Abstract
Pure magnesium aluminate (MgAl2O4) nanoparticles were successfully synthesized by novel sol–gel method with the aid of Mg(NO3)2.6H2O, Al(NO3)3·9H2O, and starch without adding external surfactant. Moreover, starch plays role as capping agent, reducing agent, and natural template in the synthesis MgAl2O4 nanoparticles. The structural, morphological and optical properties of as obtained products were characterized by techniques such X-ray diffraction, energy dispersive X-ray microanalysis, scanning electron microscopy, and ultraviolet–visible spectroscopy. The sample indicated a ferromagnetic behavior, as evidenced by using vibrating sample magnetometer at room temperature. To evaluate the photocatalyst properties of nanocrystalline magnesium aluminate, the photocatalytic degradation of methyl orange under ultraviolet light irradiation was carried out.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nanoparticles have gained much attention among materials, because the nanocrystal properties not only depend on their composition but also depend on their size, shape, and size distribution [1–7]. Global industrialization (such as textile, refineries, leather, paper, chemical, and plastic industries) has used different types of dyes resulted in the release of large amounts of toxic compounds into environment [8]. Generally, 30–40 % of these dyes remain in the waste waters. Additionally, presence of these dyes diminishes the photosynthesis and causes many serious health problems for humanity. To overcome these problems, the waste water from those industries must be treated before their discharge. Various physical and chemical methods have been used for color removal from waste waters. One of these methods is semiconductor photocatalysis and it has proven to be an effective in treating waste water pollution since it is an environmentally friendly, low-cost, and sustainable treatment methodology [9–11]. The search for low cost and efficient photocatalysts is still continuing. Some spinel-type oxides such as BaCr2O4 [12], NiFe2O4 [13], CaBi2O4 [14], ZnGa2O4 [15], CuGa2O4 [16], ZnFe2O4 [17] and CuAl2O4 [18–20] used as photocatalysts are semiconductor materials with narrow band high and these materials have been proven to be an efficient in the degradation of pollutants and/or the production of photocatalytic hydrogen. Many methods for preparation of nano-sized spinels have been reported such as co-precipitation [21], sol–gel [14–18] sonochemical [16], microemulsion [12] and solution combustion [20]. However, combustion method has many advantages compared to these methods as will be mentioned latter. Additionally, in the combustion technique, nitrates are used as oxidizers, and some organic compounds such as glycine, sucrose, sorbitol, and others are used as fuel. In which the heat released due to the combustion reaction between the oxidizers and the fuel which is exothermic cause can the preparation of the target nanomaterials [21]. Magnesium aluminate spinel (MgAl2O4) is one of the most famous ceramic material type of metal-oxide with special properties such as high melting point (2135 °C), good thermal shock resistance, high mechanical strength, low thermal expansion coefficient, excellent resistance to acid and bases, and also having catalytic and optical properties [22, 23]. Due to these desirable properties, it found widely application in metallurgical, electrochemical, radiotechnical and chemical industries [24]. Thus, the preparation of magnesium aluminate powders with high purity, chemical homogeneity, control of stoichiometry, fine particle size, narrow particle size distribution, and minimum particle agglomeration with high sinter activity has received considerable attention in order to improve the material properties [25]. In many of applications, especially as catalyst support, there has been growing interest to meet the properties such as high surface area, small crystallite size and more active sites in the synthesis of MgAl2O4 spinel [26]. In recent years, various techniques such as wet chemical techniques and solid-state reaction method have been employed for the production of pure MgAl2O4 spinel powders. The fabrication of the high purity dense sintering body by conventional solid-state reaction method is often difficult [27] and high temperature calcinations, longer reaction time in this synthesis triggers the grain growth and hard agglomeration [28]. Also inhomogeneity, lack of stoichiometry control, high temperature is the disadvantages of solid-state routes. But various wet chemical techniques have been applied for the synthesis of pure spinel powders at relatively low temperatures to improve the sinterability and fabrication fine particles including hydroxide or carbonate salts co-precipitation, classical sol–gel route, spray drying, freezedrying, mechanochemical synthesis, hydrothermal, microwave-assisted combustion processing, microemulsion and etc. [29–36]. Among the wet chemical routes, Sol–gel technique has been used widely because it has the advantage of producing pure, ultrafine powders at low temperatures, High surface area and pore size distribution. In this report, for the first time, we had presented the preparation of MgAl2O4 nanoparticles by novel sol–gel method at 800 °C in the presence of starch without adding external surfactant. This approach is simple, low energy consumption and friendly to the environment. A green approach for MgAl2O4 nanoparticles synthesis by utilizing natural template permits the reaction to proceed usually in milder conditions. Although existing chemical approaches have effectively produced well-defined MgAl2O4 nanoparticles, these processes are generally costly and include the employ of toxic chemicals. The photocatalytic degradation was investigated using methyl orange (MO) under ultraviolet light irradiation [37, 38].
2 Experimental
2.1 Characterization
Magnesium nitrate hexahydrate (Mg(NO3)2·6H2O), aluminium nitrate nonahydrate (Al(NO3)3·9H2O), were purchased from Merck Company and used without further purification. X-ray diffraction (XRD) patterns were recorded by a Philips-X’PertPro, X-ray diffractometer using Ni-filtered Cu Kα radiation at scan range of 10 < 2θ < 80. Scanning electron microscopy (SEM) images were obtained on LEO-1455VP equipped with an energy dispersive X-ray spectroscopy. The EDS analysis with 20 kV accelerated voltage was done. The magnetic measurement of samples were carried out in a vibrating sample magnetometer (VSM) (Meghnatis Daghigh Kavir Co.; Kashan Kavir; Iran) at room temperature in an applied magnetic field sweeping between ±10,000 Oe. UV–Vis diffuse reflectance spectroscopy analysis (UV–Vis) was carried out using Shimadzu UV–Vis scanning spectrometer.
2.2 Synthesis of MgAl2O4 nanoparticles
At first, 1.80 g of Mg(NO3)2·6H2O was dissolved in 50 mL of distilled water. Then, 6.08 of starch was subsequently added to the above solution under magnetic stirring at 70 °C for 30 min. Afterwards, 5.28 g of Al(NO3)3·9H2O was dissolved in 50 mL of distilled water and was added to the above solution under magnetic stirring. A solution was obtained and further heated at 100 °C for 1 h to remove excess water. During continued heating at 110 °C for 1 h, the solution became more and more viscous to become a gel. Finally, the obtained product was calcinated at 800 °C for 2 h in a conventional furnace in air atmosphere and then cooled it to room temperature.
2.3 Photocatalytic experimental
The methyl orange (MO) photodegradation was examined as a model reaction to evaluate the photocatalytic activities of the magnesium aluminate nanoparticles. The photocatalytic experiments were performed under an irradiation ultraviolet light. The photocatalytic activity of nanocrystalline MgAl2O4 obtained was studied by the degradation of methyl orange solution as a target pollutant. The photocatalytic degradation was performed with 50 mL solution of methyl orange (0.0005 g) containing 0.1 g of MgAl2O4. This mixture was aerated for 30 min to reach adsorption equilibrium. Later, the mixture was placed inside the photoreactor in which the vessel was 15 cm away from the visible source of 400 W mercury lamps. The photocatalytic test was performed at room temperature. Aliquots of the mixture were taken at definite interval of times during the irradiation, and after centrifugation they were analyzed by a UV–vis spectrometer. The methyl orange (MO) degradation percentage was calculated as:
where A0 and A are the absorbance value of solution at A0 and A min, respectively.
3 Results and discussion
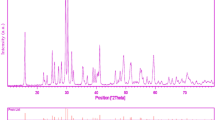
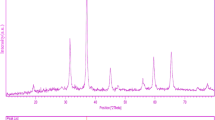
Figure 1 shows a typical XRD pattern (10° < 2θ < 80°) of MgAl2O4 nanoparticles. Based on the Fig. 1, the diffraction peaks can be indexed to pure cubic phase of MgAl2O4 (face-centered cubic, Fd–3 m with lattice size of 8.0831°A, JCPDS No. 73-1959). No other crystalline phases were detected. From XRD data, the crystallite diameter (Dc) of MgAl2O4 nanoparticles was calculated to be 33 nm using the Scherer equation:
where β is the breadth of the observed diffraction line at its half intensity maximum (400), K is the so-called shape factor, which usually takes a value of about 0.9, and λ is the wavelength of X-ray source used in XRD. The morphology of the MgAl2O4 nanoparticles has been examined by SEM image (Fig. 2). According to the Fig. 2, it is seen that the products composed of small spherical shapes nanoparticles with average size of about 50 nm. The EDS analysis measurement was used to investigate the chemical composition and purity of MgAl2O4 nanoparticles. According to the Fig. 3, the product consists of Mg, Ti, and O elements. Furthermore, neither N nor C signals were detected in the EDS spectrum, which means the product is pure and free of any capping agent or impurity. The hysteresis loop of MgAl2O4 nanoparticles was studied to examine their magnetic properties (Fig. 4). At 300 K the remanent magnetization (Mr) is 0.001 emu/g, the coercive field (Hc) is 82 Oe and the magnetization at saturation (Ms) is estimated to be only .006 emu/g (the saturation magnetization Ms was determined from the extrapolation of curve of H/M vs H). The room temperature UV–vis absorption spectra of MgAl2O4 nanoparticles were also measured in the range of 200–600 nm. Figure 5a shows the diffuse reflection absorption spectra of the MgAl2O4 nanoparticles calcinled at 800 °C. The figure indicates that the MgAl2O4 nanoparticles shows absorption maxima at 337 nm, the direct optical band gap estimated from the absorption spectra for the MgAl2O4 nanoparticles is shown in Fig. 5b. An optical band gap is obtained by plotting (αhν)2 versus hν where α is the absorption coefficient and hν is photon energy. Extrapolation of the linear portion at (αhν)2 = 0 gives the band gaps of 2.85 eV for MgAl2O4 nanoparticles. Photodegradation of methyl orange under UV light irradiation (Fig. 6a–c) was employed to evaluate the photocatalytic activity of the as-synthesized MgAl2O4. No methyl orange was practically broken down after 80 min without using UV light irradiation or nanocrystalline MgAl2O4. This observation indicated that the contribution of self-degradation was insignificant. The probable mechanism of the photocatalytic degradation of methyl orange can be summarized as follows:
Using photocatalytic calculations by Eq. (1), the methyl orange degradation was about 50 % after 80 min irradiation of UV light, and nanocrystalline MgAl2O4 presented high photocatalytic activity (Fig. 6a). The spectrofluorimetric time-scans of methyl orange solution illuminated at 510 nm with nanocrystalline MgAl2O4 are depicted in Fig. 6b. Figure 6b shows continuous removal of methyl orange on the MgAl2O4 under UV light irradiation. It is generally accepted that the heterogeneous photocatalytic processes comprise various steps (diffusion, adsorption, reaction, and etc.), and suitable distribution of the pore in the catalyst surface is effective and useful to diffusion of reactants and products, which prefer the photocatalytic reaction. In this investigation, the enhanced photocatalytic activity can be related to appropriate distribution of the pore in the nanocrystalline MgAl2O4 surface, high hydroxyl amount and high separation rate of charge carriers (Fig. 6c). Furthermore, this route is facile to operate and very suitable for industrial production of MgAl2O4 nanoparticles. The synthesis pathway of MgAl2O4 nanoparticles is shown in Scheme 1.
a Photocatalytic methyl orange degradation of MgAl2O4 nanoparticles under ultraviolet light, b fluorescence spectral time scan of methyl orange illuminated at 510 nm with MgAl2O4 nanoparticles and c reaction mechanism of methyl orange photodegradation over MgAl2O4 nanoparticles under ultraviolet light irradiation
4 Conclusions
In this work, magnesium aluminate nanoparticles were successfully synthesized by a novel sol–gel method at 800 °C for 120 min. The stages of the formation of MgAl2O4, as well as the characterization of the resulting compounds were done using X-ray diffraction and energy dispersive X-ray spectroscopy. The products were analyzed by scanning electron microscopy (SEM), and ultraviolet–visible (UV–Vis) spectroscopy to be round, about 50 nm in size and Eg = 2.85 eV. VSM analyzes indicates a ferromagnetic behavior for the synthesized nanoparticles. When as-prepared nanocrystalline magnesium aluminate was utilized as photocatalyst, the percentage of methyl orange degradation was about 50 % after 80 min irradiation of UV light.
References
M. Rahimi-Nasarabadi, J. Nanostruct. 4, 211 (2014)
F. Beshkar, M. Salavati-Niasari, J. Nanostruct. 5, 17 (2015)
M. Ahmadzadeh, M. Almasi-Kashia, A. Ramazani, J. Nanostruct. 5, 97 (2015)
F.S. Ghoreishi, V. Ahmadi, M. Samadpourc, J. Nanostruct. 3, 453 (2013)
A. Rahdar, M. Aliahmad, Y. Azizi, J. Nanostruct. 5, 145 (2015)
E. Khosravifard, M. Salavati-Niasari, M. Dadkhah, G. Sodeifian, J. Nanostruct. 2, 191 (2010)
M. Najafi, H. Haratizadeh, M. Ghezellou, J. Nanostruct. 5, 129 (2015)
J. Safaei-Ghomi, S. Zahedi, M. Javid, M.A. Ghasemzadeh, J. Nanostruct. 5, 153 (2015)
J.S. Piccin, C.S. Gomes, L.A. Feris, M. Gutterres, Chem. Eng. J. 183, 30 (2012)
M.N. Chong, B. Jin, C.W.K. Chow, C. Saint, Water Res. 44, 2997 (2010)
A.A. Firooz, A.R. Mahjoub, A.A. Khodadadi, M. Movahedi, Chem. Eng. J. 165, 735 (2010)
D.F. Wang, Z.G. Zou, J.H. Ye, Chem. Phys. Lett. 373, 191 (2003)
Z.R. Zhu, X.Y. Li, Q.D. Zhao, H. Li, Y. Shen, G.H. Chen, Chem. Eng. J. 165, 64 (2010)
J.W. Tang, Z.G. Zou, J.H. Ye, Angew. Chem. Int. Ed. 43, 4463 (2004)
V.B.R. Boppana, D.J. Doren, R.F. Lobo, Chemsuschem 3, 814 (2010)
K. Gurunathan, J.O. Baeg, S.M. Lee, E. Subramanian, S.J. Moon, K.J. Kong, Int. J. Hydrogen Energ. 33, 2646 (2008)
S.W. Cao, Y.J. Zhu, G.F. Cheng, Y.H. Huang, J. Hazard. Mater. 171, 431 (2009)
Y.Y. Jiang, J.G. Li, X.T. Sui, G.L. Ning, C.Y. Wang, X.M. Gu, J. Sol-Gel. Sci. Technol. 42, 41 (2007)
D.S. Mathew, R.S. Juang, Chem. Eng. J. 129, 51 (2007)
W.Z. Lv, B. Liu, Q. Qiu, F. Wang, Z.K. Luo, P.X. Zhang, S.H. Wei, J. Alloys Compd. 479, 480 (2009)
D. Kovacheva, H. Gadjov, K. Petrov, S. Mandal, M.G. Lazarraga, L. Pascual, J. Mater. Chem. 12, 1184 (2002)
M. Sindel, N.A. Travitzky, N. Claussen, J. Am. Ceram. Soc. 73, 2615 (1990)
H. Zhang, X. Jia, Y. Yan, Z. Liu, D. Yang, Z. Li, Mater. Res. Bull. 39, 839 (2004)
X. Su, X. Du, S. Li, J. Li, J. Nanopart. Res. 12, 1813 (2010)
B. Alinejad, H. Sarpoolaky, A. Beitollahi, A Saberi, S.H. Afshar, Mater. Res. Bull. 43, 1188 (2008)
J.J. Guo, H. Lou, H. Zhao, D. Chai, X. Zheng, Appl. Catalyst A: Gen. 273, 75 (2004)
P.Y. Lee, H. Suematsu, T. Yano, K. Yatsui, J. Nanopart. Res. 8, 911 (2006)
D. Domanski, G. Urretavizcaya, F. Castro, F. Gennari, J. Am. Ceram. Soc. 87, 2020 (2004)
M.M. Rashad, Z.I. Zaki, H. El-Shall, J. Mater. Sci. 44, 2992 (2009)
Z. Haijun, J. Xiaolin, L. Zhanjie, L. Zhenzhen, J. Mater. Lett. 58, 1625 (2004)
X.L. Pan, S.S. Sheng, G.X. Xiong, K.M. Fang, S. Tudyka, N. Stroh, H. Brunner, Coll. Surf. A: Physicochem. Eng. Asp. 179, 169 (2001)
C.T. Wang, L.S. Lin, S.J. Yang, J. Am. Ceram. Soc. 75, 2240 (1992)
K. MacKenzie, J. Temuujin, T. Jadamba, M. Smith, P. Angerer, J. Mater. Sci. 35, 5529 (2000)
X. Zhang, J. Mater. Ch. Ph. 116, 415 (2009)
J. Bai, J. Liu, Ch. Li, G. Li, Q. Du, J. Adv. Pow. Tech. 22, 72 (2011)
F. Meyer, A. Dierstein, Ch. Beck, W. Hrtl, R. Hempelmann, S. Mathur, M. Veith, J. Nanostruct. Mater. 12, 71 (1999)
M. Riazian, J. Nanostruct. 4, 433 (2014)
G. Nabiyouni, D. Ghanbari, S. Karimzadeh, B. Samani Ghalehtaki, J. Nanostruct. 4, 467 (2014)
Acknowledgments
Authors are grateful to council of University of Borujerd for providing financial support to undertake this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Talebi, R., Khademolhoseini, S. & Rahnamaeiyan, S. Preparation and characterization of the magnesium aluminate nanoparticles via a green approach and its photocatalyst application. J Mater Sci: Mater Electron 27, 1427–1432 (2016). https://doi.org/10.1007/s10854-015-3907-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-015-3907-1