Abstract
Aluminium silvering is widely used for creating high current joints between two busbar connectors to transfer electrical power. Silver properties improved joints quality and long term conductivity. The most commonly used technique is the laborious electroplating, which produces a lot of toxic sludges. As an alternative, the authors elaborated new technology of silvering busbar connectors. The new method of aluminium silvering consists of well-known screen printing process of properly prepared paste with silver nanoparticles. Use of nanoparticles gives an opportunity to print and sinter the silver layer on aluminium base. Another advantage of this technology is that the layer can be deposited and sintered in outdoor conditions, using acetylene torch or hot air gun. In this paper, several technological parameters, as well as obtained results, are discussed. Different paste compositions, grain size of silver nanoparticles and sintering method were measured. The adhesion between silver layer and aluminium base was measured. The contact resistivity of joints between silvered electrodes were tested and compared to electroplated joints or pure aluminium contacts. The measurements results are shown and discuss. The screen printed layer was less dependent on joint pressure than those from pure or electroplated aluminium.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Aluminium busbar joints provide high electrical current connections between metal connectors and are widely used in electrical power industry. They are formed by clamping two or more plates of flat aluminium with the use of bolts and nuts. They are supposed to be rigid and maintenance free and could be detached and reattached. Unfortunately, the plain aluminium can’t be used because of its high susceptibility to form aluminium oxide, especially in high current joints, what was precisely described by Schoft [1]. To prevent that situation, a surface of aluminium can be electroplated with silver. This technique is commonly used in industry, but due to the difficulty of covering the aluminium surface with silver, it’s necessary to use intermediate layers like copper or nickel. The intermediate layer is made with the use of electroplating process. The electroplating process consists in immersing the aluminium in bath, which contains proper metal salt. Then the current is applied to the bath. Plated material becomes a cathode, causes metal ions to migrate to the aluminium plate and deposits on it. This decreases joint resistance and prevents aluminium from oxidation. The main disadvantage of that technique is that it causes a lot of toxic, dangerous for natural environment wastes. What’s more, electroplating is a time-consuming method, which can be used only to cover conductive surfaces. The use of intermediate layers increases the process duration and creates additional costs. The ion concentration in metal salt causes the change of process effectiveness and that is why metal salt ion concentration should be checked and compensated to the same level. Moreover, it is difficult to provide suitable bath and containers to perform electroplating in outdoor conditions to repair silver coating on big aluminium elements. On the other hand, electroplating is a long known and thoroughly researched technique, used for years and described in many patents [2, 3].
In present paper authors proposed an alternative to electroplating method of silver deposition on aluminium. It can be done by screen printing a paste containing nano-sized silver on aluminium substrate. Then the silver layer is dried in 120 °C for 10 min and sintered in a furnace. This method can be easily applied during outdoor repair with no need of sophisticated tools. Due to high surface energy of nanoparticles, sintering of layers occurs in temperatures much below silver melting point (961 °C) and, what is more important, below melting point of aluminium (660 °C). In recent paper [4] the authors proved, that well sintered layers can be obtained on dielectric substrates, i.e. glass, cerami, and Kapton foil in temperatures below 300 °C, which, in case of standard silver paste, would be insufficient. In present work the authors applied paste with silver nanoparticles to low melting metal substrate to enhance reliability of joints, which is described in European patent [3]. Because of the special condition, in which joint works, it’s needed to be tested in respect of join resistance and adhesion between silver and aluminium. There are many different types of adhesion, but the main one for those tests is practical adhesion [5]. It is hard to examine it with the use of conventional tests due to layer thickness [6]. To overcome that problem, some special methods are developed, like Modified Button Shear Test [7]. Finally, the scratch test was chosen as a suitable method for measuring, estimating the adhesion of thin silver layer [8–10].
2 Materials
2.1 Powders
Silver nanopowder can be obtained by various methods like laser ablation [11], polyol synthesis [12] or thermal decomposition of silver palmitic acid. Due to its easy upscaling and good efficiency, the last method was chosen. Grain size distribution was evaluated basing on SEM and image analysis. It was close to Gaussian plot with an average 30.5 nm and standard deviation 5.4 nm. Powder contained 20–25 % of palmitic acid chains as an organic coating. This residue prevents nanoparticles from agglomeration, because palmitic acid chains behave as natural surface-active medium. More details and the procedure of obtaining this powder was presented in [1]. The second solid content applied was the powder with silver flakes from Amepox AX20LC. Verified average particle thickness for silver flakes was 2–4 μm. Maximum agglomerate size was 6–12 μm and average flake size was 100 nm.
2.2 Pastes
The pastes contained 80–85 wt% of a silver powder and 15–20 wt% of an organic vehicle. Two types of silver powders were used. In paste “nano-Ag” the only solid component was the silver nanopowder. In “nano/flake-Ag” paste the two types of powders were used, silver nanopowder and commercial Amepox AX20LC silver microflakes in weight portions ratio 1:3. The mixture of two powders with different sizes and shapes i.e. nano-powder and silver micro flakes allows to obtain higher packing densities and therefore better sintering than with using a single-sized powder. For single-sized spheres the maximum theoretical packing density for ordered arrangement of uniformly sized spheres is 0.74. Due to some randomness in grain arrangement and deviations of real grains the packing densities of the real powders are about 0.65. The mixing of powders differing in sizes can result in significant improvement of packing densities. For the size ratio of particles 0.02 the packing densities 0.85 can be achieved if the volumetric content of finer particles in the mixture is 25 % (1:3). [13, 14]. This ratio between grain provides nearly the highest packing densities even for other size ratios in the range 0.02–0.6. Powders were weighed and suspended in toluene. Precipitation was conducted by means of ultrasonic agitation with frequency of 35 kHz for 2, 5 h.
Then the silver powders were dispersed in a simple organic vehicle by grounding with the use of ultrasonic agitation and a three-roll mill. The rheological properties suitable for screen-printing technology, along with the ability to decompose and evaporate in 300 °C, were provided by the composition of an organic vehicle. The organic vehicle consisted of two components: a resin and a solvent. As a resin a Polymethyl methacrylate with weight average molar mass Mw ~350,000 measured by GPC (Sigma–Aldrich) was chosen. As a solvent a butyl carbitol acetate was applied. The content of a resin in a solvent was 8 wt%. This vehicle, after thermal treatment at 300 °C for 1 h, leaves 70 ppm of residues only.
2.3 Substrates
The substrates were metal bars with dimensions 95 × 50 × 6 mm made from aluminium. The EDX analysis of substrate revealed the existence of impurities, mainly Cu, Si and Mg.
The surface of the substrates were chemically etched before silvering. The surface roughness of the substrate was measured before etching and at each stage. The 3D scanning profile-meter, Veecoo Dektak 150, was used. The initial surface roughness of the unetched substrate was 0.02 µm and no surface damages were noticed as shown in Fig. 1.
Several etching baths were prepared: They are enumerated in Table 1. The three different etching procedure were tested as follows:
-
1.
Samples “1T” were etched for 1 min in bath no. “1” at 40 °C. After etching, the surface roughness increased very slightly to 0.05 µm, however the 0.15 µm deep trenches appeared. They were distributed randomly with mean density of 300 per square mm. The surface roughness outside the trenches was nearly not affected by etching. They were created by higher speed of etching impurities, like silicon and by creating a local electrochemical cell around the Cu and Mg impurities.
-
2.
Samples “2T” were etched for 1 min in bath no. “1” at 40 °C (like sample “1T”) then etched for 3 min in bath no. “2” at 40 °C.
The surface roughness has increased even further to 0.13 µm. More trenches has appeared, some of which were even 0.60 µm deep distributed on the surface with density 500 per square mm. It was the consequence of copper, magnesium and other compounds removal.
-
3.
Samples “3T” were etched for 1 min in bath no. “1” at 40 °C then etched for 3 min in bath no. “2” at 40 °C (like sample “2T”) and finally etched for 3 min in bath no. “3” at 40 °C.
The surface morphology changed entirely. The surface roughness achieved 0.26 µm. The whole surface became rough without any flat spots as shown in Fig. 2.
2.4 Silver printing
The pastes can be deposited by a film bar applicator, screen printing or stencil printing. Due to a large area of an open window in a stencil the squeegee has to be rigid. Screen printing in this paper was chosen due to requirements of a scratch test on an even film thickness, that the stencil or applicator cannot provide on large area substrates. The pastes were deposited on aluminium substrates by screen printing technique through steel screen with resolution 200 mesh, then dried at 120 °C for 10 min. The series of samples were described in Table 2. Most of the samples were sintered in belt furnace except sample “O” which was thermally treated with hot air gun rated for 550 °C of an output air and sample “V”, which was treated with acetylene torch adjusted towards excess oxygen (very lean mixture). The last two methods simulated outdoor repair conditions. After sintering the layer thickness and roughness were measured.
2.5 Joint resistance
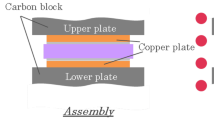
Two samples were placed with the silver layers facing each other with 5 mm of overlap. Then both were pressed toward each other with pneumatic press. Maximum pressure obtained was 13 MPa. The joint resistance during pressing was measured. The schematic measuring setup is shown in Fig. 3, and the results are shown in Fig. 4. The contact resistivity versus joint ascending and descending pressure was measured for current density 0.2 A/mm2. After detaching the layers were optically inspected to find delamination defects.
2.6 Adhesion measurements
Samples after resistance measurements were scratch tested on Revetest Scratch Tester. The force was set from 1 to 10 N and the increase was 1 N/mm. Samples were silvered by using two different pastes. One with nanoparticles and second with mixture nanoparticles and flakes. The SEM picture for aluminium surface silvered by nano/flake-Ag paste was shown in Fig. 5, while in Fig. 6, the SEM for sample silvered with nano-Ag was shown. For the interface research between the nano/flake-Ag layer and substrate, sample were broken and then fracture was examine by SEM, which is shown in Fig. 7.
3 Conclusion and summary
The experiment results (Fig. 4) have shown, that joint resistance between electrodes covered with silver layer is less dependent to joint pressure than joints between pure aluminium. These joints stay reliable even under low pressures, whereas the joint resistance of pure aluminium rises significantly. That phenomena can solve the common problem with sudden joint failure due to aluminium creep, that causes lowering joint pressure during exploitation [15]. The investigation also showed, that increasing annealing temperature of joints from 400 °C up to 500 °C decreased joint resistance by 50 %. Increased annealing temperature causes interdiffusion between silver and aluminium, which improves joint adhesion and conductivity. Samples with nano-Ag layer (“A” and “B”) were susceptible to delamination effects no matter which temperature of sintering was chosen. Delamination is usually results from insufficient adhesion of sintered layer to the substrate. The problem was solved in two steps. By applying a mixture of nano/flakes-Ag (Sample “D”) the delamination was significantly reduced, but still existent. The 3 stage aluminium etching before layer printing “3T” have solved the problem entirely (Sample “C”). The drawback was a higher joint resistivity of samples with nano/flakes mixture than samples with nano-Ag due to higher surface roughness and thickness (Table 1). The hot air gun did not provide enough temperature to sinter the layer (Sample “O”). Therefore the color of cured layer was still brown and defect occurred after attach/detach test. The acetylene torch was sufficient to sinter silvered layer and resulted in matte white color. This method of sintering is recommended for outdoor repair.
The information about adhesion was obtained from SEM images by analyzing the scratched surface. Scratch line created on nano/flakes-Ag layer is presented in Fig. 5 parted in three sections. The continuous detachment is shown in Fig. 5c at the end of the test after 6 mm of distance which is approximately 6 N. Some silver layer parts have been crushed by diamond indenter and broken off, but didn’t fall out completely, what is shown in Fig. 5b, c. That means that the layer adheres to aluminium substrate. The area of detachment is define by flakes-Ag agglomeration size. The higher applied stress was the more conglomerate flakes broken away. Silver layer transverse plastic deformation is observed even after 600 µm (0.6 N) as transverse cracks in indenter track. Figure 6 shows scratched nano-Ag layer. Transverse cracks were observed after 670 µm (0.67 N). The Ag coating fall out completely after 1.168 mm (1.17 N). The last part of SEM image (Fig. 6) reveals that the layer do not adhere to aluminium base. After all the researches were made the entire silver coating fall out completely from specimen. That lead to the conclusion that the presence of silver flakes significantly increase the adhesion between layer and aluminium base.
The fracture image (Fig. 7) proved that the nano/flakes-Ag layer adhesion to aluminium substrate looks properly. The coating completely adhere to aluminium base and does not make the open air space between materials. The silver flakes are clearly visible in the image because they made a layer base structure. All the researches proves that silver coating with paste contains of nano/flakes-Ag shows relevant properties for use as high current joints.
References
S. Schoft, Joint resistance depending on joint force of high current aluminium joints, in Electrical Contacts (2004), pp. 502–510
L. Woods, US4430169A (1984)
M. Jakubowska, M. Jarosz, O. Jeremiasz, A. Młożniak, M. Teodorczyk, EP2447313 (2013)
M. Jakubowska, M. Jarosz, K. Kiełbasinski, A. Młożniak, New conductive thick-film paste based on silver nanopowder for high power and high temperature applications. Microelectron. Reliab. 51(7), 1235–1240 (2011)
K.L. Mittal, Adhesion measurement of thin films. Electrocompon. Sci. Technol. 3, 21–42 (1976)
S.C. Joo, D.F. Baldwin, Analysis of adhesion and fracture energy of nano-particle silver in electronics packaging applications. IEEE Trans. Adv. Packag. 33(1), 48–57 (2010)
S. Joo, D.F. Baldwin, Interfacial adhesion of nano-particle silver interconnects for electronics packaging application, in Electronic Components and Technology Conference (2008), pp. 1417–1423
Li Jiang, Thermo-mechanical reliability of sintered-silver joint versus lead-free solder for attaching large-area devices, Virginia Polytechnic Institute, 8 Dec 2010
K.-S. Kim, Y. Kim, S.-B. Jung, Microstructure and adhesion characteristics of a silver nanopaste screen-printed on Si substrate. Nanoscale Res. Lett. 7, 49 (2012)
R. Jaworski, L. Pawlowski, F. Roudet, S. Kozerski, F. Petit, Characterization of mechanical properties of suspension plasma sprayed TiO2 coatings using scratch test. Surf. Coat. Technol. 202, 2644–2653 (2008)
A.D.Albert, Nanostructured Ag produced by LAMA, The University of Texas at Austin
A. Gautam, G.P. Singh, S. Ram, A simple polyol synthesis of silver metal nanopowder of uniform particles. Sci. Direct Synth. Metals 157, 5–10 (2007)
K.W. Chan, A.K.H. Kwan, Evaluation of particle packing models by comparing with published test results. Particuology 16, 108–115 (2014)
J. Zheng, W.B. Carlson, J.S. Reed, The packing density of binary powder mixtures. J. Eur. Ceram. Soc. 15, 479–483 (1995)
J. Kindersberger, H. Löbl, S. Schoft, Plastic deformation and loss of joint force by creep in high current joints, in Proceedings of 20th International Conference on Electrical Contacts, Stockholm Sweden (2000), pp. 367–372
Acknowledgments
This work is financed by the National Center for Research and Development, Project No. 0891/R/T02/2010/10.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kiełbasiński, K., Krzemiński, J., Młożniak, A. et al. New technology of silvering aluminium busbar joints with the use of printable paste containing nano-size Ag particles. J Mater Sci: Mater Electron 26, 1832–1837 (2015). https://doi.org/10.1007/s10854-014-2618-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-014-2618-3