Abstract
This work reports the study on magnetic properties of Mg1−xFexO (x = 0, 0.05 and 0.07) nanoparticles prepared by the polymer pyrolysis method. Phase, valence states and magnetic properties of these samples were characterized using X-ray diffraction (XRD), transmission electron microscopy, X-ray absorption near edge structure (XANES) and vibrating sample magnetometer (VSM), respectively. The XRD results show that the samples have a phase nature of the cubic structure with constant crystallite size of 25 ± 2 nm. XANES spectra reveal the existence of Fe3+ ions in the samples. VSM measurements exhibit the room temperature ferromagnetism with a maximum magnetization of 1.60 emu/g for x = 0.07. The origin of ferromagnetism is fitted on the bound magnetic polaron (BMP) model to indicate the numbers of BMPs involving overlapping of polarons via oxygen vacancy defects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Oxide-based dilute magnetic semiconductors (O-DMSs) and insulator oxides which can exhibit a ferromagnetic (FM) with Curie temperature (T c) above room temperature (RT), have attracted much due to its potential application in spintronic devices [1, 2]. These O-DMSs and insulating such as ZnO, TiO2, SnO2, In2O3, CeO2, CaO or MgO display RT-FM with just a few percent of a transition-metal (TM) doping such as V, Cr, Mn, Fe, Co or Ni when grown as thin film [3–6] or in powder form [7, 8], etc. Several models of RT-FM have been proposed for these semiconductors and insulators, including a new exchange mechanism involving donor electrons in an impurity, the carrier (electron) mediated exchange mechanism and F-center exchange (FCE) as on defect mediated mechanism, especially oxygen vacancies (VO) [7, 9, 10]. Some experimental and theoretical results report that the VO plays an important role in the magnetic origin for wide band gap oxides/insulators [11]. However, this is still not well understood and a unified framework capable of explaining this is needed.
Magnesium oxide (MgO) is an insulator with a wide band gap of 7.8 eV and exhibit diamagnetism in bulk form. Early work on MgO based was focused on nanocrystalline form due to the capability in the magnetic property improvement. For example, the RT-FM can be observed in nanocrystalline MgO with particle size of ~10–50 nm [11–14]. The authors suggested that this behavior was attributed to the Mg vacancy defect at the surface of the nanoparticles. Recently, Maoz et al. [15] also found RT-FM in MgO nanosheet, which originated from VO. This suggested that the RT-FM in this sample could be necessarily induced by Mg vacancy and/or VO based on FCE. Further, the substitution on Mg ions by various transition metal such as Mg1−xFexO [16], Mg1−xNixO [17], Mg1−xMnxO [18] and Mg1−xCrxO [19] can improve the magnetic properties. Therefore, it is of great interest to study the magnetic properties in nanocrystalline Mg1−xFexO powders as promising ferromagnetic materials for the understanding of future spintronic devices.
In this work, we report the magnetic properties of Fe-doped MgO (Mg1−xFexO, x = 0, 0.05 and 0.07) nanoparticles prepared by the polymer pyrolysis method. The crystallinity, morphology, and valence states of Mg1−xFexO samples were characterized by X-ray diffraction (XRD), transmission electron microscopy (TEM) and X-ray absorption near edge spectroscopy (XANES). The magnetic properties of Mg1−xFexO samples were investigated using a vibrating sample magnetometer (VSM).
2 Experimental
Nanoparticles of Fe-doped MgO (Mg1−xFexO, x = 0, 0.05 and 0.07) were synthesized by the polymer pyrolysis method according to our previous work [20]. 0.006 mol of Mg(NO3)2·6H2O (Aldrich), Fe(NO3)3·9H2O (Kanto) and 70 ml of acrylic acid (C3H4O2) were dissolved in deionized water to promote the polymerization. The precursor solution was stirred and heated at 30 °C until it dried. Then the final product was pre-calcined in a furnace at 400 °C for 2 h to form the polyacrylate salt. The precursor was then calcined at a temperature of 700 °C for 3 h in air to obtain the cubic phase of MgO.
The prepared samples were characterized using XRD, XANES and VSM. Bruker D2 Phaser X-ray diffractometer with Cu Kα radiation (λ = 0.154184 nm) was used to study the phases of the Mg1−xFexO samples. The morphology and crystal structure of the samples were performed using TEM (TEM Ziess, EM902, Germany). XANES spectra of Fe K edges were studied using X-ray absorption near edge spectroscopy in transmission mode at the BL5.2 line at Siam Photon (Synchrotron Research Institute) in Nakhon Ratchasima, Thailand. The magnetic measurements were performed at room and low temperature using a vibrating sample magnetometer (Versa Lab VSM, Quantum Design) in the field cooling (FC) mode with an applied field of 500 Oe.
3 Results and discussion
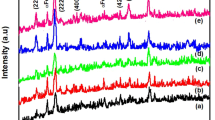
Figure 1 shows the XRD patterns of Mg1−xFexO nanoparticles with x = 0, 0.05 and 0.07. All samples exhibit peaks consistent with the cubic structure of MgO in the standard JCPDS 78-0430 and the diffraction peak corresponding to the impurity phase of Fe3O4 is observed in sample of x = 0.07. It also shows that Fe ions might have been substituted into the MgO lattice due to no impurity phase of Fe3O4 in the sample of x = 0.05. The Rietveld analysis confirms the cubic structure [space group Fm-3m (225)] of all samples, as shown in Fig. 2. The average crystallite sizes (D) of all samples were calculated from the X-ray line broadening using Scherrer’s equation. The crystallite size of 25 ± 2 nm is remained with increasing Fe content (as listed in Table 1). The effect of Fe doping on the lattice parameter (a) as obtained from the Rietveld analysis is observed to be decreased from 0.4217(4) to 0.4213(4) nm with increasing Fe content, as shown in Table 1. This is possibly due to the replacement of the larger Mg2+ ions (0.89 Å) by the smaller Fe3+ ions (0.78 Å). This causes a change in the Mg–O bond length (lattice distortion) and the overall lattice parameter, which is in good agreement with earlier reports [18, 21].
Refined X-ray diffraction patterns of Mg1−xFexO nanoparticle calcined at 700 °C for samples with a x = 0, b x = 0.05 and c x = 0.07. The experimental data are indicated by the dots and the calculated data by the solid line overlaying them; the bottom curve shows the difference between the experimental and calculated data
The morphology and structure of Mg1−xFexO nanoparticles are investigated by TEM with the selected area electron diffraction (SAED) patterns, as shown in Fig. 3. TEM bright field images show aggregated nanoparticles. The average particle size of the samples was evaluated from the histogram in the inset of Fig. 3 to be 25 ± 1 nm, which is consistent with the XRD results. It can be also seen from the inset in Fig. 3 that a single particle of the samples is in the range of 24–26 nm. The SAED patterns of the samples showed the spotty ring patterns suggesting a formation of polycrystalline structure. This also agrees well with the XRD results.
The valence states of Fe in Mg1−xFexO samples are obtained from the XANES measurements. The XANES spectra at Fe K-edge were measured at room temperature. Figure 4 shows edge energies of the Fe foil, FeO (Fe2+) standard, Fe3O4 (Fe2+, Fe3+) standard, Fe2O3 (Fe3+) standard, and Mg1−xFexO samples with (a) x = 0.05 (b) x = 0.07. The structure of shift of the edge position can be used to determine the valence state of Fe in the Mg1−xFexO samples. From Fig. 3, the edge position of FeO (Fe2+) standard is approximately 7120 eV, while that of Fe3O4 (Fe2+, Fe3+) standard is approximately 7122 eV. In addition, edge position of Fe2O3 (Fe3+) standard is approximately 7124 eV. These can be simply used as a fingerprint of phases and valence states. It is seen that the edge positions of all samples are quite similar to that of the Fe3+ standard, indicating the Fe3+ valence state for most of the Fe ions in all samples.
Figure 5a shows the magnetic field dependence of the specific magnetization (M–H curve) obtained from VSM measurements for MgO nanoparticles measured from 50 to 300 K (room temperature, RT) with magnetic field (H) in the range of ±30 kOe. Each sample exhibits ferromagnetism (FM) component with large hysteresis loop and diamagnetic increased at higher H. Therefore, the magnetization (M) is subtracted in terms of the Eq. (1):
where χ p is the slope at high H and then saturated magnetization (M S) from subtraction is M f = M − χ p H [22]. The inset shows the subtraction of MgO sample measured at 300 K with the M S value of 0.035 emu/g (as summarized in Table 1). The sample shows well FM and largest hysteresis loop measured at 50 K as shown in Fig. 5b. The M S value decreases with increasing measuring temperature, due to the thermal fluctuations causing the randomization of polarization direction. However, the slight decrease of M S measured at 300 K compared to that measured at 50 K can contribute to the Curie temperature (T C) above 300 K. This observed T C corresponds to the obtained result from field cooling (FC) curve, as shown in the Fig. 7. This observed ferromagnetism in this MgO nanoparticle is of great interest because bulk MgO is diamagnetic ordering [23]. Based on previous analysis of FM component in MgO nanoparticles, it is suggested that this FM behavior results from the vacancy defects [e.g., oxygen vacancies (VO) and/or Mg vacancies (VMg)] at the surfaces of the nano-crystals [18, 24, 25]. The M s value of 0.035 emu/g (~25 nm in size) of our MgO nanoparticles is much higher than that of 0.0026 emu/g for MgO nanoparticles (~11.9 nm) synthesized by precipitation method [18] and the value of 0.025 emu/g for MgO nanoparticles (~20 nm) synthesized by co-precipitation method [24]. The difference in the M value of MgO materials depends on the preparation conditions as originated from vacancy defects at the surface of nanoparticles. This behavior has been also observed in other oxides as well as in ZnO [26–28], TiO2 [29], and CeO2 [30]. Therefore, for ferromagnetism in oxides, the defects are also the important effect.
The M–H curves obtained from VSM measurements at RT for Mg1−xFexO nanoparticles with H in the range of ±30 kOe are shown in Fig. 6. All samples show well FM with increasing of M s from 0.14 to 1.60 emu/g due to the increase of Fe content, as summarized in Table 1. The Fe substitution causes a decrease of the coercive field (H c ) compared to that of the pure MgO nanoparticle. This RT-FM behavior in our Mg1−xFexO nanoparticles can be explained by the existence of oxygen vacancies (VO) based on bound magnetic polarons (BMP). The VO is naturally formed to ensure charge neutrality, which is sensitive to any disorder in the oxygen sublattice resulting from thermal, doping and/or substitution technique and preparation conditions (e.g., high temperature heating in vacuum or different atmospheres or ion-bombarding of the surface) [31]. According to the BMP model, the large density of VO results more BMPs and neighboring BMPs can overlap in the long-range FM coupling. To consider the number of BMPs, the M–H curves can be well fitted using a simple BMPs model Eq. (2) [32]:
where the first term is from BMP contribution and the second term is due to paramagnetic matrix contribution. M 0 = Nm s , N is the number of BMPs involved and m s is the effective spontaneous moment per BMP. L(x) = coth(x) − 1/x is the Langevin function with x = m eff H/(k B T), where m eff is the true spontaneous moment per BMP, and at higher temperature it can be approximated to m s = m eff . The values of the parameters M 0, N and χ m of the samples are listed in Table 1. The M–H curves fitted with BMP model for Fe-doped MgO samples are plotted in the insets of Fig. 6, which exhibit a good fit for each sample. The parameters are found to increase with increasing of Fe content to be from 0.08 to 1.26 emu/g, 9.3 × 1014 to 1.32 × 1016 cm−3 and 4.3 × 10−6 to 1.6 × 10−5 egs for M 0, N and χ m , respectively. The number of BMPs in our samples is close to that of Ni-doped ZnO nanoparticles (N = 1015 cm−3) [32] and Co-doped ZnO nanoparticles (N = 1017 cm−3) [33], which is required for a long-range FM order. However, the difference in the number of BMPs depends on the availability of free carriers of various materials. Thus, the RT-FM in our results can be explained by VO interaction based on the BMP model.
Figure 7 shows the temperature dependence of magnetization (M–T curve) for the samples with x = 0 and 0.05 measured under the H of 500 Oe between 50 and 400 K in the FC mode. Both samples show similar behavior of FC curve and no evidence of secondary phase can be detected. The T C of samples can be determined from the derivative of the magnetization of dM FC (T)/dT = 0 [34]. In this case, the result of the magnetization curve shows that the T C of both samples is above 400 K. Thus, it is necessary to design FM oxide materials with high T C above RT and high thermal equilibrium stability during the device fabrication process.
4 Conclusion
Mg1−xFexO nanoparticles with the crystallite sizes of approximately 25 ± 2 nm have been prepared by the polymer pyrolysis method. Structural characterization showed the cubic phase of MgO and detected the impurity phase of Fe3O4 with sample of x = 0.07. Bright field TEM images showed the agglomeration of nanoparticles with size of 25 ± 1 nm, corresponding to the stable size obtained from the XRD results. The valence state of Fe3+ ions were observed in the Mg1−xFexO nanoparticles and higher concentration doping of Fe3+ caused of as high as the defects/oxygen vacancy. This oxygen vacancy induced ferromagnetic behavior at room temperature in Mg1−xFexO nanoparticles based on BMP model. Additionally, the high T C of nanoparticles was observed above 400 K. It is suggested that the stable room temperature ferromagnetism in Mg1−xFexO nanoparticles can potentially be useful for the future spintronics and transparent nanodevices.
References
T. Dietl, H. Ohno, F. Matsukura, J. Cibert, D. Ferrand, Science 287, 1019 (2000)
S.J. Pearton, W.H. Heo, M. Ivill, D.P. Norton, T. Steiner, Semicond. Sci. Technol. 19, R59 (2004)
J.M.D. Coey, Curr. Opin. Solid State Mater. 10, 83 (2006)
N.H. Hong, J. Sakai, W. Prellier, A. Hassini, A. Ruyter, F. Gervais, Phys. Rev. B 70, 195204 (2004)
C.B. Fitzgerald, M. Venkatesan, L.S. Dorneles, R. Gunning, P. Stamenov, J.M.D. Coey et al., Phys. Rev. B 74, 115307 (2006)
A. Tiwari, V.M. Bhosle, S. Ramachandran, N. Sudhakar, J. Narayan, S. Budak, A. Gupta, Appl. Phys. Lett. 88, 142511 (2006)
J. Philip, A. Punnoose, B.I. Kim, K.M. Reddy, S. Layne, J.O. Holmes, B. Satpati, P.R. LeClair, T.S. Santos, J.S. Moodera, Nat. Mater. 5, 298 (2006)
S. Phokha, S. Pinitsoontorn, S. Maensiri, J. Appl. Phys. 112, 113904 (2012)
J.M.D. Coey, M. Venkateshan, C.B. Fitzgerald, Nat. Mater. 4, 173 (2005)
Q.Y. Wen, H.Y. Zhang, Q.H. Yang, Y.Q. Song, J.Q. Xiao, J. Magn. Magn. Mater. 321, 3110 (2009)
J.F. Hu, Z.L. Zhang, M. Zhao, H.W. Qin, M.H. Jiang, Appl. Phys. Lett. 93, 192503 (2008)
M. Sterrer, E. Fischbach, T. Risse, H.J. Freund, Phys. Rev. Lett. 94, 186101 (2005)
N. Kumar, D. Sanyal, A. Sundaresan, Chem. Phys. Lett. 477, 360 (2009)
D.D. Wang, Z.Q. Chen, C.Y. Li, X.F. Li, C.Y. Cao, Z. Tang, Phys. B 407, 2665 (2012)
B.M. Maoz, E. Tirosh, M. Bar Sadan, G. Markovich, Phys. Rev. B 83, 161 (2011)
A. Shalimov, K. Potzger, D. Geiger, H. Lichte, G. Talut, A. Misiuk, H. Reuther, F. Stromberg, S. Zhou, C. Baehtz, J. Fassbender, J. Appl. Phys. 105, 064906 (2009)
S. Ramachandran, J. Narayan, J.T. Prater, Appl. Phys. Lett. 90, 132511 (2007)
S. Azzaza, M. El-Hilo, S. Narayanan, J.J. Vijaya, N. Mamouni, A. Benyoussef, A. El Kenz, M. Bououdina, Mater. Chem. Phys. 143, 1500 (2014)
F. Stavale, N. Nilius, H.J. Freund, New J. Phys. 14, 033006 (2012)
S. Phokha, S. Hunpratup, S. Pinitsoontorn, B. Putasaeng, S. Rujirawat, S. Maensiri, Mater. Res. Bull. 67, 118 (2015)
S. Suwanboon, P. Amornpitoksuk, A. Sukolrat, Ceram. Int. 37, 1359 (2011)
M.S. Seehra, S. Suri, V. Singh, J. Appl. Phys. 111, 07B516 (2012)
G. Sharma, P. Jeevanandam, Microporous Mesoporous Mater. 165, 55 (2013)
ChN Rao, U.T. Nakate, R.J. Choudhary, S.N. Kale, Appl. Phys. Lett. 103, 151107 (2013)
B. Choudhury, A. Choudhury, Mater. Res. Express 1, 025026 (2014)
A.N. Andriotis, R.M. Sheetz, M. Menon, J. Phys. Condens. Matter 22, 334210 (2010)
G.Z. Xing, Y.H. Lu, Y.F. Tian, J.B. Yi, C.C. Lim, Y.F. Li, G.P. Li, D.D. Wang, B. Yao, J. Ding, Y.P. Feng, T. Wu, AIP Adv. 1, 022152 (2011)
X. Bie, C. Wang, H. Ehrenberg, Y. Wei, G. Chen, X. Meng, G. Zou, F. Du, Solid State Sci. 12, 1364 (2010)
S.D. Yoon, Y. Chen, A. Yang, T.L. Goodrich, X. Zuo, D.A. Arena et al., J. Phys. Condens. Matter 18, L355 (2006)
S. Phokha, S. Pinitsoontorn, P. Chirawatkul, Y. Poo-arporn, S. Maensiri, Nanoscale Res. Lett. 7, 425 (2010)
I. Kosacki, T. Suzuki, V. Petrovsky, H.U. Anderson, P. Colomban, Solid State Ion. 149, 99 (2002)
G. Srinet, R. Kumar, V. Sajal, J. Appl. Phys. 114, 033912 (2013)
B. Pal, P.K. Giri, J. Nanosci. Nanotechnol. 11, 1 (2011)
X. Zhou, J. Xue, D. Zhou, Z. Wang, Y. Bai, X. Wu, X. Liu, J. Meng, ACS Appl. Mater. Interf. 2, 2689 (2010)
Acknowledgments
The authors would like to thank the Synchrotron Light Research Institute (Public Organization), Nakhon Ratchasima, Thailand for XANES facilities, the Department of Physics, Khon Kaen University for providing VSM facilities and the Advanced Materials Physics (AMPs) Laboratory for our research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Phokha, S., Klinkaewnarong, J., Hunpratub, S. et al. Ferromagnetism in Fe-doped MgO nanoparticles. J Mater Sci: Mater Electron 27, 33–39 (2016). https://doi.org/10.1007/s10854-015-3713-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-015-3713-9