Abstract
Effect of dimethyl borate (C3H9BO3) composition on the structure, morphology, thickness, elemental composition, optical absorption, photoluminescence of ZnO nanotubes and the performance of the DSSC has been studied. It was found that the structure, diameter, thickness, elemental composition, optical absorption and morphology of ZnO nanostructure are significantly influenced by the composition of dimethyl borate. The diameter and thickness of ZnO nanotubes decrease with the increase in the composition of C3H9BO3. The photoluminescence increases with the composition of C3H9BO3. The DSSC utilizing ZnO nanotubes synthesized at 1 wt% C3H9BO3 yields the J SC and η of 1.9 mA cm−2 and 0.222 %, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Doping of metal oxide materials for use as photoanode in DSSC has successfully improved its performance of [1–8]. The metal oxide films are doped with metallic and non-metallic materials in order to improve the properties of metal oxide such as optical absorption and electronic conductivity. Doping can also lower down the band gap of metal oxide. This increases the generation rate of free electron–hole pair and consequently improves the performance of the device [3–8]. The doped metal oxides that are commonly utilized in DSSC are tin doped ZnO [1], indium doped ZnO [2], cerium doped TiO2 [3], nitrogen doped TiO2 [4–7], sulfur doped TiO2 [4], graphene doped TiO2 [8], boron doped ZnO [9, 10], Sn-doped-ZnO [11], Na-doped ZnO [12] and Mg-doped ZnO [13].
Boron has been chosen as dopant since it is a good conductor, non-toxic, easily available and cheap in price. Furthermore, n-type metal oxide is produced upon doping metal oxide with boron, improving its electronic conductivity. Charge carrier transport is enhanced in DSSC utilizing boron doped metal oxide. Boron doped ZnO films was prepared via sol–gel dip coating technique on silica glass and ITO coated glass substrate, respectively [14, 15]. Spray pyrolysis was employed to prepare boron doped ZnO films on glass substrate [16, 17]. Boron doped ZnO samples was prepared by low pressure chemical vapor deposition (LP-CVD) [18, 19].
It has been reported in [9] that boron doped ZnO thin film electrodes in DSSC was synthesized via spray pyrolysis technique. The source of boron was boric acid. Boron doped ZnO as photoelectrode in DSSC synthesized via sol–gel technique has been reported in [10] for which the source of boron was also boric acid. Charge carrier transport is enhanced in DSSC utilizing boron doped metal oxide, consequently improving the performance of DSSC. In this work, we have introduced another source of boron that is dimethyl borate (C3H9BO3) which is added into a growth solution containing Zn(NO3)2 and HMT via hydrothermal technique. The aim of the work is to investigate the influence of C3H9BO3 composition on the structure, morphology, thickness, elemental composition, optical absorption, band gap energy and photoluminescence of ZnO films. These properties are then related to the performance parameters of the DSSC utilizing boron doped ZnO samples.
2 Experimental
The ZnO nanostructures were grown on FTO substrate via simple seed mediated hydrothermal technique. This method involved two route steps, namely, seeding process by deposition of ethanolic zinc acetate dihydrate to prepare uniforms ZnO nanoseed and followed by the growth process in aqueous solution of zinc nitrate hexahydrate (Zn(NO3)·6H2O) and hexamethylenetetramine (HMT). Reagent grade of dimethyl borate, Zn(NO3)·6H2O and HMT powders (≥99.0 % in purity) were used without purification.
2.1 Seeding process
The substrates were cleaned using acetone, 2-propanol and ethanol for 15 min in ultrasonic bath, respectively. The substrates were then dried under nitrogen flow. After cleaning, the substrates were used immediately for growth process. ZnO nanoseeds on the FTO surface were prepared using an alcohol-thermal seeding method. A thin layer of ethanolic solution of 10 mM zinc acetate dihydrate (Zn(CH3COO)2·2H2O) on a cleaned FTO surface was firstly prepared by two-steps spin-coating process for 6 s at 400 rpm and followed for 30 s at 3000 rpm in order to make sure the solution was evenly distributed on the substrate and to get the sufficient thickness of the seed growth on the substrate. The sample was then dried at 100 °C on a hot-plate for 15 min and then cooled down to 50 °C. These procedures were repeated three times in order to get appropriate thickness of ZnO nanoseeds. The sample was annealed at 350 °C for 1 h in air condition in a horizontal tube furnace.
2.2 Growth process
The growth of ZnO nanostructures from the nanoseeds was carried out by immersing the nanoseeds-attached FTO in a equimolar solution, containing 0.040 M Zn(NO3)·6H2O and 0.040 M HMT. Then, 1 wt% C3H9BO3 was added into the solution and the growth reaction was carried out at 90 °C for 8 h inside an electric oven. The substrate with ZnO seed layer was put into the growth solution with the angle between the substrate and beaker bottom was 45°. After heating for 8 h at 90 °C, the solution was subsequently cooled down to 50 °C and the reaction was left for 16 h. After the growth process, the samples were then taken out and washed several times using pure water in order to remove any precipitation on their surface and dried using a flow of nitrogen gas for characterizations. Finally, the sample was annealed at 250 °C for 1 h to burn out the organic part. These procedures were repeated for preparing the samples with 3.0, 5.0 and 7.0 wt% C3H9BO3.
The structure and phase structure of the samples were examined by X-ray diffraction (XRD) model Bruker D8 Advance. The morphology of the samples was observed using field-emission scanning electron microscopy (FESEM) analysis (Zeiss Supra 55VP FESEM) with the magnification of 50,000×. The outer diameter of the ZnO nanotubes was estimated by using the scale located at the lower corner of the FESEM micrographs. The thickness of the nanotubes was estimated from the cross-sectional view of the FESEM micrographs. Energy dispersive X-ray (EDX) spectrometer was employed for the elemental analysis. Optical spectrophotometer UV–Vis Lambda 900 Perkin Elmer was employed to study the optical absorption and reflection of the samples and coated N719 dye samples. Photoluminescence spectrophotometer was employed to study the photoluminescence of the samples.
2.3 Fabrication and performance study of DSSC
ZnO samples were immersed into an ethanolic solution of 0.3 mM N719 dye for 2 h. The samples were then taken out, rinsed gently with fresh ethanol and then dried under a flow of nitrogen gas. Platinum film as a counter electrode was prepared by sputtering platinum pellets on the ITO substrate. An electrolyte containing 0.5 M LiI/0.05 M I2/0.5 M TBP in acetonitrile was used as a redox couple of I−/I3 −. A DSSC was fabricated by sandwiching the parafilm between ZnO sample and platinum counter electrode and clamped in order to optimize the interfacial contact of the cell components. The electrolyte was injected into the cell via a capillary. The performance study of the cell was carried out by observing the current–voltage under illumination using an AM 1.5 simulated light with an intensity of 100 mW cm−2. The illuminated area of the cell was 0.23 cm2. The current–voltage curves were recorded by a Keithley high-voltage source model 237 interfaced with a personal computer. Figure 1 shows a schematic illustration of the DSSC utilizing boron-doped ZnO as photoanode.
3 Results and discussion
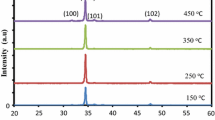
Figure 2 shows the XRD patterns of the samples with various compositions of C3H9BO3. All samples are crystalline with three wurtzite phase peaks at 33.90°, 36.39°, and 47.48°, respectively. This results agree well with those reported in [15, 16, 20]. According to the JCPDS (file no. 36-1451), the peaks are attributed to (002), (101) and (102) plane. The peak intensity at all planes is about the same. There is no peak shift due to boron doping since the doping level is low that is maximum of 7 % only. Boron doping into ZnO does not affect the peak shift. The full width half maximum (FWHM) at all planes is also about the same, indicating that the crystallite size of the samples is almost the same.
Figure 3 shows the FESEM micrographs of ZnO samples synthesized at various C3H9BO3 compositions. It is observed from the micrographs that the nanoparticle shape for all samples is nanotube. It is also seen the morphology of the samples are homogenous since the nanotubes size is almost identical. The morphology of the nanotubes is incompact since pores are observed to present between the nanotubes [21]. The diameter of the nanotubes was found to decrease with the composition of dimethyl borate as illustrated in Table 1. This is due to the fact that during the addition of C3H9BO3 into the growth solution, C3H9BO3 etches the nanotubes causing its size becomes smaller. This result is consistent with that reported in [18] for which the grain size of ZnO:B films decreased with the composition of diborane (B2H6). The boron source was B2H6.
Figure 4 depicts the FESEM cross-sectional view of the ZnO nanotubes synthesized at various C3H9BO3 compositions. It is clearly seen from the images that the nanotubes are aligned on the FTO substrate. The length or thickness of the nanotubes was found to decrease with the composition of C3H9BO3 as illustrated in Table 1. This is due to upon the addition of C3H9BO3 into the growth solution, C3H9BO3 etches the nanotubes causing its thickness becomes smaller [18].
Figure 5 illustrates the EDX spectra for ZnO nanotubes with various compositions of C3H9BO3. From the spectra, it is observed that the element of boron present in all samples. This indicates that boron has successfully been doped into ZnO nanotubes. As the composition of C3H9BO3 increases, the boron atomic percentage also increases.
Figure 6 depicts the optical absorption spectra of ZnO samples synthesized at various C3H9BO3 compositions. The spectra show similar pattern. The light was strongly absorbed in the ultraviolet region corresponding with the wavelength range from 300 to 370 nm, while much lower absorption was observed in visible region. This is because uncoated dye ZnO sample absorbs less light in visible region. 1 wt% C3H9BO3 sample possesses the highest absorption while 7 wt% C3H9BO3 sample shows the lowest peak absorption. The absorption intensity decreases with the composition of C3H9BO3. Figure 7 shows the reflectance spectra of the samples synthesized at various compositions of C3H9BO3. The spectra shows quite similar pattern. The spectra show sharp peaks at 400 nm for 3 and 7 wt% C3H9BO3 sample. 1 wt% sample shows the peak at 385 nm, while 5 wt% C3H9BO3 sample shows the peak at 390 nm. It is noticeable that the reflection increases with the composition of C3H9BO3, opposite to that of absorption. Also, from the spectra, it is observed that the band gap energy of the sample does not change with the concentration of C3H9BO3. It is confirmed by all tangent lines drawn on the shoulders of the curves, falling at the wavelength of 380 nm corresponding with the band gap energy of 3.27 eV. This result agrees well with that reported in [14, 17].
Figure 8 shows the photoluminescence spectra of the samples synthesized at various compositions of C3H9BO3. The photoluminescence spectra show some sharp peaks at 390, 440 and 470 nm which are in visible region [14, 22]. 1 and 5 wt% sample possesses the lowest and highest peak intensity, respectively at these wavelengths. This indicates that 1 and 5 wt% samples possess the lowest and highest recombination electron–hole rate, respectively. In other words, 1 and 5 wt% samples possess the lowest and highest excitonic state, respectively. Excitonic state is also called as bound state of electron and hole.
Figure 9 shows the current density–voltage (J–V) curves under 100 mW cm−2 light of the DSSC utilizing ZnO samples prepared with various C3H9BO3 compositions. The device with 1 wt% C3H9BO3 yields the highest output power, while the one with 7 wt% C3H9BO3 generates the lowest power. The output power decreases with the composition of C3H9BO3. The photovoltaic parameters are illustrated in Table 1. According to the table, it was found that the device with 1 wt% C3H9BO3 demonstrates the highest J SC and η. This is due to 1 wt% boron doped ZnO sample possesses the highest optical absorption and lowest photoluminescence in visible region as shown in Figs. 6 and 8, respectively. The device with 5 wt% sample shows the lowest η since it shows the highest photoluminescence in visible region as illustrated in Fig. 7. According to Table 1, the V OC and FF do not significantly with the composition of C3H9BO3. Also, from the table, the fill factor is low since the area of maximum power rectangles drawn from the J–V curves is much smaller than that of the J–V curves. The highest η produced from this work is slightly lower than that reported in [22] which was 0.29 %. The boron source in [22] was boric acid. Moreover, it is much smaller than those reported in [10, 14] which were 1.53 and 1.56 %, respectively. Meanwhile, the DSSC based on Mg-doped ZnO nanorods exhibited the efficiency of 0.14 % [13] and the DSSC based on Sn doped-ZnO thin film exhibited the efficiency of 0.74 % [11]. The low efficiency of the DSSC from this work is due to the insufficient dipping time of boron doped ZnO samples into N719 dye solution. This is also due to the insufficient thickness of ZnO samples as shown in Table 1, leading to small amount of the dye adsorbed onto ZnO films.
4 Conclusions
The element of boron was successfully doped into the ZnO films by adding C3H9BO3 into the growth solution. The diameter and thickness of ZnO nanotubes decreases with the composition of C3H9BO3. The optical absorption of boron doped ZnO films increases with the decrease in the composition of C3H9BO3. The photoluminescence of the sample increases with the composition of C3H9BO3. The DSSC utilizing the sample synthesized at 1 wt% C3H9BO3 demonstrates the highest J SC and η of 1.9 mA cm−2 and 0.222 %, respectively. This is due to the sample possesses the highest optical absorption and lowest excitonic state.
References
S. Ameen, M.S. Akhtar, H.K. Seo, Y.S. Kim, H.S. Shin, Influence of Sn doping on ZnO nanostructures from nanoparticles to spindle shape and their photoelectrochemical properties for dye sensitized solar cells. Chem. Eng. J. 187, 351–356 (2012)
A. Tubtimtae, M.-W. Lee, ZnO nanorods on undoped and indium-doped ZnO thin films as a TCO layer on nonconductive glass for dye-sensitized solar cells. Superlattice Microstruct. 52, 987–996 (2012)
J. Zhang, W. Peng, Z. Chen, H. Chen, L. Han, Effect of cerium doping in the TiO2 photoanode on the electron transport of dye-sensitized solar cells. J. Phys. Chem. C 116, 19182–19190 (2012)
J.C. Zhang, Z.Y. Han, Q.Y. Li, X.Y. Yang, Y. Yu, W.L. Cao, N, S-doped TiO2 anode effect on performance of dye-sensitized solar cells. J. Phys. Chem. Solids 72, 1239–1244 (2011)
M.Y.A. Rahman, A.A. Umar, S.K.M. Saad, M.M. Salleh, A. Ishaq, Effect of dye on the performance of nitrogen doped TiO2 solar cell prepared via ammonia treated liquid phase deposition technique. J. New Mater. Electrochem. Syst. 17, 33–37 (2014)
T. Ma, M. Akiyama, E. Abe, High-efficiency dye-sensitized solar cell based on a nitrogen doped nanostructured titania electrode. Nano Lett. 5, 2543–2547 (2005)
C.K. Lim, H. Huang, C.L. Chow, P.Y. Tan, X. Cheng, M.S. Tse, O.K. Tan, Enhanced charge transport properties of dye-sensitized solar cells using TiNxOy nanostructure composite photoanode. J. Phys. Chem. C 116, 19659–19664 (2012)
Y.-B. Tang, C.-S. Lee, J. Xu, Z.-T. Liu, Z.-H. Chen, Z. He, Y.-L. Cao, G. Yuan, H. Song, L. Chen, L. Luo, H.-M. Cheng, W.-J. Zhang, I. Bello, S.-T. Lee, Incorporation of graphenes in nanostructured TiO2 films via molecular grafting for dye-sensitized solar cell application. ACS Nano 4, 3482–3488 (2010)
B.N. Pawar, G. Cai, D. Ham, R.S. Mane, T. Ganesh, A. Ghule, R. Sharma, K.D. Jadhava, S.H. Han, Preparation of transparent and conducting boron-doped ZnO electrode for its application in dye-sensitized solar cells Sol. Energy Mater. Sol. Cells 93, 524–527 (2009)
V. Kumar, N. Singh, V. Kumar, L.P. Purohit, A. Kapoor, O.M. Ntwaeaborwa, H.C. Swart, Doped zinc oxide window layers for dye sensitized solar cells. J. Appl. Phys. 114, 134506 (2013)
P. Dhamodharan, C. Manoharan, S. Dhanapandian, M. Bououdina, S. Ramalingam, Preparation and characterization of spray deposited Sn-doped ZnO thin films onto ITO substrate as photoanode in dye sensitized solar cell. J. Mater. Sci. Mater. Electron. 26, 4830–4839 (2015)
İ. Polat, Effects of Na-doping on the efficiency of ZnO nanorods-based dye sensitized solar cells. J. Mater. Sci. Mater. Electron. 25, 3721–3726 (2014)
İ. Polat, S. Yılmaz, E. Bacaksız, Y. Atasoy, M. Tomakin, Synthesis and fabrication of Mg-doped ZnO-based dye-synthesized solar cells. J. Mater. Sci. Mater. Electron. 25, 3173–3178 (2015)
S. Jana, A.S. Vuk, A. Mallick, B. Orel, P.K. Biswas, Effect of boron doping on optical properties of sol–gel based nanostructured zinc oxide films on glass. Mater. Res. Bull. 46, 2392–2397 (2011)
M. Caglar, S. Ilican, Y. Caglar, F. Yakuphanoglu, Boron doped nanostructure ZnO films onto ITO substrate. J. Alloys Compd. 509, 3177–3182 (2011)
B.J. Lokhande, P.S. Patil, M.D. Uplane, Studies on structural, optical and electrical properties of boron doped zinc oxide films prepared by spray pyrolysis technique. Phys. B 302–303, 59–63 (2001)
B.N. Pawar, S.R. Jadkar, M.G. Takwale, Deposition and characterization of transparent and conductive sprayed ZnO:B thin films. J. Phys. Chem. Solids 66, 1779–1782 (2005)
S.Y. Myong, J. Steinhauser, R. Schluchter, S. Fay, E. Vallat-Sauvain, A. Shah, C. Ballif, A. Rufenacht, Temperature dependence of the conductivity in large-grained boron-doped ZnO films. Sol. Energy Mater. Sol. Cells 91, 1269–1274 (2007)
S. Fay, U. Kroll, C. Bucher, E. Vallat-Sauvain, A. Shah, Low pressure chemical vapour deposition of ZnO layers for thin-film solar cells: temperature-induced morphological changes. Sol. Energy Mater. Sol. Cells 86, 385–397 (2005)
I. Iwantono, W. Nurwidya, L.R. Lestari, F.Y. Naumar, S. Nafisah, A.A. Umar, M.Y.A. Rahman, M.M. Salleh, Effect of growth temperature and time on ZnO films properties and the performance of dye-sensitized solar cell. J. Solid State Electrochem. 19, 1217–1221 (2015)
L. Roza, M.Y.A. Rahman, A.A. Umar, M.M. Salleh, Direct growth of oriented ZnO nanotubes by self-selective etching at lower temperature for photo-electrochemical (PEC) solar cell application. J. Alloys Compd. 618, 153–158 (2015)
M.Y.A. Rahman, L. Roza, A.A. Umar, M.M. Salleh, Effect of acid boric composition on the properties of ZnO thin film nanotubes and the performance of dye-sensitized solar cell (DSSC). J Alloys Compd. 648, 86–91 (2015)
Acknowledgments
This work was supported by The Ministry of higher Education of Malaysia under research Grant FRGS/2/2013/SG02/UKM/02/5, FRGS/2/2013/SG02/UKM/02/8 and GUP-2013-030.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Rahman, M.Y.A., Roza, L., Umar, A.A. et al. Effect of dimethyl borate composition on the performance of boron doped ZnO dye-sensitized solar cell (DSSC). J Mater Sci: Mater Electron 27, 2228–2234 (2016). https://doi.org/10.1007/s10854-015-4015-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-015-4015-y