn·2H2O], and manganese acetate tetrahydrate [(CH3COO)2Mn·4H2O] dissolved in the deionized water and glycine solution was added slowly drop by drop to it, which acts as a chelating agent. All reagents used were of analytical grade and used without further purification. The solution was stirred continuously for 3 h at room temperature. Further, the homogenous solution was heated to 80 °C with stirring to ensure homogeneity until the gel was formed. The obtained gel was dried overnight in a vacuum oven at 120 °C. The gel precursors were decomposed at 300 °C for 5 h to eliminate the organic constituents present. Finally, the decomposed powder was slightly ground and sintered in the microwave furnace at 850 °C for 3 h. Again the obtained ZnMn2O4 was ground into fine powder before subjecting it to further analysis.
The phase formation of the synthesized material were investigated by X-ray diffraction (XRD, Brucker D8 Advance, Cu Kα radiation, λ = 1.5406 Å) with a range of 2θ from 10° to 80° with a step size of 0.02 s−1 at a scan rate of 2 min−1. The Fourier transform infrared spectroscopy (FT-IR) spectra obtained by JASCO 400 Infrared spectrometer from 4000 to 400 cm−1. The surface morphology and compositions of the samples investigated by scanning electron microscopy (Carl Zeiss EVO-185H, UK) equipped with an energy-dispersive X-ray spectrometer (EDX). The synthesized ZnMn2O4 powder converted to cylindrical pellets for electrical measurements by coating with the silver paste for ohmic contact. The dielectric measurements were carried out by an LCR meter (HIOKI 3532-50LCR meter HITESTER) in the frequency range from 50 Hz to 5 MHz for the temperature ranging from 303 to 573 K.
3 Results and discussion
3.1 Structural and morphological analysis
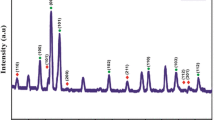
Figure 1 illustrates the XRD pattern for the synthesized ZnMn2O4 which attributes to the phase formation and crystal structure. It is observed from the XRD pattern that all the diffraction peaks clearly corresponds to the standard JCPDS 77-0470 with space group I41/amd and hkl values indexed accordingly. XRD peaks were sharp and very intense, indicating the good crystalline nature of the material without any secondary phase formation. The unit cell parameters calculated from PowderX software [14] were a = b = 5.71749 Å and c = 9.23527 Å.
The average particle size of the synthesized ZnMn2O4 was estimated using the FWHM values of the major peaks in the XRD pattern from Scherrer’s formula [15].
where D is crystallite size, k = 0.9 is the particle shape factor, λ = 1.5405 Å is the X-ray wavelength used, β is the full-width half maximum of the major XRD peaks, and θ is the Bragg’s angle. The average crystallite size was found to be around 55–95 nm.
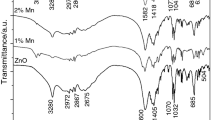
Figure 2 illustrates the FT-IR spectroscopy recorded in the range of 400–4000 cm−1. It observed strong absorption peak between 500 and 700 cm−1 region which corresponds to the formation of metal–oxygen bonds of tetrahedral and octahedral sites i.e. Zn–O and Mn–O bonds. These bands confirm the formation of spinel compounds for the synthesized material [2, 4].
The surface morphology of ZnMn2O4 SEM images are shown in the Fig. 3. SEM images in Fig. 3a–c observes the agglomerated spherical particles. It is evident that the particles are distributed uniformly in size. The microstructure of the sample showed well-defined grains and grain boundaries in the SEM images. The elemental compositions of the sample are obtained from EDX spectrum as shown in Fig. 3d. The EDX spectrum shows the elemental mapping along with the percentage of composition of each element as shown in the inset of Fig. 3d. It confirms the absence of any impurity in the synthesized sample indicating the formation of exact composition.
3.2 Dielectric studies
The electric parameters such as real part of dielectric constant (ε′), the imaginary part of dielectric constant (ε″) and ac-conductivity were calculated using the equation below.
and
where C is capacitance in farad, d is the thickness of the pellet in mm, A is cross-sectional area of the pellet in m2, ε0 is constant of permittivity in free space, ω = 2πf is the angular frequency, and tan δ is dielectric loss [16]. Figure 4a, b shows the variation in the real (ε′) and imaginary (ε″) part of dielectric constant obtained using the Eqs. (2) and (4). These values decrease gradually with increase in the frequency at low-frequency region whereas it is almost independent of the frequency in the high-frequency region. This type of behaviour is explained by Maxwell–Wagner model [17]. According to this model, highly conducting grains are separated by low conducting grain boundary at lower frequencies [16, 18]. At higher frequencies, piling up of charges at grain boundaries increases resulting in the space charge polarization effect in the material. This pattern of variation in the dielectric constant with frequency is due to the grain boundary, defects, hopping of electrons between Mn3+ and Mn4+, oxygen vacancy, space charge polarization effect, voids, etc. At very low frequency, the higher value of dielectric constant with the increase in temperature observed due to the thermal activation of the charges in ZnMn2O4.
Figure 5 shows the frequency dependent dielectric loss tangent (tan δ) for different temperatures from 303 to 373 K. As the frequency increases dielectric loss tends to become almost zero and is independent of frequency. At lower frequency region, the high value of the dielectric loss is due to the low conducting phase of grain boundaries which signifies more energy required for electron exchange between the Mn3+ and Mn4+ ions indicating maximum energy loss. The low value of the dielectric loss observed with the increase in frequency. As a result, a small amount of energy is sufficient for electron exchange. As temperature increases, the high value of the dielectric loss is seen at the lower frequency which is due to thermally activated charges and accumulation of charge carriers at the interfaces [19, 20].
3.3 Impedance studies
The frequency and temperature dependent real (Z′) and imaginary (Z″) part of impedance is calculated using the equation given below.
where G is the conductance (S), Cp is the static capacitance in parallel equivalent circuit mode (F) of the material.
Variation in the real part of impedance (Z′) with frequency for different temperature is shown in Fig. 6. It observed that the real part (Z′) of impedance remains constant at lower frequency region, and merging of Z′ occurs for various temperatures with the increase in frequency that is due to the space charge effects. It was also observed that the real part of impedance Z′ decreases with increase in temperature denoting the lower density of trapped charges and enhancement of the immobile species at lower frequency [21].
Figure 7a shows the variation of imaginary part (Z″) of impedance with frequency for different temperatures. It clearly observed from Fig. 7a that the peak decreases and shift’s towards the higher frequency with the increase in temperature denoting the temperature dependent relaxation process in the material. Usually, the relaxation process in oxide materials is due to the presence of oxygen vacancy. At lower temperature the relaxation process is due to the oxygen vacancy and higher temperature as a result of defects in the material. The relaxation time for the imaginary part of impedance is obtained from the maxima peak using the formula
Arrhenius equation to calculate the activation energy is
where τ0 is the pre-exponential factor, Ea is activation energy, KB is Boltzmann constant, and T is temperature. Arrhenius plot for temperature dependent relaxation time is shown in Fig. 7b. The activation energy (Ea) obtained is 1.2061 eV that is in good agreement with the absolute value of activation energy of 1.2 eV [9, 22]. However, this value attributes to the grain bulk and grain boundary conduction. This value of activation energy is due to the depletion in the oxygen vacancies at the adjacent space charge regions of the grain boundaries and also due to the hopping mechanism between Mn3+ and Mn4+.
Nyquist plots of the impedance spectra at different temperature for ZnMn2O4 are shown in Fig. 8a, b. It is one of the influential techniques to analyze the electrical properties with individual contribution from the grains, grain boundaries and interface effects over a wide range of frequency. Nyquist plot consists of one or two semicircles depending upon the property of the material. The semicircle at the lower frequency is due to the grain boundary and the same at high frequency is due to grain conduction. It observed from the Fig. 8a that the impedance spectra for the temperature ranges from 363 to 423 K clearly shows the overlapping of two semicircles with a spike attached to it which signifies the grain and grain boundaries resistances and spikes to the interfacial effects. Impedance plot for temperatures from 403 to 423 K in Fig. 8a shows the single semicircle with a spike attached attributing to the dominance of grain resistance in the material. Nyquist plot shown in the Fig. 8b indicates the single semicircle of grain resistance along with the grain boundary resistance effect which is very small, as there are no clear semicircles but a spike attached to the semicircle. From the Nyquist plot, it is observed that the centre of the semi-circle is depressed with the rise in temperature signifying the decrease in the impedance value which influences the conductivity and which results in the thermal activation of the material [23]. It also confirms the decrease in the grain and grain boundary resistance of the material with the increasing temperature which attributes to the behaviour of negative temperature co-efficient of resistance for NTC thermistor applications. The value of grain resistance (Rg), grain capacitance (Cg) and relaxation time (τg) are calculated from the Nyquist plot. Further, activation energy calculated from the Arrhenius equation for the relaxation time that is shown in the Fig. 8c. Activation energy (Ea) is found to be 1.1957 eV that is again in good agreement with the existence of oxygen vacancy and defects in cations that obtained from the imaginary part of impedance (Z″) [10]. Ionic conductivity of the material is obtained using the grain resistance (Rg) concerning rise in temperature by the formula
where σ is ionic conductivity, d is the thickness of the pellet and S is the surface area of the pellet. Figure 8d shows the ionic conductivity against 1000/T plot where it observed that the ionic conductivity increases with increase in temperature and activation energy obtained to be 1.0506 eV. This activation energy corresponds to the diffusion of oxygen vacancies as well as the oxygen vacancy complexes or defect dipoles [9].
3.4 Electrical modulus
The values of real (M′) and imaginary (M″) part of electrical modulus is calculated using the relation
where M′ and M″ are real and imaginary part of the electric modulus, C0(=ε0A/d) is the vacuum capacitance of the measuring pellet and the pellet thickness d, area of cross-section A, the permittivity of free space ε0, and the angular frequency ω. Figure 9 shows the variation of real part of the electric modulus (M′) with frequency for different temperatures of ZnMn2O4. It observed that the minimal value of M′ for all temperature at lower frequency region signified the temperature independence in the real part of modulus and consequent dispersion with increasing frequency for all temperatures. This behaviour indicates the suppression in the electrode polarization effect at lower frequency region [24].
Figure 10a shows the imaginary part of the electric modulus (M″) with frequency for different temperatures. The shift in the peak of the modulus towards the higher frequency for higher temperature signifies that the relaxation is thermally activated in the material and also confirms the earlier discussions on relaxation. Also, the asymmetric broadening of the modulus peaks denotes the spreading of relaxation time and non-Debye type of relaxation [25]. The Arrhenius plot of the temperature dependence of relaxation time for variation in M″ with frequency shown in Fig. 10b. The activation energy obtained is 1.14506 eV that is in the range of the oxygen vacancy justifying the earlier discussion on the material. This result in the diffusion of oxygen vacancies as well as the defect dipoles or oxygen vacancy complexes composed of oxygen and other cation vacancies. In this material the hopping between Mn3+ and Mn4+ also creates oxygen vacancy that results in the relaxation process.
Complex electric modulus explains the difference between grains, grain boundary and electrode polarization effect in the material. Figure 11 shows the complex modulus plot for various temperatures. At lower temperature, it clearly shows the two semicircles indicating the grain and grain boundary conduction in the material that gradually suppresses denoting demolition of the grain boundary effect in the material. At higher temperature hopping of ions within the Mn site results in the suppression of semicircular arc into an arc [26].
4 Conclusion
Nanocrystalline ZnMn2O4 material was synthesized by the sol–gel method by using glycine as a chelating agent with microwave assisted sintering. XRD pattern clearly confirmed the formation of tetragonal ZnMn2O4 with the space group I41/amd. SEM image showed the grain and grain boundaries in the synthesized material. The existence of grain boundaries, defects, hopping of electrons between Mn3+ and Mn4+, oxygen vacancy and space charge polarization effect played a major role in the dielectric relaxation. The impedance and electric modulus studies as a function of frequency for different explained the strong correlation between the grains and grain boundaries. Detailed studies of impedance and modulus have provided insight into the electrical properties and relaxation process in the material. The activation energy was obtained using Arrhenius plot under different perspectives of impedance and modulus. It confirmed the existence of the oxygen vacancies in the adjacent space charge regions of the grain boundaries and also due to the hopping mechanism between Mn3+ and Mn4+. Nyquist plot of impedance confirms the reduction in the resistance with increasing temperature, which further signifies its potential application as NTC thermistor.
References
R. Gherbi, Y. Bessekhouad, M. Trari, Structure, optical and transport properties of Mg-doped ZnMn2O4. J. Alloys Compd. 655, 188–197 (2016). doi:10.1016/j.jallcom.2015.09.192
P. Zhang, X. Li, Q. Zhao, S. Liu, Synthesis and optical property of one- dimensional spinel ZnMn2O4 nanorods. Nanoscale Res. Lett. 6, 323 (2011). doi:10.1186/1556-276X-6-323
S.V. Lakshmi, S. Pauline, Structural, morpological and optical properties of hetarolite—ZnMn2O4 nano particle by hydrothermal method. Int. J. Sci. Res. 3, 8–9 (2014)
Y. Deng, S. Tang, Q. Zhang, Z. Shi, L. Zhang, S. Zhan et al., Controllable synthesis of spinel nano-ZnMn2O4 via a single source precursor route and its high capacity retention as anode material for lithium ion batteries. J. Mater. Chem. 21, 11987 (2011). doi:10.1039/c1jm11575h
R.N. Jadhav, V. Puri, Effect of film thickness and pH of zinc manganite on microwave absorption and complex permittivity. Synth. React. Inorg. Met. Org. Nano-Met. Chem. 44, 1426–1428 (2014). doi:10.1080/15533174.2013.809744
F. Méndez-martínez et al., Znx−1CuxMn2O4 spinels; synthesis, structural characterization and electrical evaluation. J. Mex. Chem. Soc. 54(1), 2–6 (2010)
M. Khairy, M.A. Mousa, Electrical and catalytic properties of gamma-irradiated and unirradiated ZnMn2O4 nanoparticles. Am. J. Chem. 2(6), 306–311 (2012)
J. Takahashi, A. Miura, H. Itoh, K. Sawayama, T. Akazawa, Phase change and electrical resistivity of Zn–Mn–Ni–O-based NTC thermistors produced using IZC powder recycled from used dry batteries. Ceram. Int. 34, 853–857 (2008). doi:10.1016/j.ceramint.2007.09.035
C.C. Wang, C.M. Lei, G.J. Wang, X.H. Sun, T. Li, Oxygen-vacancy-related dielectric relaxations in SrTiO3 at high temperatures. J. Appl. Phys. 094103, 1–10 (2013). doi:10.1063/1.4794349
C. Elissalde, J. Ravez, Ferroelectric ceramics: defects and dielectric relaxations. J. Mater. Chem. (2001). doi:10.1039/b010117f
Y. Bessekhouad, M. Trari, Photocatalytic hydrogen production from suspension of spinel powders AMn2O4 (A = Cu and Zn). Int. J. Hydrogen Energy 27, 357–362 (2002)
J. Xu, Z. Yang, Y. Zhang, X. Zhang, H.U.A. Wang, Bipolar resistive switching behaviours in ZnMn2O4 film deposited on p+-Si substrate by chemical solution deposition. Bull. Mater. Sci. 37, 1657–1661 (2014)
P. Li, J. Liu, Y. Liu, Y. Wang, Z. Li, W. Wu et al., Three-dimensional ZnMn2O4/porous carbon framework from petroleum asphalt for high performance lithium-ion battery. Electrochim. Acta 180, 164–172 (2015). doi:10.1016/j.electacta.2015.08.095
C. Dong, PowderX: Windows-95-based program for powder X-ray diffraction data processing. J. Appl. Crystallogr. 32(4), 838 (1999)
J. Langford II, A.J.C. Wilson, Scherrer after sixty years: a survey and some new results in the determination of crystallite size. J. Appl. Crystallogr. 11(2), 102–113 (1978). doi:10.1107/S0021889878012844
N. Kumari, V. Kumar, S.K. Singh, Structural, dielectric and magnetic investigations on Al3+ substituted Zn ferrospinels. RSC Adv. 5, 37925–37934 (2015). doi:10.1039/C5RA03745J
H. Rahmouni, M. Smari, B. Cerif, E. Dhahri, K. Khirouni, Conduction mechanisum, impedance spectroscopic investigation and dielectric behavior of La0.5Ca0.5-xAgxMnO3 manganites with the composition below the concentration limit of silver solubility in perovskites (0 < x<0.2). Dalton Trans. (2015). doi:10.1039/C5DT00444F
S. Singh, S.E. Shirsath, Structural phases and Maxwell–Wagner relaxation in magnetically soft-ZnFe2O4 and hard-Sr2Cu2Fe12O22 nanocomposites. Ceram. Int. (2015). doi:10.1016/j.ceramint.2015.10.023
O. Padmaraj, M. Venkateswarlu, N. Satyanarayana, Structural, electrical and dielectric properties of spinel type MgAl2O4 nanocrystalline ceramic particles synthesized by the gel-combustion method. Ceram. Int. 41, 3178–3185 (2015). doi:10.1016/j.ceramint.2014.10.169
T. Javed, A. Maqsood, Structural, electrical and dielectric properties of Co–Mn spinel nanoferrites prepared by co-precipitation technique. J Supercond. Nov. Magn. 4, 2137–2144 (2011). doi:10.1007/s10948-011-1168-7
C. Sujatha, K.V. Reddy, K.S. Babu, A.R. Chandra, M.B. Suresh, K.H. Rao, Effect of Co substitution of Mg and Zn on electromagnetic properties of NiCuZn ferrites. J. Phys. Chem. Solids 74, 917–923 (2013). doi:10.1016/j.jpcs.2013.02.005
M. Gerstl, E. Navickas, M. Leitgeb, G. Friedbacher, F. Kubel, J. Fleig, The grain and grain boundary impedance of sol-gel prepared thin layers of yttria stabilized zirconia (YSZ). Solid State Ion. 225, 732–736 (2012). doi:10.1016/j.ssi.2012.02.012
M. Gerstl, E. Navickas, G. Friedbacher, F. Kubel, M. Ahrens, J. Fleig, The separation of grain and grain boundary impedance in thin yttria stabilized zirconia (YSZ) layers. Solid State Ion. 185, 32–41 (2011). doi:10.1016/j.ssi.2011.01.008
W. Chen, W. Zhu, C. Ke, Z. Yang, L. Wang, X.F. Chen et al., Impedance spectroscopy and conductivity mechanism of CoFe2O4–Pb(Zr 0.53Ti0.47)O3 composite thick films. J. Alloys Compd. 508, 141–146 (2010). doi:10.1016/j.jallcom.2010.08.029
A. Mekap, P.R. Das, R.N.P. Choudhary, Dielectric, magnetic and electrical properties of ZnFe2O4 ceramics. J. Mater. Sci.: Mater. Electron. 24, 4757–4763 (2013). doi:10.1007/s10854-013-1470-1
L.S. Lobo et al., Investigation of electrical studies of spinel FeCo2O4 synthesized by sol-gel method. Superlattices Microstruct. (2015). doi:10.1016/j.spmi.2015.09.010
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lobo, L.S., Ruban Kumar, A. Investigation of structural and electrical properties of ZnMn2O4 synthesized by sol–gel method. J Mater Sci: Mater Electron 27, 7398–7406 (2016). https://doi.org/10.1007/s10854-016-4714-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-4714-z