Abstract
In this comprehensive work, we focus sensitively on the changes of microstructural, electrical, superconducting and mechanical properties belonging to the bulk Bi1.7Pb0.4Sr2.0Ca2.0ZrxCu3.1Oy materials with the different Zr nanoparticles (0 ≤ x ≤ 1.0) inserted in the superconducting matrix. The characterizations of the materials prepared are experimentally performed by bulk density, dc resistivity (ρ–T), X-ray powder diffraction (XRD), scanning electron microscopy (SEM), electron dispersive X-ray (EDX) and Vickers microhardness (H v ) investigations. It is found that all the characteristics given above (especially superconducting properties) degrade considerably with the increment in the Zr addition level, and in fact they reach to the global minimum points in case of the maximum dopant level. The main reason of the rapid decrement in the electrical and superconducting properties stems from the presence of the localization problem in the Cu–O2 consecutively stacked layers. Likewise, the Zr foreign addition increases the artificial random defects, dislocations and grain boundary weak-links in the superconducting system, even being favored by the SEM examinations. At the same time, the EDX surveys indicate that the Zr nanoparticles in the tetravalent state prefer to substitute for the divalent states of the Cu inclusions through the crystal structure as a result of their close ionic radius values (86 pm for Zr4+ ion and 87 pm for Cu2+ ion). Thus, the differentiation of the electronegativity reduces the mobile hole concentration in the Cu–O2 slabs. Additionally, the XRD experimental findings show that there is a systematic decrement in the Bi-2223 (high) phase up to the Zr concentration level of x = 0.70 beyond which new characteristics peaks of ZrO2 appear immediately. This is in relation to the fact that the solubility limit corresponding to the Zr foreign impurity in the Bi-2223 system is about x = 0.70. Similarly, the regular decrement in both the c-axis length and crystallite size with the dopant confirms the deterioration of the superconducting properties. Furthermore, the dramatic reduction of the H v values stems from the suppression of the crystallinity and connection quality in the intergrain coupling of the superconducting samples exhibiting typical Indentation Size Effect behavior due to the existence of both elastic and plastic deformations in the system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Importance of materials science has seriously increased in the last half century. Developing technology has made compulsory the use of high performance materials. Materials exhibiting permanent and better mechanical properties are needed in a wide range from electronics to the automotive industry and from the aerospace to defense industry. Overall, the mechanical properties emerge as the decisive factor in the process, that the raw material (in bulk form) can be used to form (sheets, plates, wires, etc.) in the industry. It is well known that the mechanical properties of materials depend on production conditions and hence they are related to their microstructure [1]. Generally, mechanical characterization techniques are mainly based on the study of the behavior of materials under the external force, and they are the main method of assessment in the performance of the end products for industry needs.
However, the produced materials and developments in the field of superconductivity are quite interesting in terms of providing a major contribution to the above-mentioned sectors. When wire and strip production are considered, it is also of great importance to investigate the mechanical properties as well as structural, electrical and magnetic properties of superconductors. Mechanical properties provide useful information on deformation characteristics and strength of materials [2].
In determining mechanical properties in different experimental techniques, hardness test method is the most commonly used [3–6]. Especially Vickers hardness testing is one of the most appropriate method for estimating the mechanical properties. Hardness is the mechanical parameters associated powerfully with the structure and the composition of the solid [7].
The causes of BSCCO (from high critical temperature parents) superconductor’s preferation are the high transition temperature among high-temperature superconductors (except Hg-based superconductors), the lower hardness than YBCO, absence of Cu–O chains in structure of BSCCO, no depletion of oxygen leading to show the insulating behavior problems in YBCO material [8], ability to increase the transition temperature by forming different phase, and the first compounds in high temperature superconductors (HTSc) don’t contain rare earth element. Moreover, they are examined in the smaller research laboratory because of not only the relatively easy preparation of metal oxide, very high critical temperature (Tc) and critical magnetic fields (Hc) but also great technological importance. At the same time, the understanding of their properties and mechanisms being responsible for the superconducting behavior are very ambitious dealing areas for theoretician [9]. In addition, the HTSc with zero resistance and perfect diamagnetism have the Cu–O layer being in charge of the carrying current, extremely high anisotropy and perovskite structure. The compounds with granular and ceramic structure exhibit undesirable mechanical properties such as fragile and inelastic as a consequence of the ceramic structure [10–12]. In the current work, we survey the change of the microstructural, mechanical, electrical and superconducting properties belonging to the Bi-2223 (a special group in the BSCCO parents) with the Zr foreign impurity level by means of the standard characterization techniques such as the bulk density, dc electrical resistivity, X-ray powder diffraction examination, scanning electron microscopy, electron dispersive X-ray and Vickers microhardness investigations. It is found that all the characteristic properties degrade considerably with the Zr concentration level due to the increased artificial random defects, grain boundary weak-interactions and distortion between superconducting Cu–O2 planes.
2 Experimental procedure
The pure and Zr added Bi-2223 superconducting materials are sensitively prepared in atmospheric air by means of the conventional solid-state reaction technique from the high-purity chemical powders of Bi2O3, PbO, ZrO2, SrCO3, CaCO3 and CuO (Alfa Aesar Co., Ltd. with the purity of 99.99 %). All the oxides and carbonates are accurately weighed in stoichiometric proportion by an electronic balance and are pound in an agate mortar for half an hour to achieve much smaller particle sizes of the chemicals. Then, the resultant powders are exposed to the milling process for homogeneous mixture during the duration of 8 h. The chemical mixture is pressed into a rectangular bar of 1.5 × 0.4 × 0.4 cm3 at a constant load of 300 MPa in the air atmosphere. After that, the solidified powders are subjected to the heat treatment process (3 °C/min for heating rate and 5 °C/min for cooling rate) at 800 °C in a Protherm (Model PTF 12/75/200) programmable tube furnace for 24 h. After taking from the furnace, the rectangular bars solidified in blackish color are again mixed for about 1.5 h in the agate mortar and pelletized at the same environmental conditions. As for the last annealing process, the materials are tempered at 840 °C for the duration of 48 h in the air atmosphere. The bulk Bi1.7Pb0.4Sr2.0Ca2.0ZrxCu3.1Oy materials (x = 0, 0.1, 0.2, 0.3, 0.5, 0.7 and 1) produced will hereafter be denoted as Zr0, Zr1, Zr2, Zr3, Zr4, Zr5 and Zr6 respectively.
The identification of the pure and Zr added Bi-2223 superconducting samples is performed by means of the available experimental measurements techniques including the bulk density, powder X-ray diffraction, dc resistivity (ρ–T), scanning electron microscopy (SEM), electron dispersive X-ray (EDX) and Vickers microhardness (H v ) surveys.
The bulk density examinations belonging to the superconducting compounds are experimentally surveyed by means of the Archimedes water displacement technique. Besides, the bulk density parameters achieved from the measurements enable us to theoretically determine the relative densities and the variation of the porosities (degree of granularity) with the Zr dopant level in the crystal lattice.
The electrical and superconducting findings are taken from the dc resistivity versus temperature measurements. Through the experiments, the dc current of 5 mA is applied to the surface of the samples. The (typical four-probe) measurements are carried out by a Keithley 220 programmable current source and Keithley 2700 nano-voltmeter in the He gas contact cryostat (CRYO Industries) with the provision for the vacuum in the temperature range of 25–115 K.
Moreover, the X-ray powder diffraction measurements are exerted with the aid of a Rigaku Multiflex + XRD 2 kW diffractometer with CuKα target including the monochromatic beam with the wavelength of 1.54 Å° in air atmosphere at the room temperature. The experimental evidences are recorded in the 2θ range from 3° to 60° at a scan speed of 3°/min with step increase of 0.02. We also examine the volume fractions, crystallite size and lattice cell parameters (a and c a-axis lengths within the accuracy of ±0.01 Å) for the pure and Zr added Bi-2223 superconducting compounds.
At the same time, the surface identification (morphology, crystallinity, porosity, melting, voids, cracks and interactions between the grains) of the bulk superconductors is exerted using a Jeol scanning electron microscope (SEM, JEOL 6390-LV), operated at the external voltage of 20 kV with the resolution power of ≈3 nm. Furthermore, local element composition and distribution of the compounds are quantitatively surveyed via SEM equipped with an Oxford X-ray micro-probe (EDX) including IXRF System Model 550i analyzer.
Moreover, the mechanical properties are examined by means of the digital microhardness tester (SHIMADZU, HVM-2) at room temperature under air atmosphere. The indentation load is applied throughout the different locations on the specimen surfaces for 10 s in the load range of 0.245–2.940 N. The reasonable mean diagonal length is measured in the accuracy of ±0.1 μm. At the same time, the microhardness curves enable us to determine the Vickers hardness, elastic modulus and yield strength values.
3 Results and discussion
3.1 Dc electrical resistivity findings
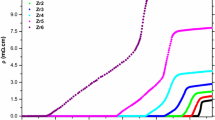
In this part of the paper, the variation of electrical resistivity against temperature in the range from 25 to 115 K is explored to determine the fundamental changes in the superconducting and electrical properties of the poly-crystalline Bi-2223 compounds with the Zr foreign impurity. The dc electrical resistivity findings are graphically displayed in Fig. 1 in detail. According to the measurement results, it is pertinent to mention here that the increment in the Zr inclusions damages seriously both the electrical and superconducting properties belonging to the Bi-2223 system. In more detail, each bulk material transits into the superconducting state as the metallic behavior with the positive linear (dρ/dt) temperature-dependent resistivity [13–15]. One must, however, keep in mind that the existence of the Zr addition in the Bi-2223 superconducting system damages the metallic character due to disorder of the metallic interaction between the grains. Similarly, the room temperature resistivities tend to enhance with decreasing the mobile hole carrier concentration in the Cu–O2 consecutively stacked layers. Namely, the pure bulk material exhibits the minimum room temperature resistivity value of about 3.69 mΩ cm while the Zr6 sample obtains the largest value of about 13.16 mΩ (Inset of Fig. 1). Based on the result, it is tempting to speculate that the Zr nanoparticles in the tetravalent state might be substituted for the divalent states of the Cu decorations, even being favored by the EDX experimental evidences. At the same time, we can evaluate the onset critical transition (T onset c ) and offset critical transition (T offset c ) temperatures from the ρ–T curves from Fig. 1. The maximum T onset c /T offset c values of 110.8/108.7 K are observed for the pristine sample whereas the Zr-6 material obtains the global minimum values 84.9 and 40.8 K for T onset c and T offset c values, respectively (Fig. 1). The main reason of the rapid reduction of the T offset c values results from the decrement in both the mobile hole concentration in the Cu–O2 consecutively stacked layers (known as localization problem) [16], and average crystallite size due to the enhancement in the porosity and weakening of the intergrain coupling [17]. This can be observed as the pseudo-transition from high (Bi-2223) phase to low (Bi-2212) phase in macroscopic world (Inset of Fig. 1). Moreover, the considerable degradation of critical temperatures can be explained by the presence of the increased artificial random defects, grain boundary weak-interactions and especially distortion between superconducting Cu–O2 planes in the Bi-2223 system [18–21]. What stands out clearly here is that the Zr addition causes significantly the enhancement in the degree of broadening (ΔT c = T onset c − T offset c ) as a consequence of the change of homogeneities in the oxidation states. One can easily encounter from Fig. 2 that the pure sample exhibits the largest ΔT c value of 2.1 K while the Zr6 solid material with the ΔT c parameter of 44.1 K dwells rapidly in the top point (Fig. 2). In other words, the Zr doping is ploughed to promote the growth of the high (Bi-2223) phase (Table 1).
3.2 XRD measurement evidences
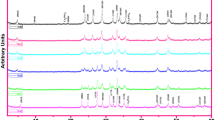
This part focuses on the change in the crystal structure, phase fracture, crystallinity, average grain size and lattice constant parameters of the bulk Bi-2223 superconducting materials with the Zr concentration level inserted in the crystal lattice. In this respect, the powder X-ray diffraction (XRD) patterns are taken in the range of 2θ = 3–60° at the room temperature in the atmospheric air. Characteristic peaks of the high phase and low phase are assigned to be (hkl) H Miller indices and (hkl) L Miller indices as provided in Fig. 3, respectively. Based on the figure, it is fair to conclude that the high (Bi-2223) phase degrades considerably whereas the low (abbreviated as Bi-2212) phase becomes stronger and stronger with the enhancement of the Zr impurities in the Bi-2223 crystal structure. In fact, it would be more precise to say that the new peaks of (028) L, (11 15) L and (002) L belonging to the low phase emerge immediately while the high phase peaks of (008) H, (028) H, (11 19) H and (016) H disappear with the increase of the Zr foreign impurities. In case of x = 1.00, new characteristics peaks pertaining to the ZrO2 compound are observed at 2θ = 30.24° and 50.46° (Fig. 4). Thus, the solubility limit of the Zr nanoparticles along with the Bi-2223 crystal lattice is determined to be about x = 0.70 [22, 23]. As for the characteristics peak intensities, the same trend is valid for the intensities. Namely, the characteristics peak intensities of the Bi-2223 (Bi-2212) phase retrograde (increase) noticeably with the increment in the Zr additives in the crystal structure. The change in the peak intensities enables us to calculate the volume fraction values by using the following relations:
in the equations, I is related to the peak intensity of the present phase. The computations are numerically listed in Table 2. It can be inferred from the table that the Bi-2223 phase monotonously decreases from 91.6 to 77.8 % with the increment in the Zr concentration level up to x = 0.50, after this critical point the value tends to hastily suppress towards to the value of 52.4 %. On the other hand, the relative percentage value belonging to the Bi-2212 phase increases significantly to 47.6 %. It is another probable result deduced from the present work that the change of the Zr content level in the crystal lattice affects the lattice cell parameters computed from the least square method along with d values and (hkl) planes for the tetragonal unit cell structure. The lattice constant parameters are inserted in Table 2. It is apparent from the table that the enhancement in the Zr addition level leads to expand the a-axis length values but contract in the c cell parameter as a result of the partial replacement of tetravalent Zr inclusions for the divalent Cu nanoparticles in the Bi-2223 system. Namely, the presence of the different aliovalent substitutions through the Bi-2223 crystal structure results in the positive charge in the oxygen deficient Bi–O double layers, and so the positive charge appeared needs to be balanced by the valency/oxygen for the charge neutrality [24–28]. Hence, the interlayer distances between the Cu–Ca–Cu and Ca–Sr sites in the layered structure enlarge; however, the interlayer spaces of the Sr–Bi, Bi–Bi and Sr–Bi–Bi–Sr sites shorten automatically, so does c cell parameter [29]. Moreover, it is natural to confirm that the required extra energy for the bond formation gives rise to the contraction (from 37.02 to 30.72 Å) of the c-axis length [30]. At the same time, as well known that the Cu–O bond length in the Cu–O2 consecutively stacked layers arranges the size of the basal plane [31]. In the current work, the Zr foreign impurities enlarge the a lattice cell parameter from about 5.26–5.44 Å as a consequence of the enhancement in both the Cu–O bond length and effective Cu valence [31–33]. As part of XRD measurement findings, we calculate the average crystallite size values for each sample by means of the following formula [34];
in this relation, t corresponds to the crystal thickness and λ displays the wavelength of the incident X-rays. B denotes the full width at half maximum (FWHM) of the Bragg peak when θ B provides Bragg angle. All the computations are given in Table 2. According to the table, one can be convinced quickly that the average grain size values significantly decrease from 69.2 to 42.6 nm with the increase of the Zr ingredients in the Bi-2223 crystal lattice due to the deterioration of the crystallinity and connection between the superconducting grains.
3.3 SEM surface morphology examinations
In the current work, the SEM investigation plays an important role on the determination of the changes in surface morphology, crystallinity, porosity, melting, voids, cracks and connections between the superconducting grains with the Zr addition inserted in the main matrix. The micrographs taken from the virgin and Zr doped Bi-2223 superconducting specimen surfaces are imaged in the secondary electron image mode at the magnification of 5000×. The photos belonging to the materials are demonstrated in Fig. 5a–g. It is apparent form the figure that the morphological characteristics are found to be strongly dependent upon the Zr content level in the layered structure. The pristine compound exhibits even smoother and denser surface appearance (clear and characteristic slabs) with the finer interaction between the superconducting grains (Fig. 5a). At the same time, the sample presents more uniform surface appearance with larger average crystallite distribution, lower porosity, better texturing and crystallinity. With the increase of the Zr inclusions embedded the surface morphology gets worse and worse due to the beginning of the voids, porosity, cracks and partial melting on the corners, leading to the damages on the metastability of the bulk Bi-2223 materials. In this respect, the Zr6 material exhibits the worst surface morphology as shown in Fig. 5g. To be honest, the Zr dopant content diminishes significantly the characteristic flaky layers of large platelet-like structure throughout the samples [35]. The bulk (Bi, Zr)-2223 compounds with distorted/damaged microstructures are totally inadequate for the potential engineering and technological applications.
3.4 Experimental EDX results
The local elemental composition survey is quantitatively carried out by the electron dispersive X-ray (EDX) experimental method to discuss more detailed information about the material microstructures. The experimental evidences achieved for the pure and Zr doped Bi-2223 superconducting materials are numerically displayed in Table 3. Based on the table data depicted, one fairly evident fact is that neither the extra findings (except for the Zr peaks) nor different phases including any cation in the diagrams confirm the introduction of all the elements (O, Ca, Sr, Zr, Cu, Pb and Bi) into the Bi-2223 crystal lattice utterly [36, 37]. Moreover, it is another probable discussion extracted from the EDX examinations that the values belonging to Pb and especially Cu elements reduce drastically with the existence of the Zr foreign impurities in the superconducting matrix. This is attributed to the fact that the Zr nanoparticles inserted may mostly be substituted for the Cu inclusions, confirming why the superconducting properties regress or two-stage transition begins with the existence of the foreign inclusions in the crystal structure. At the same time, the pictures of Fig. 6a–g show the element composition/distribution mappings obtained from the nano-scale grains on the specimen surfaces. It is visible from the figure that the element distribution maintains to be almost homogeneous (uniform) on the whole area scanned for the virgin and Zr added Bi-2223 superconducting materials. However, it is necessary to underline that the Cu element (symbolized by green dots) distributions begins to decrease regularly with the Zr concentration level. In fact, the Cu element distribution is observed to be the lowest level for the Zr6 superconducting compound (Fig. 6g). Thus, the measurement of EDX mappings relies on the fact that the Cu element may partly be substituted by the Zr foreign impurities. Consequently, electrons are less attracted due to the different electronegativity between the Zr (1.33 Pauling scale) and Cu (1.90 Pauling scale) atoms as compared to before, causing to decrease the mobile hole concentration (per Cu ion) in copper oxide planes. This explains that why the superconducting characteristics degrade with the Zr dopant. That the slight reduction of the Pb distribution may also affect on the deterioration of the superconductivity is another interesting point deduced form the mappings.
3.5 Density and porosity parameters pertaining to superconductors
In this part, we investigate the bulk density parameters of the materials to support the experimental findings of the dc resistivity measurements. In this respect, the differentiation of the connectivity between the superconducting grains and the transport properties of the grain boundaries in the Bi-2223 system with the Zr content level is surveyed via the Archimedes water displacement method. The relative densities are determined based on the density of pure BSCCO system (≈6.302 g/cm3) [38, 39] and all the computations are numerically listed in Table 1 clearly. One can see from the table that the bulk density values decrease monotonously with the enhancement of the Zr concentration level. Numerically, the highest density is determined to be 6.127 g/cm3 for the pure superconducting compound while the Zr6 sample exhibits the lowest value of 5.624 g/cm3. According to the data obtained, the connectivity between the superconducting grains in the crystal system gets worse and worse with the increment in the dopant level, being favored by the other experimental techniques. Additionally, we make typical computations including the relative density values to discuss the degree of granularity (porosity) belonging to the virgin and Zr added polycrystalline materials. Two main methods having been defined previously in are used for the calculations of the granularity degrees [40, 41]. The data calculated are tabulated in Table 1. According to the calculations performed, the porosity values tend to enhance regularly with increasing the Zr foreign decorations in the Bi-2223 layered structure. In fact, it would be more affirmative to claim that from the Zr concentration level of x = 0.50 onwards in the Bi-2223 superconducting matrix, the porosities increase more and more rapidly and dwell in the maximum point of 10.76 %. This is related to the fact that the presence of Zr impurities in the Bi-2223 crystal system abates the interaction between the grains, even being supported by the SEM examinations clearly.
3.6 Vickers micro hardness measurement analysis
Up to this part of the paper, we verify that the Zr nanoparticles inserted in the superconducting matrix are ploughed to improve the surface morphology, grain connectivity, flux pinning ability, electrical and superconducting characteristics of the bulk Bi-2223 materials with the aid of the experimental investigations. Here, we deal with the change of the mechanical performance belonging to the Bi-2223 system by means of the Vickers microhardness (H v ) measurements exerted at different indentation test loads in the range of 0.245 N–2.940 N. The real (true) microhardness values are calculated from the relation as the following:
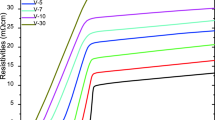
through the equation, H v is in relation to the Vickers microhardness value, d denotes the mean diagonal length belonging to the indentation impression when F shows the applied indentation test load. The experimental data obtained are sensitively given in Fig. 7. Similar to the findings obtained from other parts, the Vickers microhardness (H v ) parameters tend to degrade considerably as the Zr addition level increases in the Bi-2223 system as a result of the parallel increment in the specimen cracking/porosity, and irregular grain orientation distribution and grain boundary problems [42, 43]. At the same time, one can realize that the prepared materials exhibit non-linear microhardness behavior due to the decrement of the H v values with the applied indentation test load. Namely, the inorganic compounds each present the typical Indentation Size Effect (ISE) nature under the applied test load [44]. This is accordance with the fact that the elastic recovery plays dominant character on the pure and Zr added Bi-2223 superconducting materials due to the existence of both elastic (reversible) and plastic (irreversible) deformations in the system [45]. Numerically, the H v values are found to be in a range of about 0.871–0.690 and 0.467–0.221 GPa under the applied test load of 0.245–2.940 N for the pure and Zr6 materials, respectively. It is another probable result that the abrupt decrease in the independent microhardness values substitutes for the constant variation after the critical test load of about 2 N due to the presence of the saturation regions. The main reason of the plateau regions results from the increased impurity phases, grain boundary weak-connections, disorder, cracking/porosity and irregular grain orientation distribution in the crystal lattice [46, 47]. Even, the similar results including the change of the elastic properties are verified from the X-ray crystal structures pertaining to the inorganic compounds. We also perform the theoretical calculations for elastic (Young’s) modulus (E) and yield strength (Y) by means of the following formulas:
One can discuss the theoretical findings depicted in Table 4 that the Zr dopant in the Bi-2223 layered structure leads to degrade considerably owing to the suppression of the crystallinity and the strength in the connection between the superconducting grains. Even, the decrement in the Young’s modulus and yield strength parameters with the applied load points out the ISE nature of the pure and Zr doped superconducting materials studied in this work.
Additionally, we examine the combination of the Young’s modulus and bulk density to clarify the change of the crystallite size and connection quality between the superconducting grains by utilizing from the experimental bulk density dependence of elastic modulus as given in Fig. 8. It is apparent from the figure that every graph obtained displays the similar trend due to the typical ISE behavior. Namely, the E values obtained tend to improve (retrograde) parabolically with the enhancement of the bulk densities (Zr content level), confirming that the higher a material exhibits the bulk densities, the sample obtains the better the crystallite size and interaction between the superconducting grains. It is another important point deduced from the curves that at the smaller applied test loads the variation in the modulus values against the experimental bulk density is far greater than those at the higher loads. This fact presents that both the reversible and irreversible deformations are produced together in the virgin and Zr added materials at the applied test load of 0.245 N after which the plastic deformation starts to play dominant character on the materials, however. In fact, the change of the E parameters versus the bulk densities at the test load of 1.960 N is consistence with that of the E parameters under the 2.940 N applied load as a result of the totally dominant character of the plastic deformation. The long and short of it is that the Zr inclusions inserted in the superconducting system are inadequate for the applications of the Bi-2223 materials in engineering areas.
4 Conclusion
Throughout this study, the effect of the Zr impurities on the electrical, microstructural, mechanical and superconducting characteristics belonging to the Bi-2223 superconducting ceramics is examined by bulk density, dc resistivity, powder X-ray diffraction, scanning electron microscopy, electron dispersive X-ray and Vickers hardness experimental measurements. It is observed that the characteristic properties strongly depend on the Zr concentration level and the major findings to be inferred from this work are as follows:
Dc electrical resistivity findings show that the presence of the Zr impurities in the Bi-2223 crystal lattice significantly damages the metallic character and room temperature conductivity as a result of both the increased disorder of metallic interaction between the superconducting grains, and the decrement in the mobile hole carrier concentration in the Cu–O2 consecutively stacked layers (localization problem). In fact, the room temperature resistivity enhances nearly by four-fold from 3.69 until 13.16 mΩ cm. As for the superconducting temperature parameters, both the T onset c and especially T offset c values diminish considerably due to the increased artificial random defects, grain boundary weak-link interactions and distortion between superconducting slabs in the Bi-2223 crystal structure. The T onset c values are found to be about in the range from 110.8 K (for the pure sample) to 84.9 K (for the Zr6 compound) whereas the T offset c ones are obtained to regress from 108.7 to 40.8 K with the Zr concentration level in the system. This rapid degradation in the latter parameter leads to the retrogression in the degree of broadening as a consequence of the damage of homogeneities in the oxidation states.
-
As for the bulk density and porosity (degree of granularity) investigations, the density value decreases systematically from 6.127 to 5.624 g/cm3 with the Zr dopant due to the enhancement in the porosity, demonstrating that the intergrain coupling gets worse and worse.
-
The XRD measurement findings present that the Zr nanoparticles do not stabilize the high phase as a consequence of the reduction in the characteristic peak intensities. In this respect, the relative percentage of the Bi-2223 phase decreases from 91.6 until 52.4 %. In case of the doping level of x = 1.00, new characteristics peaks belonging to the ZrO2 tetragonal phase appear at 2θ = 30.24° and 50.46°. This is related to the fact that the solubility limit of the Zr impurities in the Bi-2223 crystal structure is about x = 0.70. Additionally, the XRD patterns verify the considerable decrement/increment in the c/a lattice cell parameter. The decrease in the former parameter stems from the extra valency in the oxygen deficient Bi–O planes and the required extra energy for the bond formation. On the other hand, the expansion of the latter parameter is due to the enhancement in the effective Cu valence in the Cu–O2 consecutively stacked layers. It is another crucial result obtained from the XRD patterns that the crystallite size tends to degrade robustly from 69.2 until 42.6 nm with the enhancement of the Zr nanoparticles. This confirms the undermining of the crystallinity and interaction between the grains.
-
Moreover, the SEM micrographs of the pure and Zr doped Bi-2223 superconducting materials illustrate that both the surface morphology (crystallinity, porosity, melting, voids, cracks and connectivity between the superconducting grains) and average crystallite distribution degrade significantly with the dopant.
-
Additionally, the EDX surveys display that the Zr inclusions in the tetravalent state may mostly be substituted for the divalent states of the Cu nanoparticles along with the crystal lattice. Thus, the differentiation of the electronegativity decreases the mobile hole concentration (per Cu ion) in copper oxide layers.
-
Moreover, the microhardness measurements demonstrate that although every sample prepared exhibits the typical Indentation Size Effect behavior due to the presence of both elastic and plastic deformations in the system, the H v values dramatically degrade with the Zr dopant concentration level, pointing out the suppression of the crystallinity and connection quality in the intergrain coupling. Even, this fact is favored by the changes in the Young’s modulus and yield strength parameters.
-
Based on the considerable findings, the Zr addition results in two trap levels for holes separated by a small energy interval. Namely, the metastability of the superconducting materials gets worse and worse with Zr foreign decorations in the Bi-2223 superconducting matrix.
References
O. Uzun, T. Karaaslan, M. Gögebakan, M. Keskin, J. Alloys Compd. 376, 149–157 (2004)
N. Güçlü, U. Kölemen, O. Uzun, S. Çelebi, Physica C Supercond. 433(1–2), 115–122 (2005)
J. Gong, H. Miao, Z. Zhao, Z. Guan, Mater. Sci. Eng A. 303, 179–186 (2001)
R. Tickoo, R.P. Tandon, K.K. Bamzai, P.N. Kotru, Mater. Sci. Eng. B 110(2), 177–184 (2004)
K. Sangwall, B. Surowska, P. Blaziak, ‘Mater. Chem. Phys. 80, 428–437 (2003)
O. Sahin, O. Uzun, U. Kölemen, B. Düzgün, N. Uçar, Chin. Phys. Lett. 22, 3137–3140 (2005)
N.K. Saritekin, M. Dogruer, G. Yildirim, C. Terzioglu, J Mater Sci: Mater Electron 25, 3127–3136 (2014)
N.K. Saritekin, Y. Zalaoglu, G. Yildirim, M. Dogruer, C. Terzioglu, A. Varilci, O. Gorur, J. Alloys Compounds. 610, 361–371 (2014)
M. Yilmazlar, Karadeniz Technical University, Institute of Natural Sciences, Ph.d. Thesis, (2002), Trabzon, Turkey
K. Çolakoğlu, Serway, vol. 3, Palme Press, p: 1318 (1996)
P. Bordet, C. Chailout, Nature 327, 687–691 (1987)
D.C. Harris, M.E. Hills, T.A. Hewston, J. Chem. Educ. 64, 847–850 (1987)
P.B. Allen, Y.E. Picket, H. Krakauer, Phy. Rev. B 37, 7482–7490 (1988)
S. Martin, M. Gurvitch, C.E. Rice, A.F. Hebard, P.L. Gammel, R.M. Fleming, A.T. Fiory, Phys. Rev. B 39, 9611–9613 (1989)
D.M. Newns, P.C. Pattnaik, C.C. Tsuei, Phys. Rev. B 43, 3075–3084 (1991)
M.B. Turkoz, S. Nezir, C. Terzioglu, A. Varilci, G. Yildirim, J. Mater. Sci: Mater. El. 24, 896–905 (2013)
S.B. Guner, O. Gorur, S. Celik, M. Dogruer, G. Yildirim, A. Varilci, C. Terzioglu, J. Alloy. Compd. 540, 260–266 (2012)
K. Kocabas, O. Ozkan, O. Bilgili, Y. Kadıoglu, H. Yılmaz, J. Supercond. Nov. Magn. 23, 1485–1492 (2010)
D. Mangapathi, T. Rao, V. Somaiah, Y.C. Haribabu, Venudhar. Cryst. Res. Technol. 28, 285–298 (1993)
A. Ianculescu, M. Gartner, B. Despax, V. Bley, R. Th Lebey, M.Modreanu Gavrila, Appl. Surf. Sci. 253, 344–348 (2006)
A.I. Abou-Aly, S.A. Mahmoud, R. Awad, M.M.E. Barakat, J. Supercond. Nov. Magn. 23, 1575–1588 (2010)
S. Vinu, P.M. Sarun, A. Biju, R. Shabna, P. Guruswamy, U. Syamaprasad, Supercond. Sci. Technol. 21, 045001–045005 (2008)
R. Shabna, P.M. Sarun, S. Vinu, A. Biju, U. Syamaprasad, Supercond. Sci. Technol. 22, 045016–045022 (2009)
R. Shabna, P.M. Sarun, S. Vinu, A. Biju, P. Guruswamy, U. Syamaprasad, J. Appl. Phys. 104, 013919 (2008)
R. Shabna, P.M. Sarun, S. Vinu, U. Syamaprasad, J. Alloy. Compd. 493, 11–16 (2010)
S. Bazargan, H. Javanmard, M. Akhavan, Physica C 466, 157–162 (2007)
G.I. Harus, A.I. Ponomarev, T.B. Charikova, A.N. Ignatenkov, L.D. Sabirzjanova, N.G. Shelushinina, V.F. Elesin, A.A. Ivanov, I.A. Rudnev, Physica C 383, 207–213 (2002)
P. Starowicz, J. Sokowski, M. Balanda, A. Szytua, Physica C 363, 80–90 (2001)
C. Nguyen-Van-Huong, C. Hinnen, J.M. Siffre, J. Mater. Sci. 32, 1725–1731 (1997)
Y. Zalaoglu, G. Yildirim, C. Terzioglu, O. Gorur, J. Alloy. Compd. 622, 489–499 (2010)
P.M. Sarun, S. Vinu, R. Shabna, A. Biju, U. Syamaprasad, J. Alloy. Compd. 472, 13–17 (2009)
A. Biju, P.M. Sarun, R.P. Aloysius, U. Syamaprasad, J. Alloy. Compd. 454, 46–51 (2008)
R.J. Sanderson, K.C. Hewitt, Physica C 425, 52–61 (2005)
A. Yildiz, K. Kocabas, G.B. Akyuz, J. Supercond. Nov. Magn. 25, 1459–1467 (2012)
M.T. Malachevsky, C.A. Dovidio, Supercond. Sci. Technol. 18, 289–293 (2005)
X. Yang, T.K. Chaki, Supercond. Sci. Technol. 6, 343–348 (1993)
O. Ozturk, E. Asikuzun, M. Erdem, G. Yildirim, O. Yildiz, C. Terzioglu, J. Mater. Sci: Mater. El. 23, 511–519 (2012)
Y. Zalaoglu, G. Yildirim, H. Buyukuslu, N.K. Saritekin, A. Varilci, C. Terzioglu, O. Gorur, J. Alloy. Compd. 631, 111–119 (2015)
K. Kocabas, M. Ciftcioglu, Phys. Status Solidi A 177, 539–545 (2000)
C.J. Poole, H.A. Farach, R. Creswick, Superconductivity (Academic Press, San Diego, 1995)
R.R. Reddy, M. Murakami, S. Tanaka, P.V. Reddy, Physica C 257, 137–142 (1996)
O. Ozturk, G. Yildirim, E. Asikuzun, M. Coskunyurek, M. Yilmazlar, A. Kilic, J. Mater. Sci: Mater. El. 24, 4643–4654 (2013)
A. Sedky, Physica C 468, 1041–1046 (2008)
M. Dogruer, C. Terzioglu, G. Yildirim, O. Gorur, J. Supercond. Nov. Magn. 27, 755–761 (2014)
E. Asikuzun, O. Ozturk, H.A. Cetinkara, G. Yildirim, A. Varilci, M. Yılmazlar, C. Terzioglu, J. Mater. Sci: Mater. El. 23, 1001–1010 (2013)
M. Dogruer, Y. Zalaoglu, G. Yildirim, A. Varilci, C. Terzioglu, J. Mater. Sci: Mater. El. 24, 2019–2026 (2013)
H.C. Ling, M.F. Yan, J. Appl. Phys. 64, 1307–1311 (1988)
Acknowledgments
This study is totally supported by Abant Izzet Baysal University Scientific Research Project Coordination Unit (Project No: 2015.09.05.824).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saritekin, N.K., Terzioglu, C., Pakdil, M. et al. Solubility limit of tetravalent Zr nanoparticles in Bi-2223 crystal lattice and evaluation of fundamental characteristic properties of new system. J Mater Sci: Mater Electron 27, 1854–1865 (2016). https://doi.org/10.1007/s10854-015-3964-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-015-3964-5