Abstract
The polycrystalline sample of Ba2BiNbO6 (a member of double perovskite family) was synthesized by a high-temperature solid-state reaction method (calcination at 910 °C, and sintering at 950 °C). Preliminary analysis of room temperature X-ray data pattern of the sample confirms the formation of a single phase compound in orthorhombic crystal system. The room temperature scanning electron microscope image of the pellet sample clearly shows the uniform distribution of grains and formation of a high density sample. Detailed studies of dielectric and polarization characteristics with temperature confirmed the existence of ferroelectricity in the material. Studies of electrical properties exhibits a strong correlation with the micro-structure and resistive properties of the material. The electrical transport shows the existence of non-exponential-type of conductivity relaxation in the material. This lead-free ferroelectric has many interesting characteristics as compared to that of their lead-based counterpart, Pb2BiNbO6.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Most of the ferroelectric oxides have BO6 oxygen octahedra, even though they have different crystal structures, electrical and mechanical properties, transition temperature (Tc) and remnant polarization (Pr) [1]. There are four different types of structures of ferroelectric oxides with oxygen octahedra namely; perovskite, tungsten-bronze, layer and pyrochlore. For the last few decades, there has been extensive investigation on ferroelectric oxides particularly on oxygen octahedral type because of various industrial and technological applications. Although a number of TB-type (complex perovskite) ferroelectrics have been studied, a lot of interest has been paid on ferroelectric niobates and tantalates for their electro-optic and other related applications. The first reported ferroelectric compounds of TB-type complex perovskite structure are lead metaniobate PbNb2O6 [2, 3] and lead metatantalate PbTa2O6 [4]. The transition temperature of these two compounds was reported to be 575 and 260 °C respectively. After the discovery of ferroelectricity in these two compounds, lots of compounds of the family were studied because of their many interesting properties useful for devices. Interestingly, single crystal or ceramic sample of lead barium niobate (PBN) shows high dielectric constant, pyroelectric, piezo-electric and electro-optic coefficients which are useful for many devices such as surface acoustic wave (SAW), pyroelectric and memory devices around the morphotoric phase boundary (MPB) [5–10]. The TB-type double perovskites with general formula A2BB′O6, (with, A = an alkaline earth, B and B′ are transition metals) have been studied because of their attractive physical properties [11], which depend on structural distortions and the characteristic properties of B and B′ cations. This chemical configuration results in a lot of opportunities to combine different elements of the periodic table, generating the possibility of synthesizing new materials. The BaTiO3 which is simple perovskite is well known because of its ferroelectric character [12]. Partial substitutions of Ti by the Zr cation have been done by many authors, showing the evolution of the electric behavior from a ferroelectric response for low concentration of Zr, to a ferroelectric relaxor [13] and a dielectric behavior for high concentrations of Zr [14]. However, the position of the substitutive cations in the crystallographic cell of the material is not usually reported. Location of all ions in the lattice is important for inferring the possibility of obtaining spontaneous polarization, in order to give rise to ferroelectric behavior. In the case of polycrystalline samples, the ferroelectric behavior depends greatly on the grain size, because the effects of imperfection frequently dominate the ferroelectric response of small grains, where a significant fraction of the material volume may be influenced by grain boundaries. It is known that cationic disorder produces substantial changes of the ferroelectricity of complex perovskites [15].
Nowadays, attempts are being made to keep the environment eco-friendly and free from toxic materials such as lead. There are a good number of lead-based ferroelectrics namely, PZT, PMN, PFN etc., which are having much superior quality as compared to that of non-ferroelectrics, are widely used for various types of sensors. Therefore, it is impossible to replace the above lead based compound completely; however, continuous efforts are being made to search for new lead-free ferroelectrics with comparable characteristics for the desired purpose. In view of this we have a synthesized lead-free tungsten bronze compound with chemical formula Ba2BiNbO6 by mixed oxide route, and studied its ferroelectric and electrical characteristics for possible devices.
2 Experimental
2.1 Sample preparation
The polycrystalline samples of Ba2BiNbO6 (BBN) were synthesized by a high-temperature mixed oxide route using high-purity (AR grade) ingredients: BaCO3, Bi2O3, and Nb2O5 (>99 %, M/s LOBA Chemie Pvt. Ltd. India) as per the required stoichiometric proportions. The oxides and carbonate with the following composition were mixed in dry (air) and wet (methanol) medium for 3 h in agate mortar. Based on the repeated firing and grinding at different high temperatures (close to melting/decomposition temperature of ingredients) the calcinations temperature for formation of the material (Ba2BiNbO6) was decided. Finally the material was calcined at 910 °C for 4 h.
The calcined fine powder of the material was then cold pressed into the form of cylindrical pellets of diameter 10 and 1–2 mm thickness under a uniaxial pressure of 4 × 106 nm−2 using a hydraulic press. Polyvinyl alcohol (PVA) was used as a binder to prepare pellets. The pellets were then sintered at an optimized temperature of 925 °C (based on repeated firing) in air atmosphere for 4 h.
2.2 Characterization techniques
An X-ray diffraction (XRD) pattern data of calcined powder was recorded at room temperature using X-ray powder diffractometer (PAN Analytical XPERT) with CuKα radiation (λ = 1.5405 Å) in a wide range of Bragg’s angle (θ) (20° ≤ 2θ ≤ 80°) at a scanning rate of 2°/min. The sintered pellets were electroded with high-quality silver paste, and dried at 150 °C for 4 h before taking dielectric and electrical measurements. The microstructures of the pellet samples were recorded by Jeol JSM-6480 LV SEM at room temperature. Compositional analysis of the pellet sample was carried out by the energy dispersive X-ray spectroscopy (EDS) attached with the SEM of above model.
The dielectric (capacitance, dissipative factor), impedance and inductance parameters of the sintered pellet were measured as a function of frequency (1 kHz–1 MHz) at different temperatures (25–500 °C) using a computer-controlled phase sensitive meter (PSM 1735 LCR 4NL, UK) with a laboratory-designed and fabricated sample holder and furnace. A chromel–alumel thermo-couple and Rishab digital milli-voltmeter were used to record the temperatures. The polarization (hysteresis loop) of the material on the poled sample (electric field = 6 kV/cm, time = 8 h) was obtained at different temperatures using hysteresis loop tracer (M/S Marine India, New Delhi).
3 Results and discussion
3.1 Structural study
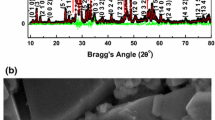
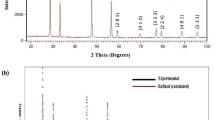
Figure 1 shows the room temperature XRD pattern of Ba2BiNbO6. The diffraction pattern of the compound consists of sharp and single diffraction peaks, which are different from that of the ingredients of the prepared compounds confirming the formation of a single-phase new compound [16]. As most of tungsten bronze structure has tetragonal or orthorhombic structure, all the observed peaks of the pattern were indexed in these crystal systems with different unit cell configurations using a standard computer program package “POWD” [17]. On the basis of best agreement between observed and calculated inter planar spacing (d), an orthorhombic unit cell was selected. The selected unit cell parameters of the sample (as given in the figure) were refined using least-squares refinement sub-routine of the above computer program. The estimated standard deviation of cell parameters was 0.0018 Å. These values are found to be very much consistent with those of some reported compounds of similar structure [18].
The scattered crystallite or particle size (P) of the compound was calculated using the broadening of some widely spread (over Bragg angles) strong and medium reflections in the Scherrer’s equation: \( P_{hkl} = \frac{k\lambda }{{\beta_{1/2} \cos \theta_{hkl} }} \) [19], where k (constant) = 0.89, λ = 1.5405 Å and β1/2 = full width at half maximum (in radians). The average value of Phkl is found to be 28 nm.
Figure 1(inset) shows the surface morphology of BBN recorded on the gold plated bulk pellet sample using a SEM. The nature and size of the microstructures of the sample suggest that surface is highly dense due to uniformly distribution of grains. In spite of sintering at optimized high temperature some small voids of irregular shape and dimension are seen in the compound. The calculated relative density of the sintered pellet is 5.85.
The elemental identification of the composites was done with EDX technique. The EDX spectra of the composites are shown in Fig. 2. The absence of extra elements other than expected (Ba, Bi, Nb and O) in the EDX spectra reveals the purity of the sample and confirms the formation of single phase i.e., orthorhombic double perovskite structure of ferroelectric phase in the compound.
3.2 Dielectric study
The temperature variation of relative dielectric constant (εr) and tangent loss (tanδ) at different frequencies (1, 10, 100 kHz and 1 MHz) of the sample are shown in Fig. 3. It is noticed that εr increases up to a temperature (referred as transition temperature (Tc ~ 350 °C)), and then it decreases suggesting ferroelectric-paraelectric phase transition at this temperature. The temperature variations of tan δ at different frequencies also follow the similar pattern. The room temperature εr and tan δ for BBN are 812 and 0.0219 respectively. The higher and smaller values of εr and tan δ of BBN respectively at room temperature, are useful for capacitors, transducer and microwave applications. The increase in εr can be ascribed to the electron–phonon interaction which is mainly observed in niobates. Above 430 °C, the increase in εr can be ascribed to the thermally activated transport of space charges [20]. The higher value of εr at lower frequency (1 kHz) of the compound is due the charge accumulation at grain boundaries [21]. Above Tc there is monotonous increase in tan δ value which can be attributed to thermal transport of oxygen vacancies, Bi+3 and Nb+4 ions which act as donor centers initiating extrinsic n-type conduction in compound. A sharp increase in tan δ at higher temperature may be due to scattering of thermally activated charge carriers and some defects in the compound. At higher temperatures the conductivity begins to dominate, which in turn, is responsible for rise in tan δ. Also, at higher temperatures the contributions of ferroelectric domain walls to tan δ is less, which causes the rise in value of tan δ [22]. The nature of frequency dependence of dielectric parameters of the material can be explained by the Maxwell–Wagner and Koop’s phenomenological models [23]. As per this model, dielectric medium contains highly conducting grains and poorly conducting grain boundaries. At higher frequencies the grain are more effective while the grain boundaries are found to be more effective at lower frequencies and higher temperatures. The decreasing nature of εr on increasing frequency may be considered due to the electron hopping between Nb+5 and Nb+4 at octahedral sites which cannot follow the alteration of ac electric field at higher frequencies. Therefore, electrons have to pass through the well conducting grains and the poorly conducting grain boundaries. As the grain boundaries have large resistance, the electrons pile up there, and produce large space charge polarization. Therefore, εr has larger value in the low-frequency range. On further increase of frequency, the electrons change their direction of motion rapidly, which hinders the movement of electrons inside the dielectric material and thus accumulation of charges at the grain boundaries decreases resulting in the decrease of εr.
3.3 Polarization study
Figure 4 shows hysteresis loop of the sample at two different temperatures. The remnant polarization (2Pr) of the compound at 30 and 50 °C was found to be 5.74 and 5.12 µC/cm2 respectively. The coercive field (2Ec) at these temperatures was found to be 0.11 and 0.1 kV/cm respectively. It is observed that the value of remnant polarization and coercive field decreases with rise in temperature. Again, the area of the room temperature loop was found to be decreased with rise in temperature which confirms the existence of ferroelectric properties in the material.
3.4 Impedance and modulus spectroscopy
Complex impedance spectroscopy (CIS) technique is widely used to study frequency-temperature dependence of the electrical properties of dielectrics and ionic conductors. By using this technique, one can able to distinguish (1) bulk, (2) grain boundary and (3) electrode polarization effect of the material. When an ac signal is applied across the pellet sample, its output response is measured. The impedance measurements of the materials give us data having both real (resistive) and imaginary (reactive) components. For this purpose some basic equations/formulism of impedance and electrical modulus are used in this technique such as: complex impedance \( Z^{\ast} = Z^{\prime } + jZ^{\prime \prime } \), complex modulus \( \frac{1}{\varepsilon^{\ast}} = M^{\ast} = M^{\prime } + jM^{\prime \prime } = j\omega C_{o} Z^{\ast} \), and complex permittivity \( \varepsilon^{\ast} = \varepsilon^{\prime } - j\varepsilon^{\prime \prime } \). Using above equations we will get following impedance and modulus formalism to calculate and study the detail electrical properties of the materials.
\( Z^{2} = Z^{{'^{2} }} + Z^{{''^{2} }} \), \( M^{\prime } = - \omega C_{o} Z^{\prime \prime } ,M^{\prime \prime } = \omega C_{o} Z^{\prime } \) where Co is the geometrical capacitance, j = √−1.
3.4.1 Impedance analysis
Figure 5 shows variation of Z′ ~ Z″ (Nyquist plot) at different temperature of sample BBN. All the semicircles exhibit some depression instead of a semicircle centered on the real axis. This behavior is indicative of non-Debye type of relaxation and it also manifests that there is a distribution of relaxation time instead of a single relaxation time in the studied material. The value of bulk resistance (Rb) at different temperatures has been obtained from the intercept of the semicircular arcs on the real axis (Z′). It clearly shows that the parameters Rb decreases with rise in temperature suggesting the NTCR behavior of the material. In an ideal Debye like response, a perfect semi-circle with its center at Z′ axis is observed for which equivalent circuit to fit the experimental data is parallel combinations of (CR). Since semicircles in the studied material at different temperature are depressed type suggesting non-Debye type of relaxations, for which we have number of relaxation time instead of single relaxation time.
Figure 6 shows the variation of Z″ with frequency at selected temperatures. It is observed that the value of Z″ decreases with rise in frequency as well as temperature in the sample. The value of Z″ increases with rise of frequency and attains a maximum at certain frequency (Z″max). This is due to the presence of relaxation in the sample [20]. The frequency at which Z″ attains maximum value is known as relaxation frequency (fr). Inverse of fr is known as relaxation time of the sample. Again, relaxation time decreases with rise in temperature suggesting the temperature dependence of relaxation phenomena. Generally, in dielectric material relaxation process occurs due to the presence of immobile charges at low temperatures and defects/vacancies at higher temperatures [24].
3.4.2 Modulus analysis
The analysis of electrical modulus, measured from impedance analyzer or LCR meter, is very useful to detect parameters such as electrode polarization, grain boundary conduction effect, bulk properties electrical conductivity and relaxation time. Figure 7 shows the variation of M″ with frequency at selected temperatures. Once again, M″ spectroscopy studies provide nature of relaxation phenomena in the material. The peak or maximum value of M″ (M″max) shifts to higher frequency side which suggests the existence of hopping mechanism in which charge carriers dominates intrinsically and hence mechanism is considered as thermally activated process. Asymmetric broadening of the peaks indicates spread of relaxation with different time constants, which once again suggests non- Debye type [15].
The reactive part of impedance (Z″) and modulus (M″) spectroscopy plot is shown in Fig. 8. Sinclair et al. [25] reported that the combine plot of Z″ and M″ as a function of frequency is used to detect the presence of smallest capacitance and largest resistance. According to them, if peaks of Z″ and M″ ~ frequency are occur at different frequencies then motion of charge carrier is short range whereas for long range movement of charge carrier peaks are occur at same frequencies. In the studied compound there is mismatch of peaks at fixed temperatures suggesting short range motion of charge carriers and departure from ideal Debye-like behaviour [26].
3.5 Conductivity study
3.5.1 Ac conductivity
Studies of frequency-temperature dependence of ac conductivity are generally carried out to understand the type of electrical conduction mechanism and the nature of charge carriers in the material. The ac electrical conductivity (σac) is calculated using an empirical relation, σac = ωεrε0tanδ, where ε0 is permittivity in free space and ω is angular frequency. To understand the effect of frequency on conduction mechanism of the material, we have to use Jonscher’s universal power law [27]: \( \sigma_{T} (\omega ) = \sigma (0) + \sigma_{1} (\omega ) = \sigma_{0} + A\omega^{n} \) where σ (0) is the frequency independent term giving rise to dc conductivity and σ1(ω) is the purely dispersive component of ac conductivity. The exponent n can have a value between zero and one which represents the degree of interaction between mobile ions and lattices around them whereas A determines the strength of polarizability.
Figure 9 shows the variation of σac of the material with frequency at different temperatures. At higher temperatures the conductivity curves show frequency independent plateau in the low-frequency region whereas at higher frequencies σac α ωn, thus frequency dispersion is still maintained in the material. It is obvious that σac increases with rise in frequency but it is nearly independent at low frequency. Therefore, extrapolation of this part towards lower frequency side gives σdc. The increasing trend of σac with rise in frequency may be attributed to the disordering of cations between neighboring sites, and presence of space charges [28]. In the high frequency region the curves approach to each other. The nature of conductivity plots reveals that the curves exhibit low frequency dispersion phenomena obeying the Jonscher’s power law. According to Jonscher [27], the origin of the frequency dependence of conductivity lies in the relaxation phenomena arising due to mobile charge carriers. When a mobile charge carrier hops to a new site from its original position, it remains in a state of displacement between two potential energy minima obeying above power law. The value of n < 1 signifies that the hopping motion involves a translational motion with a sudden hopping whereas n > 1 means that the motion involves localized hopping without the species leaving the neighborhood. The frequency at which change in slope takes place is known as hopping frequency of the polarons (ωp), and is temperature dependent. The material obeys universal power law which is confirmed by a fit of above equation to the experimental data (Fig. 9) where solid line correspondence to fitted curve and symbols are experimental data. From non-linear fitting it is found that the motion of charge carriers in the samples is translational one because of small value of n (<1). A close look on the plot reveals value of conductivity increases with increase of temperature once again supports the NTCR behavior of the sample.
Figure 10 shows the variation of fitting parameter A and n with temperature. It is seen that the value of n decreases with rise in temperature and becomes minimum near Tc in BBN. Again, it increases with increase in temperature whereas pre-exponential factor A has a reverse trend. The exponent n represents interaction between mobile ions with the lattice around them. The observed minima at Tc (or decreasing trend with temperature) suggest a strong interaction between the lattice and mobile ions. According to dynamic theory [29, 30], one of the transverse optical mode (soft mode) is weakened, and the restoring force tends to be zero at transition temperature Tc. Therefore, if charge carriers coupled with soft mode, they become mobile at Tc, and thus conductivity increases. The pre-exponential factor A determines the strength of polarizability. The maximum value of A at Tc suggests the presence of high polarizability i.e., maximum dielectric constant.
3.5.2 dc conductivity
Figure 11 shows variation of σdc ~ 103/T for the compound. The nature of the plot follows the Arrhenius relation \( \sigma _{dc} = \sigma_{o} e^{{\frac{{ - E_{a} }}{{K_{B} T}}}} \) [19]. The calculated activation energy (Ea) is 0.846 eV for BBN. Conductivity increases with rise of temperature signifying NTCR behavior in the studied compound.
4 Conclusion
The preliminary structural analysis of synthesized material using X-ray diffraction data shows that the material has an orthorhombic crystal structure. Detailed studies of temperature dependence of dielectric and electric polarization conclude that the material has ferroelectric characteristics. Impedance spectroscopy studies indicate that the material exhibits (1) conduction due to bulk (grain) effect, (2) NTCR-type behavior and (3) temperature dependent relaxation phenomena. Modulus analysis indicates non–exponential type of conductivity relaxation in the material. Ac conductivity study reveals the material is semiconducting nature and obeys Jonscher’s universal power law.
References
F.W. Aiger, Modern Oxide Materials-Preparation, Properties and Device Applications (Academic Press. Inc., London, 1972)
G. Goodman, Ferroelectric properties of lead metaniobate. J. Am. Ceram. Soc. 36, 368–372 (1953)
M.H. Francombe, B. Lewis, Structural, dielectric and optical properties of ferroelectric lead metaniobate. Acta Crystallogr. 11, 696 (1958)
M.E. Lines, A.M. Glass, Principles and applications of ferroelectrics and related materials (Oxford University Press, Oxford, UK, 2001)
C.A. Randall, A.D. Hilton, D.J. Barber, T.R. Shrout, Extrinsic contributions to the grain size dependence of relaxor ferroelectric Pb(Mg1/3Nb2/3)O3: PbTiO3 ceramics. J. Mat. Res. 8, 880–884 (1993)
R. Guo, A.S. Bhalla, C.A. Randall, L.E. Cross, Dielectric and pyroelectric properties of the morphotropic phase boundary lead barium niobate (PBN) single crystals at low temperature (10–300 K). J. Appl. Phys. 67, 6405 (1990)
M. Lee, R.S. Feigelson, A. Liu, L. Hesselink, Photorefractive properties of tungsten bronze ferroelectric lead barium niobate (Pb1−x Ba x Nb2O6)crystals. J. Appl. Phys. 83, 5967 (1998)
T.R. Shrout, H. Chen, L.E. Cross, Dielectric and piezoelectric properties of Pb1−xBaxNb2O6 ferroelectric tungsten bronze crystals. Ferroelectrics 74, 317–324 (1987)
M. Lee, H. Lee, R.K. Route, R.S. Feigelson, Optical properties of lead barium niobate (Pb1−x Ba x Nb2O6) crystals. J. Appl. Phys. 81, 917 (1997)
G. Burns, F.H. Dacol, R. Guo, A.S. Bhalla, Ferroelectric (Pb, Ba)Nb2O6 near the morphotropic phase boundary. Appl. Phys. Lett. 57, 543 (1990)
V.R. Palkar, S.K. Malik, Observation of magnetoelectric behavior at room temperature in Pb(FexTi1−x)O3. Solid State Commun. 134, 783–786 (2005)
D.A. Landínez Téllez, G. Peña Rodríguez, J. Arbey Rodríguez, F. Fajardo, J. Roa Rojas, Structural, magnetic, multiferroic, and electronic properties of Sr2TiMnO6 double perovskite. Dyna 79, 111–115 (2012)
V.A. Isupov, Nature of physical phenomena in ferroelectric relaxors. Phys. Solid State 45, 1107–1111 (2003)
P. Baettig, N.A. Spaldin, Ab initio prediction of a multiferroic with large polarization. Appl. Phys. Lett. 86, 12505–12507 (2005)
C. Ang, R. Guo, A.S. Bhalla, L.E. Cross, Dielectric dispersion at microwave frequencies. J. Appl. Phys. 87, 3937–3940 (2000)
H.P. Klug, L.E. Alexander, X-ray Diffraction Procedures for Polycrystalline and Amorphous Materials (Wiley-Interscience, New York, 1974)
E.W. POWD, An interactive powder diffraction data interpretation and indexing Program, Ver 2.1 (School of Physical Science, Finders University of South Australia, Bedford Park, SA 5042, Australia)
B.N. Parida, P.R. Das, R. Padhee, R.N.P. Choudhary, A new ferroelectric oxide Li2Pb2Pr2W2Ti4Nb4O30: synthesis and characterization. J. Phys. Chem. Solids 73, 713 (2012)
B.D. Cullity, Elements of X-ray Diffraction (Addison-Wesley Publishing Co. Inc., Boston, 1978)
B.N. Parida, P.R. Das, R. Padhee, R.N.P. Choudhary, Phase transition and conduction mechanism of rare earth based tungsten-bronze compounds. J. Alloys Compd. 540, 267 (2012)
K. Jawahar, Choudhary RNP, structural and dielectric properties of Y3/2Bi3/2Fe5O12. Mater. Lett. 62, 911–913 (2008)
P.R. Das, L. Biswal, B. Behera, R.N.P. Choudhary, Structural and electrical properties of Na2Pb2Eu2W2Ti4X4O30 (X = Nb, Ta) ferroelectric ceramics. Mater. Res. Bull. 44, 1214–1218 (2009)
C.G. Koops, On the dispersion of resistivity and dielectric constant of some semiconductors at audiofrequencies. Phys. Rev. 83, 121–124 (1951)
D.P. Almond, A.R. West, Impedance and modulus spectroscopy of “real” dispersive conductors. Solid State Ionics 11, 57–64 (1983)
D.C. Sinclair, A.R. West, Impedance and modulus spectroscopy of semiconducting BaTiO3 showing positive temperature coefficient of resistance. J. Appl. Phys. 66, 3850 (1989)
M. Nobre, S. Lanfredi, Ferroelectric state analysis in grain boundary of Na0.85Li0.15NbO3 ceramic. J. Appl. Phys. 93, 5557 (2003)
A.K. Jonscher, Nature 267, 673 (1977)
N.K. Karan, D.K. Pradhan, R. Thomas, B. Natesan, R.S. Katiyar, Solid State Ionics 179, 689 (2008)
Z. Lu, J.P. Bonnet, J. Ravez, P. Hagenmuller, Solid State Ionics 57, 235 (1992)
A.K. Jonscher, Dielectric Relaxation in Solids (Chelsea Dielectric Press, London, 1983)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Panda, N., Parida, B.N., Padhee, R. et al. Structural, dielectric and electrical properties of the Ba2BiNbO6 double perovskite. J Mater Sci: Mater Electron 26, 3797–3804 (2015). https://doi.org/10.1007/s10854-015-2905-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-015-2905-7