Abstract
In this study, Cu–Sn alloy was electrodeposited from aqueous electrolytic bath onto Mo electrode. Before electrodeposition, some calculations using MATLAB software to obtain the dominant complex of Cu–citrate in different pH values and cyclic voltammetry (CV) experiments was performed. The potential range in which the alloy electrodeposition process could be carried out in a solution containing CuSO4, SnSO4, and Na3C6H5O7 was determined by CV. In addition, the effects of boric acid and cetyl trimethyl ammonium bromide (CTAB) surfactant on codeposition potential were studied. The microstructural properties and alloy composition were investigated by scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDS), respectively. Alloy composition up to 49.5 at.% of Sn was obtained. Alloy composition of 33 at.% Sn corresponding to Cu2SnS3 was obtained at solution containing 0.04 M SnSO4, 0.02 M CuSO4 and 0.4 M Na3C6H5O7 at Potential −0.75 V.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Copper alloys have a wide variety of applications due to their high thermal and electrical conductivity, good corrosion resistance, mechanical workability, electrocatalytic activity, antibacterial effect, and magnetic property [1–8].
Among these alloy systems, Cu–Sn alloy is known as one of the most commonly used in different fields, as metal coatings [9] and interconnecting materials [10–12]. Semiconducting alloy of Cu–Sn–S like Cu2SnS3 that can be prepared by codeposition of Cu and Sn, and then sulfurization of Cu–Sn alloy, has been recently considered as absorbers in solar cells thin films [13–16]. Sn–Cu alloy can be a promising alternative for Sn–Pb alloy in the soldering industry, because of worldwide raising health and environmental concerns about the toxicity of Pb [17–19]. In addition, Sn and Sn–Cu intermetallic have been suggested as anode materials for Li-ion batteries, because of their high theoretical capacity compared to the graphite [20–23]. Also, Cu–Sn alloy layer can be used as an alternative to allergenic nickel underplating for decorative gold or chromium electroplating [24]. Lower resistivity resistance of copper compared to aluminum, makes it the material of interest for interconnecting materials, however diffusion of Cu could cause electromigration and stress migration failure. This problem has been solved by alloying Cu with another metal such as Sn, Ag or Co [10–12].
Cu–Sn coatings can be deposited by electron beam evaporation [16], spray pyrolysis [25], electrodeposition [11]. Among these techniques, electrodeposition is a low-cost and simple technique that can be used for fabrication of Cu–Sn layers. Nevertheless, the significant difference between reduction potentials of Cu and Sn ions may result in poor quality coatings. In this case, the quality of layers can be improved by bringing their reduction potentials close enough to each other using different complexing agents such as cyanide, stannous, citrate, pyrophosphate, tartrate and so on [26].
Two types of bathes, acidic [11, 27–30] and alkaline [31], are used for electrodeposition of Cu–Sn alloys. The pH of acidic solutions is about one, which makes them undesirable due to etching and corroding the materials. Alkaline solutions are not compatible with photoresist materials that limit their application in MEMS [32, 33].
In this paper, citrate as complexing agent is used to deposit Cu–Sn alloy layers onto Mo substrate. The aim of this work is to examine the codeposition of Cu and Sn at pH of 6 in order to obtain conditions for fabrication of Cu2SnS3. Besides, the effect of cationic surfactant cetyl trimethyl ammonium bromide (CTAB) and boric acid on reduction potential difference of copper and tin is investigated with cyclic voltammetry.
2 Materials and methods
First, the bulk concentrations of reactants were calculated by MATLAB software on the basis of Taylor method in different pH values to obtain the dominant complex of Cu–citrate in different pH.
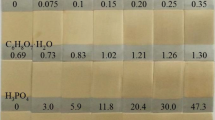
Then, cyclic voltammetry experiments were performed to obtain the codeposition potential range in the aqueous electrolytic bath containing copper sulfate pentahydrate (CuSO4·5H2O), tin(II) sulfate (SnSO4), trisodium citrate (Na3C6H5O7·2H2O). In addition, boric acid (H3BO3) and CTAB were added to the electrolytic bath and their effects on electrodeposition process were investigated. Table 1 shows the chemical concentrations and different conditions of electroplating bath. Then, a potential range obtained from CV was used to perform the potentiostatic alloy deposition experiments.
The Cu–Sn layers were fabricated from the aqueous electrolytic bath using one-step electrodeposition method at room temperature without stirring in a conventional three-electrode electrochemical cell assembly. The pH value was controlled digitally. The electrochemical cell contains an Ag/AgCl reference electrode, a platinum electrode as an inert counter electrode and Mo substrate with a deposition area of 5 × 5 mm2 as working electrode. Before electroplating, the substrate was grounded by 2,500 grit SiC waterproof paper and then cleaned ultrasonically in detergent, acetone, and distilled water to remove contaminations from the substrate.
The surface morphology and alloy composition of deposited layers were characterized by scanning electron microscope (SEM; TESCAN, Model: VEGA2, Czech Republic) and energy dispersive X-ray spectroscopy (EDS; RONTEC), respectively.
3 Results and discussion
3.1 Calculation of reactants concentration
In electrodeposition of Cu–Sn alloy, it is important to identify the dominant copper-citrate complex and its concentration in a specific pH. In the present study, trisodium citrate was used to form complexes of Cu+2 and Sn+2 ions to bring their electrodeposition potential closer and consequently allow alloy electrodepositing of Cu and Sn. Therefore, the bulk concentrations of reactants were calculated by MATLAB software on the basis of Taylor method in different pH values by using the equilibrium constants, material conservation equations, and the electroneutrality conditions according to the refs. [34, 35]. The equilibrium concentration and speciation diagram are shown in Fig. 1.
Figure 1 shows that Cu2Cit2H (−4)−2 is the dominant complex around pH 6 and contributes in reduction reaction. In the case of tin-citrate complexes, C. Han et al. [36]. Showed that only SnHCit−1 existed in solution of SnSO4 and trisodium citrate at pH = 6 and changes in the color of Sn–citrate solution lower than pH = 3 and higher than pH = 7 confirmed the presence of different complexes.
3.2 Cyclic voltammetry evaluation
3.2.1 Voltammetric studies of Cu–Sn electrodeposition
Figure 2a shows that the reduction current of Cu+2 ion starts at −0.08 V and reaches the highest value at around −0.1 V. Cyclic voltammetry of solution containing Na3C6H5O7 and CuSO4 shows two reduction peaks at −0.3 V and −1.1 V corresponding to the reduction of Cu–citrate complex and molybdenum oxide from the substrate, respectively. Molybdenum can react with H2O to produce MoOx·yH2O. Molybdenum oxide possibly forms a complex with Na3C6H5O7 and consequently reduces to molybdenum in the presence of Cu–citrate complex. An intermediate ion such as copper ion in this study is necessary to induce the reduction reaction of molybdenum oxide to molybdenum [37, 38]. Figure 2b shows cyclic voltammetry of the solution containing CuSO4 and Na3C6H5O7 at different pH values ranging from 3 to 8. As can be seen in this figure, with the increase of pH values to 6, the reduction peak and beginning of the electrodeposition shift toward more negative potentials corresponding to the presence of various complexes as discussed above.
Figure 3 shows that the reduction current of Sn–citrate complex starts at around −0.2 V and reaches the highest value at around −1 V, while according to Fig. 2b the reduction current of Cu–citrate complex starts at around −0.1 V at pH = 6 and exhibits a peak at around −0.3 V. Therefore, coelectrodeposition of copper and tin is obtained at potentials more negative than −0.2 V. The reduction peak observed at −1.1 V in Fig. 3 can be attributed to the reduction reaction of molybdenum oxide to molybdenum in presence of tin ions.
In Fig. 4, a reduction peak around −0.1 V and a reduction peak around −0.75 V are observed at CV of the solution containing 0.02 M CuSO4, 0.02 M SnSO4, and 0.2 M Na3C6H5O7 corresponding to the reduction of Cu+2 ions and Sn–citrate complex, respectively. After adding more Na3C6H5O7, the peak attributed to the reduction of Cu+2 ions disappeared, because the Cu+2 ions are formed into their complexes, i.e., Cu2Cit2H −4−2 . In addition, the reduction peak corresponding to Sn–citrate complex shifted toward the larger positive E/V values comparing to Fig. 3. It seems that the overpotential for the reduction of Sn–citrate complex on early deposited copper layer is lower than that on molybdenum substrate. Starting reduction reaction of Sn–citrate on early deposited copper layer at positive potentials can shift the reduction peak of Sn–citrate toward positive potentials. As can be seen in this figure, decrease of SnSO4 from 0.02 M to 0.01 M with the same amount of Na3C6H5O7 leads to the appearance of a reduction peak at around −0.4 V corresponding to Cu–citrate complex. It means that Sn–citrate complex is more stable than Cu–citrate complex and surplus trisodium citrate forms citrate complex of Cu+2 ions. The higher stability constant of SnHL−1 (log β = 10) [36] comparing to Cu2Cit2H −4−2 (log β = 5.87) [34] confirms our results.
Figure 5 shows that with the increase of pH values from 4 to 6, the reduction peak of Cu–citrate complex (peak around −0.35 V) shifts toward more negative E/V values due to the presence of different complexes. Consequently, a delay in reduction reaction of Cu–citrate complex results in a shift toward more negative E/V values in reduction peak of Sn–citrate complex (peak around −0.7 V).
3.2.2 Effect of CTAB surfactant and boric acid
CTAB is expected to improve the surface appearance of coatings, because it is a kind of cationic surfactant that can be adsorbed on the cathode surface. As shown in Fig. 6, when CTAB is added to the solution, all reduction reaction peaks shift toward more negative E/V values and the value of peak current decreases due to the adsorption of CTAB on the cathode surface. However, some white precipitation appeared after addition of CTAB resulting in instability of the solution. Consequently, it is not recommended to use CTAB for electrodeposition of Cu–Sn in similar conditions of our work.
Boric acid adsorbed on the surface of the electrode increases the overpotential for the hydrogen evolution on the cathode and acts as a catalyzer for electrodeposition [39–41]. As shown in Fig. 6, the cyclic voltammogram of the solution containing Sn–citrate and Cu–citrate depends on the presence of boric acid so that it lowers the codeposition overpotential of copper and tin. Therefore, boric acid can be useful for alloy electrodeposition of copper and tin.
3.3 Potentiostatic electrodeposition
Cyclic voltammetry evaluations showed that 0.4 M Na3C6H5O7 formed all 0.02 M SnSO4 and 0.02 M CuSO4 into their complexes and their solution was stable in pH 6. In addition, reduction potentials of Cu–citrate and Sn–citrate complexes shifted toward each other with the increase of pH values. In Fig. 7, pH value of potentiostatic electrodeposition solution was adjusted at 6 and the current responses were investigated. The potentials that were more negative led to higher electrodeposition current due to the incorporation of more tin ions into the deposited layer. The current responses in the potentials of −0.7 and −0.8 V were considerably different attributing to three factors:(1) the decrease of the current for double layer charging (2) the increase of the current due to the nucleation and growth of nuclei, and (3) the decrease of current as a result of overlapping of diffusion fields of nuclei and growth of layer under diffusion [42, 43]. There are two peaks in current responses of solution containing 0.04 M and 0.06 M SnSO4 before steady state step corresponding to a two-step nucleation and growth process.
3.4 Microstructure and alloy composition
SEM micrographs of coatings electrodeposited at the potentials of −0.4, −0.5, −0.6, −0.7, −0.75 and −0.8 V are shown in Fig. 8. The different microstructures were because of the different alloy compositions. The weakness of surface coverage at potentials of −0.4 and −0.8 V were due to the lower electrodeposition rate, and the pronounced growth in highly active sites, respectively. The good surface coverage was obtained at the potentials in the ranging from −0.5 to −0.7 V. The surface of the deposited layer at the potential of −0.6 V was smooth and its alloy composition corresponded to yellow miralloy. Surface morphology at the potential of −0.8 V was cauliflower-like and its alloy composition corresponded to white miralloy, speculum metal or white bronze [24]. Figure 9 shows the alloy composition deposited at potentials ranging from −0.4 to −0.8 V determined by EDS. The Sn content in alloy composition obtained from solution containing 0.02 M SnSO4 increased with the increase of electrodeposition potential, while the Cu content in alloy composition increased with the increase of electrodeposition potential up to −0.6 V and then decreased. Appearance of the deposited layers corresponded well with their composition so that it was reddish at the potentials of −0.4 and −0.5 V due to their higher content of copper, golden at the potential of −0.6 V and silver-white at the potentials of −0.7 and −0.8 V.
SEM images of Cu–Sn coatings obtained from solution containing 0.02 M CuSO4·5H2O, 0.4 M Na3C6H5O7·2H2O (a). 0.02 M SnSO4, −0.4 V (b). 0.02 M SnSO4, −0.5 V (c). 0.02 M SnSO4, −0.6 V (d). 0.02 M SnSO4, −0.7 V (e). 0.02 M SnSO4, −0.8 V, (f). 0.04 M SnSO4, −0.75 V (g). 0.06 M SnSO4,−0.75 V, duration time 45 min
The atomic percent of molybdenum in Fig. 9 depends on the thickness of the layer so that, the more amounts of molybdenum are measured when the deposited layer is thinner. As shown in Fig. 9, molybdenum content of deposited layers from solution containing 0.02 M SnSO4 decreases with the increase of potentials up to −0.7 V and increases afterward, meaning that the thickness of coatings increases with the increase of the potentials up to −0.7 V. In addition, weak surface coverage due to the pronounced growth at the potential of −0.8 V, increased molybdenum content.
In order to obtain an alloy composition for fabrication of Cu2SnS3 along with better coverage, the concentration of SnSO4 increased to 0.04 M and 0.06 M. Potentiostatic electrodeposition in these concentrations was performed at potential −0.75 V. Figure 9 shows that at concentration of 0.04 M and 0.06 M SnSO4 at potential −0.75 V the Sn content of alloy is 33 at % and 49.5 at %, respectively. At concentration of 0.04 M SnSO4, the Sn/Cu ratio and Mo/(Sn + Cu) in alloy is close to 0.5 and zero, respectively. Figure 8f shows that the surface coverage is acceptable for coating obtained from solution containing 0.04 M SnSO4 at potential −0.75 V for further investigations in order to fabricate Cu2SnS3.
4 Conclusions
Calculation of reactants concentration, cyclic voltammetry and potentiostatic experiments were performed to evaluate alloy electrodeposition of copper and tin from aqueous solution containing Na3C6H5O7 as complexing agent. In summary, the following results could be mentioned.
-
1.
Based on calculation done by MATLAB software, it was found that the dominant complex of Cu–citrate is Cu2Cit2H (−4)-2 at pH 6. So, Cu2Cit2H (−4)−2 and SnHCit−1 take part in reduction reaction to fabricate electrodeposited alloy of copper and tin.
-
2.
Na3C6H5O7 can form complex of Cu+2 and Sn+2 ions and makes the solution stable. Increasing pH up to 6 shifts reduction potential of Cu–citrate complex toward Sn–citrate one and makes alloy electrodeposition possible.
-
3.
Boric acid can make coelectrodeposition better by decreasing alloy electrodeposition overpotential. Adsorbing CTAB as cationic surfactant on the cathode surface increased overpotential for alloy electrodeposition by shifting reduction potential of Cu–citrate and Sn–citrate complexes toward negative potentials. Moreover, some white precipitation occurred after adding CTAB.
-
4.
A composition range of Cu–Sn alloy with Sn content up to 49.5 at.% was obtained through potentiostatic electrodeposition at potentials −0.4, −0.5, −0.6, −0.7, −0.75 and −0.8 V. Their appearance was also in agreement with their composition. Alloy composition for fabrication of Cu2SnS3 was obtained at solution containing 0.04 M SnSO4, 0.02 M CuSO4 and 0.4 M Na3C6H5O7 at potential −0.75 V.
References
J.H. Choi, Mater. Sci. Eng. A 550, 183 (2012)
P. De Vreese, A. Skoczylas, E. Matthijs, J. Fransaer, K. Binnemans, Electrochim. Acta 108, 788 (2013)
J. Liu, F. Li, C. Liu, H. Wang, B. Ren, K. Yang, E. Zhang, Mater. Sci. Eng. C 35, 392 (2014)
M.R. Khelladi, L. Mentar, A. Azizi, L. Makhloufi, G. Schmerber, A. Dinia, J. Mater. Sci. Mater. Electron. 23, 2245 (2012)
U. Sarac, R.M. Öksüzoğlu, M.C. Baykul, J. Mater. Sci. Mater. Electron. 23, 2110 (2012)
I. Marković, S. Nestorović, D. Marković, Mater. Des. 53, 137 (2014)
R. Lei, S. Xu, M. Wang, H. Wang, Mater. Sci. Eng. A 586, 367 (2013)
B. Zhao, Y. Zhang, J. Yang, J. Mater. Sci. Mater. Electron. 24, 4439 (2013)
Y. Sürme, A.A. Gürten, E. Bayol, E. Ersoy, J. Alloy. Compd. 485, 98 (2009)
I. Volov, X. Sun, G. Gadikota, P. Shi, A.C. West, Electrochim. Acta 89, 792 (2013)
D. Padhi, S. Gandikota, H.B. Nguyen, C. McGuirk, S. Ramanathan, J. Yahalom, G. Dixit, Electrochim. Acta 48, 935 (2003)
A. Survila, Z. Mockus, R. Juškėnas, V. Jasulaitienė, J. Appl. Electrochem. 31, 1109 (2001)
K. Junpei, C. Kotaro, A. Naoya, A. Hideaki, N. Ryota, J. Kazuo, K. Hironori, Jpn. J. Appl. Phys. 51, 10NC34 (2012)
P.A. Fernandes, P.M.P. Salomé, A.F.D. Cunha, J. Phys. D Appl. Phys. 43, 215403 (2010)
D.M. Berg, R. Djemour, L. Gütay, G. Zoppi, S. Siebentritt, P.J. Dale, Thin Solid Films 520, 6291 (2012)
N. Aihara, H. Araki, A. Takeuchi, K. Jimbo, H. Katagiri, Phys. Status Solidi. C 10, 1086 (2013)
C. Han, Q. Liu, D.G. Ivey, Electrochim. Acta 54, 3419 (2009)
W. Tang, Y. Hu, S. Huang, Met. Mater. Int. 18, 177 (2012)
E. Çadırlı, U. Böyük, S. Engin, H. Kaya, N. Maraşlı, M. Arı, J. Mater. Sci. Mater. Electron. 21, 468 (2010)
D.H. Nam, R.H. Kim, D.W. Han, H.S. Kwon, Electrochim. Acta 66, 126 (2012)
X.H. Weihua Pu, Jianguo Ren, Chunrong Wan, Changyin Jiang, in: 6th Advanced Batteries and Accumulators (ABA), 2005
J.-Y.E. Jung-Won Park, H.-S. Kwon, Int. J. Electrochem. Sci. 6, 3093 (2011)
S.D. Beattie, J.R. Dahn, J. Electrochem. Soc. 150, C457 (2003)
K.I. Murase, S. Akira, I. Takashi, S. Hiroyuki, J. Electrochem. Soc. 158, 335 (2011)
A. Mehdi, M.M. Bagheri, E. Hosein, Phys. Scr. 85, 035603 (2012)
Y.-T. Hsieh, C.-C. Tai, I.-W. Sun, Meet. Abstr. 53, MA2010 (2010)
C.T.J. Low, F.C. Walsh, J. Electroanal. Chem. 615, 91 (2008)
Y. Goh, A.S.M.A. Haseeb, M.F.M. Sabri, Electrochim. Acta 90, 265 (2013)
A.N. Correia, M.X. Façanha, P. de Lima-Neto, Surf. Coat. Technol. 201, 7216 (2007)
N. Pewnim, S. Roy, Electrochim. Acta 90, 498 (2013)
G.A. Finazzi, E.M. de Oliveira, I.A. Carlos, Surf. Coat. Technol. 187, 377 (2004)
Q.L. Chunfen Han, Douglas G. Ivey, in: CS MANTECH Conference, Austin, Texas, USA, 2007
A. He, Q. Liu, D. Ivey, J. Mater. Sci. Mater. Electron. 19, 553 (2008)
F.I.L. Tzec, G. Oskam, ECS Trans. 25, 195 (2010)
R.Y. Ying, P.K. Ng, Z. Mao, R.E. White, J. Electrochem. Soc. 135, 2964 (1988)
C. Han, Q. Liu, D.G. Ivey, Electrochim. Acta 53, 8332 (2008)
D. Sinkeviciute, J. Baltrusaitis, N. Dukstiene, J. Sol. State Electrochem. 15, 711 (2011)
E. Gómez, E. Pellicer, E. Vallés, J. Electroanal. Chem. 580, 222 (2005)
Z. Zheng-zhong, Z. Xiao-rong, Z. Chao, Wuhan Univ. J. Nat. Sci. 4, 211 (1999)
M. Rezaei, M. Ghorbani, A. Dolati, Electrochim. Acta 56, 483 (2010)
N. Zech, D. Landolt, Electrochim. Acta 45, 3461 (2000)
M. Paunovic, M. Schlesinger, Fundamentals of Electrochemical Deposition, 2nd edn. (Wiley, London, 2006)
Y.D. Gamburg, G. Zangari, Theory and Practice of Metal Electrodeposition (Springer, New York, 2011)
Acknowledgments
The authors are grateful to Amirkabir University of Technology and Esfarayen University of Technology for their support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Heidari, G., Mousavi Khoie, S.M., Abrishami, M.E. et al. Electrodeposition of Cu–Sn alloys: theoretical and experimental approaches. J Mater Sci: Mater Electron 26, 1969–1976 (2015). https://doi.org/10.1007/s10854-014-2636-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-014-2636-1