Abstract
The microstructure and electrical properties of ZnO varistor ceramics with different ZrO2 content prepared by a solid reaction route and sintered at 1100 °C were investigated. The microstructures of the varistor ceramics samples were characterized by X-ray diffraction and scanning electron microscopy; the electrical properties and current–voltage (V–I) characteristics of the varistor ceramics were investigated by DC parameter instrument. The microstructure of the prepared samples shows a decrease in grain size of ZnO phase with the ZrO2 content increase. ZrO2-doped ZnO varistor ceramics exhibit comparatively vastly superior comprehensive electrical properties with addition of 0.50 mol% ZrO2, such as the threshold voltage is 350 V/mm, the nonlinear coefficient is 25.4 and the leakage current is 2.96 μA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The ZnO varistor ceramics are extensively employed in electronic devices manufacture and especially in high voltage transmission as voltage surge protector due to its excellent nonlinear V–I characteristics and strong energy absorption ability [1–11]. ZrO2, characterized by high hardness, better wearing quality and longer service life, can largely reduce the waste of material grinding. Kim et al. [12] studied microstructure and electrical properties of ZnO varistor ceramics under different ZrO2 content and the sintering temperature. Doping with certain ZrO2 improved comprehensive electrical properties of ZnO varistor ceramics. Wang [13] studied voltage-sensitive and dielectric properties of Zr-doped SnO2 ceramics. The mechanism of the decrease of SnO2 grain size with the increase of ZrO2 concentration was discussed.
Considering that little research works of modifying the ZnO varistor ceramics by doping with ZrO2 have even been done, furthermore, Zr4+ ion (0.080 nm) is close to Zn2+ ion (0.074 nm) and doping with certain ZrO2 is very likely to exhibit unique advantages of ZnO varistor ceramics, the present work focus on the experimental investigations of the effect of ZrO2 doping on the microstructure and electrical properties by a solid reaction route. Effects of various ZrO2 content doping on the microstructure and the electrical properties of the ZnO varistor ceramics were investigated and some new results were obtained, which supply some theoretical foundation for ZrO2-doped ZnO varistor ceramics.
2 Experimental
In this paper, ZrO2 doped ZnO varistors were prepared by the solid reaction method. The samples were labeled Z0C, Z1C, Z2C, Z3C and Z4C corresponding to the different ZrO2 content x = 0, 0.50, 1.00, 1.50, and 2.00. The accurate weighted raw material (96.50 − x) mol% ZnO, 0.70 mol% Bi2O3, 1.00 mol% Sb2O3, 0.80 mol% Co2O3, 0.50 mol% Cr2O3, 0.50 mol% MnO2, and x mol% ZrO2 were milled in ethanol in a polyethylene jar for 5 h in planetary ball mill operated at 500 r/min using zirconia balls as the milling media. The mixture was dried at 70 °C for 24 h after adequate milling. The dried powder was pilled in agate mortar with the addition of 2 wt% polyvinyl alcohol binder. The powder was uniaxially pressed into pellets of 12 mm in diameter and 2 mm in thickness. The pressed pellets were sintered in air at 1100 °C for 2 h. The heating rate is 5 °C/min and the sintered the pellets were cooled inside of furnace.
The crystal structure and microstructure characteristics were investigated by X-ray diffraction (XRD, Rigaku D/max 2200, Japan) and scanning electron microscope (SEM, FEI QUANTA 400) respectively. The density of the pellets (D) was measured geometrically [14–16].
For better conducting, the silver electrodes were placed on both surfaces of the samples. The size of electrodes was 5 mm in diameter. The voltage–current (V–I) characteristics were measured using V–I source/measure unit (CJP CJ1001, China). The nominal varistor voltages (V N) at 0.1 mA (V 0.1 mA) and 1 mA (V 1 mA) were measured [17–22]. The threshold voltage (V T, V/mm), which was the breakdown voltage per unit thickness of varistor ceramic, was obtained from V T = V 1 mA/d, where d was the thickness of the sample in mm. The leakage current (I L) was measured at 0.75 V 1 mA. The nonlinear coefficient (α) was determined by the equation α = 1/lg (V 1 mA/V 0.1 mA).
3 Results and discussion
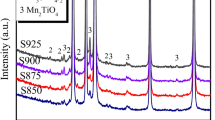
XRD patterns of ZrO2-doped ZnO varistor ceramics samples sintered at 1100 °C for 2 h are given in Fig. 1. The samples consist typically of three phases: ZnO phase, Bi-rich phase and Zn7Sb2O12 spinel phase [23, 24]; ZnO is the predominant phase [25]. Throughout the studied there are no fundamental differences in the phase evolution among the samples. The diffraction peaks of ZrO2 are not observed maybe because the amount of dopant is so little that they only account for a small fraction of the overall composition.
Figure 2 shows the SEM micrographs of the sample without addition of ZrO2 and the samples doped with different amounts of ZrO2 sintering at 1100 °C for 2 h. Those varistor ceramics samples are composed of ZnO phase, Bi-rich phase and Zn7Sb2O12 spinel phase, as determined by XRD analysis (Fig. 1). It can be seen clearly from Fig. 2 that due to the addition of ZrO2, the grain size of ZnO decreases greatly. Maybe when ZrO2 doped in large quantities, a part of the ZrO2 are dissolved in solid solution with ZnO grains. Another part of the ZrO2 presents at the grain boundaries and strongly inhibits the growth of ZnO grain.
Figure 3 shows the relative density values of ZrO2-doped ZnO varistor ceramics sintered at sintering temperature of 1100 °C for 2 h. The results represent that the sintered density of the varistor ceramics samples apparently increases and reaches the maximum value of 99 %. The most probable reason is that Zr4+ ion (0.080 nm) has a slightly larger radius than Zn2+ ion (0.074 nm). What’s more, both of the electro negativities is equivalent (relative electro negativities are 1.4 and 1.6, respectively).Thus, the secondary phase tends to form solid solution, and that is, Zr is dissolved in solid solution with ZnO grains. It results in decreasing of surface energy and chemical potential and the sintering driving force increases which leads to the densification speeding up. So, density increases with the increase of the ZrO2 content.
Figure 4 shows the leakage current of ZrO2-doped ZnO varistor ceramics samples sintered at 1100 °C for 2 h. From Fig. 4 we can find that the leakage current increases with the increase of ZrO2 content dramatically. The reason is that electric ZrO2 is filled in considerably between the ZnO grains. When applying voltage, part circuits form the pathway.
The variation of the threshold voltage of ZrO2-doped ZnO varistor ceramics samples sintered at 1100 °C for 2 h is shown in Fig. 5. It is observed that the threshold voltage firstly increases and then decreases with the increase of ZrO2 content. It reaches the maximum value of 380 V/mm at the ZrO2 content of 1.00 mol%.
As shown in Fig. 6, the nonlinear coefficients of ZrO2-doped ZnO varistor ceramics samples decrease with increase in ZrO2 content at sintering temperatures of 1100 °C for 2 h. The nonlinear coefficient as a factor of characterizing nonlinearity of a varistor, decreases with the increase of doped concentration. Its value varies from a maximum of 29.5 to a minimum of 2.1. It is seen that an increase of ZrO2 content deteriorates the nonlinear properties.
Figure 7 presents electric field–current density (E–J) curves of ZrO2-doped ZnO varistor ceramics samples sintered at 1100 °C for 2 h. The curves show that conduction characteristics are divided into two regions, namely, a linear region before the breakdown field and a nonlinear region after the breakdown field. The sharper the knee of the curves between the two regions, the better the nonlinear property is [5, 14]. In Fig. 7, E–J curves show that potential gradient and mutation degree gradually decrease, which indicate that the nonlinear coefficients of ZrO2-doped ZnO varistor ceramics samples gradually decrease and it is agree with the results of Fig. 6. When doped with 2.0 mol% ZrO2, E–J curves are almost linear and ZnO varistor ceramics present pure resistance properties.
4 Conclusions
The microstructure and electrical properties of ZnO varistor ceramics doped with different ZrO2 contents were investigated. The XRD and SEM of the samples show the presence of ZrO2. As the doping content increases, the grain size of ZnO decreases. At the same time, the nonlinear coefficient decreases. When the amount of ZrO2 is more than 0.50 mol%, doping with ZrO2 reduced its comprehensive electrical properties. The nonlinear V–I characteristics with the leakage current is 2.96 μA, the threshold voltage is 350 V/mm and the nonlinear coefficient is 25.4 could be achieved when the amount of ZrO2 is 0.50 mol% which present comparatively ideal comprehensive electrical properties of ZrO2-doped ZnO varistor ceramics.
References
P. Duran, F. Capel, J. Tartaj, C. Moure, Adv. Mater. 14(2), 137 (2002)
D. Xu, L.Y. Shi, Z.H. Wu, Q.D. Zhong, X.X. Wu, J. Eur. Ceram. Soc. 29(9), 1789 (2009)
D. Xu, X.N. Cheng, M.S. Wang, L.Y. Shi, Adv. Mat. Res. 79–82, 2007 (2009)
D. Xu, J.T. Wu, L. Jiao, H.X. Xu, P.M. Zhang, R.H. Yu, X.N. Cheng, J. Rare Earth 31(2), 158 (2013)
D. Xu, X.N. Cheng, H.M. Yuan, J. Yang, Y.H. Lin, J. Alloy. Compd. 509(38), 9312 (2011)
L.H. Cheng, G.R. Li, K.Y. Yuan, L. Meng, L.Y. Zheng, J. Am. Ceram. Soc. 95(3), 1004 (2012)
C. Huan, F. Gang, Solid State Electron. 67(1), 27 (2012)
S.A. Ansari, A. Nisar, B. Fatma, W. Khan, A.H. Naqvi, Mater. Sci. Eng. B Adv. 177(5), 428 (2012)
H. Bastami, E. Taheri-Nassaj, P.F. Smet, K. Korthout, D. Poelman, J. Am. Ceram. Soc. 94(10), 3249 (2011)
J.S. Park, Y.H. Han, K.H. Choi, J. Mater. Sci. Mater. El. 16(4), 215 (2005)
G.H. Chen, J.L. Li, X. Chen, X.L. Kang, C.L. Yuan, J. Mater. Sci. Mater. El. 26(4), 2389 (2015)
C.H. Kim, J.H. Kim, J. Eur. Ceram. Soc. 24(8), 2537 (2004)
Z.J. Wang, Z.Y. Li, L. Liu, X.R. Xu, H.N. Zhang, W. Wang, W. Zheng, C. Wang, J. Am. Ceram. Soc. 93(3), 634 (2010)
D. Xu, X.N. Cheng, G.P. Zhao, J.A. Yang, L.Y. Shi, Ceram. Int. 37(3), 701 (2011)
Z.H. Wu, J.H. Fang, D. Xu, Q.D. Zhong, L.Y. Shi, Int. J. Min. Met. Mater. 17(1), 86 (2010)
D. Xu, Q. Song, K. Zhang, H.X. Xu, Y.T. Yang, R.H. Yu, J Inorg Mater 28(11), 1270 (2013)
D. Xu, D.M. Tang, L. Jiao, H.M. Yuan, G.P. Zhao, X.N. Cheng, J. Cent, South Univ. 19(8), 2094 (2012)
D. Xu, D.M. Tang, L. Jiao, H.M. Yuan, G.P. Zhao, X.N. Cheng, T. Nonferr, Metal Soc. 22(6), 1423 (2012)
X. Dong, H. Kai, R.H. Yu, X.J. Sun, Y.T. Yang, H.X. Xu, H.M. Yuan, J. Ma, Mater. Chem. Phys. 153, 229 (2015)
H. Kai, L. Yun, R.H. Yu, J.P. Qi, X.J. Sun, Y.T. Yang, H.X. Xu, J. Ma, D. Xu, Mater. Res. Bull. 69, 98 (2015)
D. Xu, C. Zhang, Y.H. Lin, L. Jiao, H.M. Yuan, G.P. Zhao, X.N. Cheng, J. Alloy. Compd. 522, 157 (2012)
D. Xu, C. Zhang, X.N. Cheng, Y.E. Fan, T. Yang, H.M. Yuan, Adv. Mater. Res Switz. 197–198, 302 (2011)
I.O. Ozer, E. Suvaci, S. Bernik, Acta Mater. 58(12), 4126 (2010)
L. Saint Macary, M.L. Kahn, C. Estournes, P. Fau, D. Tremouilles, M. Bafleur, P. Renaud, B. Chaudret, Adv. Funct. Mater. 19(11), 1775 (2009)
J.L. He, J. Liu, J. Hu, W.C. Long, Mater. Lett. 65(17–18), 2595 (2011)
Acknowledgments
This work was financially supported by National Natural Science Foundation of China (Grant No. 51572113), Cooperative Innovation Foundation of Jiangsu Province (BY2015064-04), Jiangsu Government Scholarship for Overseas Studies (JS-2013-097) and Changzhou science and technology Program (CQ20140006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, D., He, K., Jiao, L. et al. Microstructure and electrical properties of ZrO2-doped ZnO varistor ceramics. J Mater Sci: Mater Electron 27, 767–771 (2016). https://doi.org/10.1007/s10854-015-3814-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-015-3814-5