Abstract
Anatase titanium dioxide nanoparticles were prepared from titanium oxysulphate (TiOSO4) as a precursor and the base polymer Polyvinyl Pyrrolidone is used as a capping agent. TiO2 nanoparticles were characterized by XRD, SEM, TEM, EDS, FT-IR, DLS, UV–Vis. Diffuse reflectance spectrophotometer. From XRD analysis it is seen that obtained diffraction peaks are broad which indicating that crystallite size is very small. Scanning Electron Micrograph (SEM) revealed that TiO2 nanoparticles has diameter of 10 nm calcined at 400 °C. Photocatalytic activity was checked by employing the Methyl Orange as a model pollutant under UV-light (365 nm) which shows 94 % photo-degradation in 150 min. The novelty of present approach is the large scale production of polymer capped TiO2 nanocrystals by using sol–gel process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Among the various metal oxides TiO2 play an important role in various applications such as self cleaning, gas sensors [1–3]. In particular, dye degradation is almost essential for wastewater treatment due to its toxicity. Titanium dioxide exists in three main polymorphs i.e. Anatase, Rutile and Brookite [4]. Among these, Anatase TiO2 exhibits high photocatalytic activity due to high absorption capacity towards organic, molecular oxygen and low rate of recombination of Electron Hole Pairs (EHP). TiO2 semiconductor photocatalyst has been studied for decades due to its superior photocatalytic activity under UV radiation.
Titanium dioxide nanoparticles can be prepared by various methods like hydrolysis, thermolysis, sol–gel method [5, 6], hydrothermal [7], flame synthesis [8, 9, 10]. In most of these studies, precursors like titanium (IV) alkoxides, titanium tetra isopropoxide (TTIP), titanium n-butoxide, TiCl4 have been used. But TiCl4 is toxic and corrosive while titanium (IV) alkoxide is too expensive as well as soluble in organic solvents. The method adopted for the synthesis of TiO2 has been regarded as a way for clean, economic and environmental friendly. So, large efforts are made towards the use of low cost precursor i.e. TiOSO4 [11] and also instead of organic solvent like alcohol, acetone here water is used as green solvent. Paola et al. [12] reported that TiO2 prepared by TiOSO4 and calcined at 600 °C for 10 h. The obtained results of XRD indicate the particle size is in the range of 85–95 nm, on the contrary the present method require low temperature and short time duration for TiO2 preparation.
In present work, we have focused on the synthesis and characterization of anatase TiO2 as an efficient photocatalyst by hydrolysis of titanium oxysulphate which is used by very few researchers only. To get the desired phase and size of TiO2 the various calcination temperature from 300 to 500 °C were used.
2 Experimental
Material-titanium (IV) oxysulphate (TiOSO4 × H2O) (sigma Aldrich) (729 % Ti as TiO2 basis) Ammonia solution (A.R. grade Runa chemicals 25 %) Polyvinyl Pyrollidone (PVP) K-30 (Highmedia.), deionised water was used for the synthesis of TiO2. All the reagents were analytical grade, PVP was selected on the basis of solubility in water.
2.1 Synthesis of TiO2 nanoparticles
TiO2 nanoparticles were prepared by controlled addition of TiOSO4 (0.1 M) in 100 mL distilled water with constant stirring to get clear solution. Then sufficient amount of capping agent (0.2 wt% PVP) was added and followed by dropwise addition of aqueous ammonia at room temperature to get white gelatinous precipitate. It is then washed with Millipore water for several times to ensure complete removal of sulphate ions which were checked by Barium Nitrate test. The precipitate was subjected to microwave irradiation for 20 min in microwave oven (Input 900 W, 250 MHz LG) with on–off cycle (20 on–40 off). The dried precipitate was grounded by using agate mortar pestle and calcined at different temperatures for 3 h in a temperature controlled muffle furnace. The phase purity and the degree of crystallinity of TiO2 nanoparticles were monitored by XRD technique.
2.2 Characterization of synthesized TiO2
XRD of synthesized TiO2 was recorded on analytical diffractometer with CuKα radiation (λ = 1.5406 Å) as a X-ray source over 2θ range (10°–80°). The average crystallite size of anatase TiO2 was calculated by using Scherrer’s formula. FT-IR spectrum of TiO2 was recorded on Spectrum-One (Perkin-Elmer) using KBr pellets in the range of 4000–400 cm−1. Similarly Dynamic Light Scattering (DLS) was used in order to determine the diameter of the particles. It is a simplest method for the determination of average particle size of material.
The surface morphology of TiO2 nanoparticles were scanned by scanning electron microscope (SEM) (JEOL JSM–6360) at various magnifications. The transmission electron microscopy (TEM) images were scanned using JEOL-JEM 2100-TEM equipped with high resolution gatan CCD camera. To know the elemental composition of TiO2 powder, the EDS was recorded using SEM (model JEOL–6360) connected with EDS. The UV–Vis. Diffuse reflectance spectrum was obtained by using UV–Vis spectrophotometer equipped with diffuse reflectance measurement unit, from which band edges and band gap energy of TiO2 were recorded.
2.3 Photocatalytic activity of TiO2
The photocatalytic activity of TiO2 was evaluated for the degradation of M O by UV irradiation. To evaluate optimal photocatalytic activity of TiO2 calcined at various calcinations temperature i.e. 300, 400, 500 °C were used. The photoreactor was kept open to air to get sufficient oxygen for photochemical reaction. In present work, photocatalyst TiO2 (0.1 g) was added to photoreactor containing 100 mL MO (20 ppm). Before irradiation to light, the solution was stirred for 30 min in dark to ensure an adsorption–desorption equilibrium, then exposed to light. To see the catalytic activity of as prepared TiO2 with commercial TiO2, MO degradation was carried out for both photocatalyst. At given time interval aliquots were collected from photoreactor and then centrifuged to remove photocatalyst. The centrifuged solution was used to monitor and the concentration of M O and obtained by UV–Vis. NIR double beam spectrophotometer. (UV–3600, Shimadzu).
3 Results and discussion
3.1 XRD analysis
TiO2 is used as gold standard in photocatalysis due to its remarkable photocatalytic properties. It is also used in self cleaning, gas sensors etc. [13–19] therefore green methodologies of TiO2 formation is today’s demand. For large scale production, water soluble precursors are ultimately effective, which can fulfill green solvent features as compared to precursors like TTIP [20–23]. Therefore we have selected TiOSO4 as a precursor to obtain good quality TiO2 nanopowder.
In present work, TiO2 was prepared by hydrolysis of TiOSO4 results in the formation of white precipitate of TiO(OH)2 according to the following equation [24].
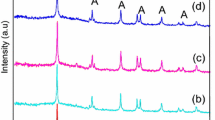
The X-ray diffraction pattern of the synthesized TiO2 calcined at 300, 400, 500 °C for 3 h are shown in Fig. 1. The determined characteristics 2θ values and [hkl] planes are 25.20 [101], 37.65 [004], 48.15 [200], 54.13 [105] and 62.38 [204] attributed to reflections of anatase TiO2 which is compared with JCPDs File No. (21–1272). The comparison of X-ray diffraction patterns of TiO2 calcined at different temperature reveals the formation of anatase phase [25]. From XRD studies it is observed that crystallinity of TiO2 increases with increase in calcination temperature up to 500 °C. At 300 °C, the crystallinity of particles begin to increase slowly and the formation of well defined crystalline anatase TiO2 was observed at 400 °C with broad peaks as compared to 500 °C were sharp diffraction peaks are obtained. The relatively broad width of peaks of XRD patterns indicates crystallites are smaller in size [26, 27]. The average crystallite size of TiO2 powder was calculated by using Scherrer’s equation [6].
where, K = Constant (0.89)
λ = Wavelength of X-ray
β = Full width at half maximum intensity (FWHM) in radian,
θ = Diffraction angle in degree.
At 400 °C an average crystallite size was found to be about 10 nm where as for 500 °C the crystallite size was increased up to 20 nm. Hence, 400 °C was chosen as the optimum calcination temperature for the synthesis of TiO2 nanoparticles. It was reported that the anatase TiO2 with small size has shown best photocatalytic activity as compared with large particle size [28, 29].
3.2 SEM images of TiO2 nanoparticles
In order to examine the surface morphology of synthesized nanoparticles, SEM analysis was performed. The SEM images of TiO2 calcined at 400 °C of different magnifications are shown in Fig. 2, which confirms that the synthesized TiO2 nanoparticles are non-uniform in shape. Here higher magnification revealed that secondary particles are agglomerated irregularly with primary particles in the range of 40–50 nm.
3.3 EDS analysis
The EDS (Fig. 3) shows two peaks which indicates presence of Ti and O at 4.5 and 0.5 keV respectively. The atomic percentage of Ti and O were 48.70 and 52.29 respectively. The present composition of Ti and O revealed the formation of non-stoichiometry TiO2 with oxygen vacancy which leads to better performance of photocatalytic activity [30]. Also the intense peak related to the bulk TiO2 and less intense peak was assigned to the surface TiO2 [31]. No any other impurities could be seen within detection limit of EDS.
3.4 TEM images of TiO2 nanoparticles
TEM images of TiO2 nanoparticles annealed at 400 °C for 3 h is shown in Fig. 4a, b, c, d which shows that the particles are not of spherical in shape. The size of particles is in the range of 10–20 nm with an average particle size from representative micrograph was found to be 10 nm which is good agreement with the result obtained from X-ray diffraction. The selected area electron diffraction pattern (SAED) indicates, TiO2 nanoparticles are in anatase phase with good crystallinity as shown in Fig. 4c with dotted concentric rings which can be assigned to non- spherical shape of TiO2. The image with higher magnification is also shown in Fig. 4d (HRTEM). The lattice fringes can be clearly indicating that the particles are nanocrystalline with anatase phase, which is also confirmed by XRD and SAED pattern.
3.5 FT-IR spectrum of TiO2 nanoparticle
FT-IR spectrum of TiO2 nanoparticles calcined at 400 °C was presented in Fig. 5. The spectrum of TiO2 capped with PVP shows diffraction peaks corresponding to the broad band centered at 543 cm−1 is a characteristic of Ti–O bending mode of vibration which confirms the formation of metal oxygen bonding [32]. The peak at 1634 was due to O–H bending vibration of adsorbed water molecule on the surface of TiO2 which may have crucial role in photocatalytic activity. The broad peak appearing at 3389 cm−1 is attributed to stretching vibration of –O–H [33]. Also there are no any other additional peaks related to organic moiety. Therefore addition of PVP did not cause any residual impurities on TiO2 surface after calcination.
3.6 Dynamic light scattering of TiO2
Dynamic light scattering is a technique that can be used to determine the size distribution profile of small particles in suspension or polymers in solution. The characterization of TiO2 nanoparticles using dynamic light scattering histogram revealed that the particle size distribution with maximum intensity at 100 nm. The increasing of particle size might be the agglomeration of the particles which is shown in Fig. 6.
3.7 UV–Vis diffuse reflectance spectrum of TiO2
Our aim is to check the photocatalytic activity; therefore band gap of material is important. The energy of incident light needed much more higher than the band gap of material used in photocatalytic process. Therefore, we have carried out to study optical nature, band gap energy absorption edge etc. by using UV–Visible diffuse reflectance spectrum of TiO2 nanoparticle calcined at 400 °C as shown in Fig. 7. The band gap energy of sample was determined by using equation
The estimated band gap energy of TiO2 was found to be 3.30 eV.
3.8 Photocatalytic degradation of methyl orange
The effect of photocatalyst calcined at different temperatures on photocatalytic degradation of MO is shown in shown in Fig. 8. From bar diagram it is revealed that, TiO2 calcined at 400 °C has more degradation efficiency which was attributed to small particle size and effectively large surface area to volume ratio than TiO2 calcined at 300 and 500 °C. So it is more favorable for photocatalysis. The effect of photocatalyst TiO2 calcined at 400 °C on degradation of M O was examined under UV light. During the experiment, the sample was analyzed at 30, 60, 90, 120, 150 min. The degradation of M O was investigated and shown in Fig. 9. It is seen that maximum 94 % degradation of M O was obtained at 1.0 g/dm3 under UV light within 150 min. Anatase TiO2 is always regarded as most effective material for photocatalytic degradation of organic pollutants in water. A 94 and 74 % MO was degraded within 150 min by anatase TiO2 and commercial TiO2 respectively. An increment of 20 % degradation efficiency was obtained for anatase TiO2 as compared to commercials TiO2. The obtained results are attributed to increase in number of active sites and photons absorbed by catalyst.
4 Conclusions
In present work, TiO2 nanoparticles were synthesized by using simple rapid and energy efficient, ecofriendly and cost effective sol gel method. The TiO2 nanoparticles calcined at 400 °C have the size in the range of 10 nm which is supported by XRD and TEM. The UV–Vis spectrum showed that absorption edge shifted in the visible region. The band gap of nanoparticles were also determined and found to be 3.12 at 300 °C and increases up to 400 °C and then decreases for higher temperature. A 94 % M O was degraded in 150 min. at 1 g/dm3 catalyst TiO2 under UV light.
References
M.R. Hoffmann, S.T. Martin, W. Choi, D.W. Bahnemann, Chem. Rew. 95, 69 (1995)
A.L. Linsebigler, G. Lu, J.T. Yates, Chem. Rew. m 95, 735 (1995)
S. Sakthivel, M.C. Hildago, D.C. Bahnemann, S.U. Geissen, V. Murugesan, A. Vogelpohl, Appl. Catal. B Environ. 63, 31 (2006)
P. Zeman, S. Takabayashi, Thin Solid Films 433, 57 (2003)
D.P. Macwan, P.N. Dave, S. Chaturvedi, J. Mater. Sci. 46, 3669 (2011)
M.A. Behnajady, H. Eskandarloo, N. Modirshahla, M. Shokri, Photochem. Photobiol. 87, 1002 (2011)
K.H. Lee, S.-W. Song, ACS Appl. Mater. Interfaces. 3, 3697 (2011)
K.K. Akuraj, A. Vital, G. Fortunato, R. Hany, F. Nueesch, T. Graule, Solid State Sci. 9, 247 (2007)
C. Chaisuk, A. Wehatoranawee, S. Preampiyawat, S. Netiphat, A. Shotipruk, O. Mekasuwandumrong, Ceram. Int. 37, 1459 (2011)
H.M. Yang, K. Zhang, R.R. Shi, X.W. Li, X.D. Dang, Y.M. Yu, J. Alloy. Compd. 413, 302 (2006)
T. Ginsbers, M. Modigell, W. Wilsmann, Chem. Eng. Res. Des. 89, 990 (2011)
A. Di Paola, M. Bellardita, L. Palmisano, R. Amadelli, L. Samiolo, Catal. Lett. 143, 844 (2013)
N.T. Nolan, M.K. Seery, S.C. Pillai, J. Phys. Chem. C 113, 16151 (2009)
N. Wetchakun, S. Phanichphant, Curr. Appl. Phys. 8, 343 (2008)
S. Ngamta, N. Boonprakob, N. Wetchakun, K. Ounnunkad, S. Phanichphant, B. Inceesungvorn, Mater. Sci. Res. Cent. Thail. 105, 76 (2013)
S. Mahshid, M. Askari, M.S. Ghamsari, N. Afshar, S. Lahuti. J. Alloys Compd. 478, 586 (2009)
R. Vijayalakshmi, V. Rajendran, Arch appl. sci. Res. 4, 1183 (2012)
M.B. Suwarnkar, R.S. Dhabbe, A.N. Kadam, K.M. Garadkar, Ceram. Int. 40(4), 5489 (2014)
H. Ogawa, A. Abe, J. Electrochem. Soc. 128, 685 (1981)
L.C. Yan, J.B. Wang, Y.Q. Wang, Chin. Phys. B 21, 9 (2012)
B.R. Li, Q. Li, W. Zhang, J.Q. Li, Y. Hao, Dev. Chem. Ind. 39, 3 (2010)
H.S. Chen, R.V. Kumar, RSC Adv. 2, 2294 (2012)
L.Y. Zhang, Y. CaO, Z.X. Liu, X.L. Yu, Z.F. Lu, J. Rare metals 35, 504 (2001)
A.S. Stanislav, T. Yoed, J. Mol. Catal. A 314, 55 (2009)
B.S. Shirke, P.V. Korake, P.P. Hankare, S.R. Bamane, K.M. Garadkar, J. Mat. Sci. Mater. Electron 22, 821 (2011)
D. Wang, L. Xiao, Q. Lau, X. Li, J. An, Y. Duan, J. Hazard. Mater. 192, 150 (2001)
T.M. Breeault, B.M. Bartlett, J. Phys. Chem. 116, 5986 (2012)
A.N. Kadam, R.S. Dhabbe, M.R. Kokate, Y.B. Gaikwad, K.M. Garadkar, Spectrochem. Acta A. 133, 669 (2014)
P.V. Korake, A.N. Kadam, K.M. Garadkar, J. Rare Earth 32, 306 (2014)
G. Liu, H.G. Yang, X. Wang, L. Cheng, H. Lu, L. Wang, G.Q. Lu, H.M. Cheng, J. Phys. Chem. 113, 21784 (2009)
M. Hamadanian, A. Reisi-Vanani, A. Majedi, Mater. Chem. Phy. 116, 376 (2009)
Y. Gao, Y. Masuda, Z. Peng, T. Yonazawa, K. Kaumoto, J. Mat. Chem. 13, 608 (2007)
J. Kunze, A. Ghicov, H. Hildebrand, J.M. Macok, L. Traveira, P. Schmuki, Z. Phys. Chem. 219, 1561 (2005)
Acknowledgments
One of the authors (KMG) thankful to DST for financial support under major research project (SR/S1/PC/0041/2010). Authors are also thankful to Director SAIF, NEHU Shillong for providing TEM facility.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khade, G.V., Suwarnkar, M.B., Gavade, N.L. et al. Green synthesis of TiO2 and its photocatalytic activity. J Mater Sci: Mater Electron 26, 3309–3315 (2015). https://doi.org/10.1007/s10854-015-2832-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-015-2832-7