Abstract
This is a detailed study of the effect of carbon impurity on the production of the oxygen-vacancy (VO) pair and its conversion to the VO2 defect, in electron-irradiated Czochralski silicon material, by means of infrared spectroscopy. Upon irradiation vacancies are trapped by oxygen atoms to form VO pairs and it was determined that the presence of carbon enhances the production of this pair. This is attributed to the tendency of carbon to capture self-interstitials, thus decreasing the annihilation rate between vacancies and self-interstitials produced during the irradiation and therefore increasing the availability of vacancies to pair with oxygen atoms in the course of irradiation. Upon annealing a number of VO pairs are captured by oxygen atoms to form VO2 defects. It was determined that the percentage of the VO converted to the VO2 defects decreases as the concentration of carbon increases. The phenomenon is discussed in terms of the various reaction channels that VO pair participates upon annealing and how the presence of carbon impacts the balance between these reactions, affecting the final products of the involved processes. Finally, an opposite trend for the conversion of the VO2 to the VO3 defect was observed. The percentage of VO2 converted to VO3 is enhanced with the increase of carbon content. This finding is discussed in terms of the effect of carbon in the oxygen diffusivity and the final impact on the reaction VO2 + Oi → VO3.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Oxygen is the dominant impurity incorporated during processing in Czochralski grown Si and the oxygen-related defects have been investigated for decades [1–3]. Oxygen atoms, located at interstitial sites in the Si lattice (Oi), are the most effective sinks for the vacancies created in the course of irradiation, leading to the formation of the VO pairs (V + Oi → VO), the well-known A-centers. There are many technological and fundamental research reasons that demand a detailed understanding of the VO defect properties and behavior: Firstly, the defect introduces an acceptor level [4–6] in the forbidden energy gap at around Ec−0.17 eV. Due to its electrical activity the A-center acts as a recombination center [7, 8], resulting in the deterioration of devices especially those operating in radiation environment, for instance Si detectors. Secondly, upon annealing VO interacts with oxygen impurities and vacancies leading to the formation [9] of various V nOm defects. Members of these families of defects cause [10] leakage currents in p-n junctions, thus affecting negatively their properties. Thirdly, VO pair has been proposed [11, 12] to act as a vehicle for oxygen aggregation processes in Si. Indeed, although the activation [13] energy for oxygen diffusion in Si is 2.53 eV, oxygen aggregation occurs with much lower activation energy [14] of 1.8 eV. To explain this, fast diffusing oxygen containing species are needed and the oxygen-vacancy pair was considered [12] as a potential candidate. Fourthly, during thermal processing of the Si material a number of VO pairs are captured by oxygen atoms to form VO2 defects. The latter defect exhibits important properties, for instance the center appears to be bistable [15] and most importantly although VO2 defect is electrically neutral, in its metastable configuration it manifests electrical activity. Precursor structures in the course of its final formation during the VO to the VO 2 conversion have also been reported [16]. More over it has been suggested that the VO2 defect acts as nuclei for oxygen precipitation [17, 18]. Aggregation and precipitation of oxygen are very important processes affecting the quality of Si-based devices. Thus, any information on the formation of the VO defects and the mechanisms that govern its conversion to the VO2 defect is considered very useful from the technological point of view, mainly for developing a fabrication process of Si-based devices with enhanced functionality. There is a fifth important reason: the formation and anneal of VO pair involves reactions with intrinsic defects (vacancies and self-interstitials) and any relative information can be used to control defect processes. To this end, the effect of other impurities, such as carbon present in the lattice, on the behavior and properties of the VO and the VO2 defects, is valuable.
Carbon is the second important impurity present in the lattice of as-grown Si [19–23]. It is an isovalent impurity in Si incorporated at substitutional sites in the lattice (Cs) and it readily traps self-interstitials produced in the course of irradiation. As a result, carbon atoms are injected at interstitial sites (Ci). Carbon interstitials exhibit in general a very interesting behavior [19, 24–26], and being very mobile at room temperature they interact with primary defects and mainly with other Cs and Oi atoms to form the CsCi and CiOi defects, respectively. The latter defect is the most significant carbon-related defect and it is expected to impact the behavior of VO defect in carbon containing Cz-Si. It is therefore important to understand how carbon affects the mechanisms of formation of the VO defect and its conversion to VO2 defect.
Notably, the VO is optically active giving rise to an IR band at 830 cm−1 in the neutral charge state and an IR band at 885 cm−1 in the negative charge state [27, 28]. Also, VO2 defect gives rise to an IR band at 888 cm−1, although it is electrically inactive [27, 29]. Therefore IR spectroscopy is a suitable tool to make proper investigation on the properties and behavior of the above oxygen-vacancy defects.
2 Experimental methods
We used samples cut from prepolished Czochralski Si wafers. Their corresponding carbon and oxygen concentrations are given in Table 1. [Cs]o and [Oi]o represent the initial concentrations of carbon and oxygen impurities prior to irradiation, although [Cs]a.i. and [Oi]a.i. the corresponding concentrations after irradiation. Three groups of samples were used, irradiated with 2 MeV electrons at three fluences: group one (samples labeled S1i, i = 1–4) with 5 × 1017 cm−2, group two (samples labeled S2i, i = 1–3) with 1 × 1018 cm−2 and group three (samples labeled S3i, I = 1–2) with 2 × 1018 cm−2, using the Dynamitron accelerator at Takasaki-JAERI (Japan). In the labels of the samples index i increases with the increase of the carbon content of the sample. The samples S12 and S13 are doped with Ge concentrations of 1 × 1017 and 1 × 1018 cm−3, respectively. The sample S23 is doped with Sn concentration of 3 × 1017 cm−3. These Ge and Sn concentrations have practically a negligible effect [30–32] on the production and evolution of VO defect and for the purpose of this work are simply considered as Cz-Si samples containing carbon. After the irradiation, all the samples were subjected to isochronal anneals up to 600 °C, in steps of ΔΤ = 10 °C and Δt = 20 min. After each annealing step, the IR spectra were recorded at room temperature by means of a FTIR spectrometer with a resolution of 1 cm−1. The two phonon background absorption was subtracted from each spectrum by using a float-zone sample of equal thickness. The concentrations of Oi, Cs, VO and CiOi defects were calculated by using the following calibration coefficients: kOi = 3.14 × 1017 cm−2, kCs = 1.0 × 1017 cm−2, k VO = 6.25 × 1016 cm−2, kCiOi = 1.1 × 1017 cm−2 respectively, as cited in ref. [30] and references therein.
3 Results and discussion
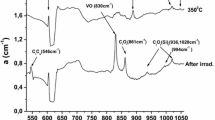
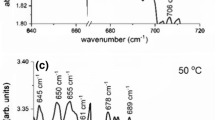
Figure 1 presents the IR spectrum of one representative sample (S22), among the groups of samples used in this experiment. It shows the spectrum after irradiation and at characteristic temperatures in the course of the isochronal anneal sequence. The above annealing temperatures were purposely chosen to focus on the production and annealing of the main irradiation-induced defects of VO (830 cm−1) and CiOi (862 cm−1) and their products upon annealing that is the VO2 (888 cm−1), VO3 (904, 968, 1000 cm−1) and the CsO2i (1048 cm−1) [19] defects. In the spectra weak bands also appear and these are attributed [19] to the CiCs (546 cm−1), CsOi (585, 637, 684 cm−1) and the CiOi(SiI) (936, 1020 cm−1) complexes. Another band [19] of CiOi at 739 cm−1 is also shown.
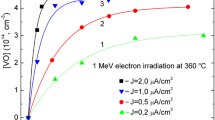
Figure 2 shows the production of the VO defect as a function of the carbon concentration for the various irradiation fluences. Figure 3 depicts how VO production varies with carbon content for each particular fluence used in the present study and in essence is an alternative representation of Fig. 2. As it is observed from Figs. 2 and 3 the concentration of VO defect increases with the increase of the carbon concentration. The relation is not linear, an indication that carbon affects indirectly the production of VO via the reactions taking place in the course of irradiation. Indeed, during irradiation V and SiI are formed. Most of them are annihilated (V + SiI → ∅). The remaining vacancies are captured by oxygen atoms to form VO defects. The presence of carbon has the following effect: Due to its strong tendency to trap SiI, carbon competes with vacancies in their capture. This leads in a decrease in the rate of the annihilation reaction (V + SiI → ∅), namely in an increase of the availability of vacancies to be captured by oxygen atoms. Thus the production of VO pairs increases, a fact verified experimentally (refer to Fig. 2). Additionally, it should be noted that in order to form a VO pair, a vacancy diffuses to an oxygen atom. It has been established experimentally that vacancies are mobile below room temperature. The onset of migration occurs [33] at ~70 K in n-type Si, at ~150 K in p-type Si and at ~200 K in high resistivity material with corresponding migration activation energies 0.18 eV for n-type, 0.32 eV for p-type and 0.45 eV for the high resistivity Si. These energies are much lower than that of 2.53 eV for oxygen diffusion [13]. The disappearance of vacancies upon annealing is accompanied in the spectra by the emergence of other signals, identified as vacancies trapped by other defects [34]. This clearly confirms [34] that the annealing occurs as a long range migration of vacancies. In this process a vacancy performs a number of jumps in the lattice until it encounters an oxygen atom. This is considered as a long-distance migration process in the course of which the vacancy travels through the lattice where it may encounter self-interstitials. In the presence of carbon this possibility is substantially decreased and the possibility of the traveling vacancy to encounter and pair with an oxygen atom is enhanced. In Fig. 3 we observe that with the increase of fluence in the range of 5 × 1017–2 × 1018 cm−2 the concentration of VO increases as expected [35, 36]. Notably, there were reports in the past [37] that variations of carbon content have no impact on VO formation in contrast with these studies. These observations can be attributed to the quality of the Cz-Si samples which ~50 years ago was inferior.
Upon thermal anneal the 830 cm−1 VO band begins to disappear from the spectra and another band at 888 cm−1 attributed to the VO2 defect [27, 29, 38, 39] begins to grow in the spectra. Figure 4 shows the evolution of the VO the VO2 and the VO3 bands for the S22 sample. It also shows the evolution of the CiOi and CsO2i bands to be discussed later. Prior to continuing to the presentation and discussion on this issue it deserves noting that for sample S22 the oxygen loss as a result of irradiation is 3 × 1017 cm−3 (see Table 1), whereas the concentrations of the VO and CiOi defects as estimated from Fig. 1 and the reported calibration coefficients are 6.2 × 1016 cm−3 and 5.6 × 1016 cm−3 respectively. Overall the concentration of the two defects is about 1.18 × 1017 cm−3 a value smaller than that of the oxygen loss, but not inconsistent with that, if one considers that additional oxygen atoms go to the formation of other oxygen-related defects, as for instance CiOi(SiI) (see Fig. 1) and other centers. Another point: Although, the fate of vacancies upon irradiation is more or less known and a lot of vacancy-related defects have been identified by EPR and IR techniques, the fate of self-interstitials is less clear. In the latter case the Ci, CiOi and CiCs defects formed in the course of irradiation act [19, 40, 41] as sites for the agglomeration of self-interstitials. Thus, IR bands of the Ci(SiI) (953, 960 cm−1), CiOi(SiI) (934, 1018 cm−1) and CiCs(SiI) (987, 993 cm−1) have been reported [19, 40, 41] previously in high dose 2 MeV electron and 5 MeV fast neutron irradiated Si material. In our case here of 1.5 MeV electron irradiation only the bands of the CiOi(SiI) defect are clearly seen in the spectra. Figure 5 shows the conversion ratio of the a VO2/a VO versus carbon concentration for various fluencies. Symbol “a” represents the absorption coefficient. Figure 6 is in essence an alternative representation of Fig. 5. It shows the conversion ratio of the a VO2/a VO for various carbon concentrations, for each particular fluence used in this study. As it is immediately seen from the latter figures, the conversion ratio a VO2/a VO decreases with the increase of the carbon concentration. To understand this behaviour one has to consider the main reactions that the VO participates upon annealing and how the formation of the VO2 defect is affected by the presence of carbon. Three main processes have been suggested in the literature [1, 24, 27, 38, 39] to contribute in the demise of the VO pair upon annealing. The first is a fast process related with the destruction of A-centers by self-interstitials:
The second is a slow process related with the migration of VO pair and its capture by immobile oxygen atoms to form the VO2 defect. Indeed the decrease of the (830 cm−1) IR band of VO in the spectra upon anneal above 300 °C, is accompanied by the emergence of a band at (888 cm−1) related [27] with the VO2 defect via the reaction
However, annealing kinetics studies and comparisons of the concentration of the VO and the VO2 defects has led to the suggestion [27, 29] that a third process should take place involving the capture of VO by some unknown sinks, or/and the reaction of VO by other defects and impurities:
It has been found [29] that X depends on the amount of carbon in Si material and it was suggested [29, 39, 42] that these unknown defects may be carbon related. Another relative suggested reaction is:
Also the following relative reactions have been considered [43]
Furthermore, it deserves noting that reactions as VO dissociation
VO pairing with itself
and VO pairing with vacancies
have also been considered and discussed in the literature [1, 38, 39]. It has been found [29] that more than half of the existed A-centers are not converted to VO2 defects. The question about the lack of the VO pairs that are not transformed to VO2 defects is still more or less unanswered and a complete picture about the fate of the lost VO pairs upon annealing has not been established so far. For instance, there are indications [42] that an electrical level Ec−0.20 eV and a Photoluminescence signal at 950-meV may be related with a defect formed upon annealing of the VO pair, but this needs further verification. Also the hypothesis that the VO pair is missing due to its capture by some infrared inactive sinks needs further information to be verified. In this work in order to understand the influence of carbon in the conversion of VO to VO2 we have firstly to take into account the reaction processes and especially those expressed by the relations (3), (4) and (5), indicating a direct or indirect role of carbon. For instance if X is carbon in reaction (3) then the formation of CVO defect is expected. Density functional theory (DFT) calculations indicate that the formation of the Cs VO defect is favourable (by −1.66 eV) [44]. This illustrates that the Cs VO defects are more bound as compared to the VO pairs (−1.32 eV). The DFT calculations also show that next nearest neighbor configurations of the Cs VO cluster are highly bound [44]. In essence when the VO pairs encounter a substitutional carbon atom at nearest neighbor or next nearest neighbor site, they will be trapped and form the larger Cs VO cluster [44]. There are experimental reports [19] about the existence of a luminescence signal produced in irradiated Si, with a zero-phonon line at 488 meV (3942 cm−1) correlated with a (CiOi + V) structure. The suggested [19] reaction scheme for the production of the center is: Cs + SiI → Ci, Ci + Oi → CiOi, (CiOi + V) → 488 meV band and this defect anneals out at about 200 °C. These assignments are hypothetical and in view of the recent DFT evidence [44] it is possible to suggest an alternative reaction scheme (VO + Cs → Cs VO) leading to the formation of the Cs VO defects. In this scheme the 200 °C annealing temperature reflects the binding energy difference (between the Cs VO and VO, i.e. −0.34 eV when considering nearest neighbor Cs VO defects) required to dissociate the VO defect from the Cs.
If X is a carbon-related defect, a potential candidate is the CiOi complex. Indeed, the reaction VO + CiOi → CsO2i has been reported previously [45]. The VO and CiOi bands anneal out together from the spectra at about 300 °C and a band at 1048 cm−1 has been correlated with the CsO2i defect. The whole process is depicted in Fig. 4. However, the annealing of the CiOi defect [24] occurs mainly by dissociation so that a small percentage is responsible for the VO loss in the course of annealing, as a consequence of reactions with carbon-related complexes. Thus other complementary reactions are taking place. In this line of thought the role of carbon in the reduction of the ratio a VO2/a VO could be mainly determined by the reaction Cs + SiI → Ci and in particular its effect on the reaction VO + Oi → VO2. The following approach is envisaged. On thermal anneals and for temperatures above 300 °C, carbon may capture only temporarily the self-interstitials. In essence, at these temperature carbon interstitials only temporarily form and then carbon atoms return back to substitutional sites releasing these self-interstitials (Ci → Cs + SiI). The idea of the conversion of Cs to Ci and vice versa via the trapping and release of self interstitials has been used in the literature [46–48] in relation with the diffusion mechanism of carbon in Si and the carbon-mediated aggregation of self-interstitials in Si. Thus, a percentage of these self-interstitials originated from the reaction (Ci → Cs + SiI) reacts with VO2 defects. In this framework, with the increase of carbon, the number of VO2 defects formed decreases. It is possible that some of the Ci’s could react with Oi atoms to form CiOi pairs, something consistent with the slight increase of the latter defect prior to annealing that is in the temperature range 250–300 °C. Of course at these temperatures most of the Ci return at substitutional sites liberating SiI’s, which react with VO2 defects as mentioned above. One may also consider that some of these self-interstitials react with VO defects (VO + SiI → Oi), besides those reacting with VO2 defects. Thus, the number of VO pairs available to be converted to VO2 defects becomes also smaller. We argue that there is a balance between these two processes so that on the whole the presence of carbon leads to less VO2 defects in relation with the initial VO defects. In other words, less VO are converted to VO2 in the course of annealing, when carbon is present. This is equivalent with saying the ratio a VO2/a VO decreases with the increase of the carbon concentration. In this framework it deserves mentioning that a defect reaction of VO2 defect with Ci has been suggested [49] to occur in high temperature (600–800 K) electron irradiations according to the reaction (VO2 + Ci → Ci VO2 or CsO2i). Again in view of the recent DFT evidence [44] it is possible to suggest alternative reaction schemes (VO2 + Cs → Cs VO2 or Oi + Cs VO → Cs VO2) leading to the formation of the Cs VO2 defects. The possibility of the existence of these extended clusters needs to be investigated further.
To explain the effect of carbon on the production of VO defect and its conversion to VO2 defect we have suggested a different role for carbon in the two cases. In the production process carbon traps self-interstitials thus suppressing the annihilation ratio with vacancies, which lead to an increase of the available vacancies leading to an enhancement of VO productivity. However, in the annealing process of VO and its conversion to the VO 2 defect, carbon acts as temporary trap for the self-interstitials which upon releasing destroy some of the VO 2 defects, leading finally to a reduction of VO2 defects, alternatively to a diminishing of the a VO2/a VO ratio. Thus, both processes, namely the production of VO defect and its conversion to the VO2 defect are limited by reactions with self-interstitials, the availability of which and their role is determined by the presence of carbon impurity.
Figure 7 shows the conversion ratio of the a VO3/a VO2 versus carbon concentration for various fluencies. The thermal evolution of the VO3 bands is shown in Fig. 4. The curve in Fig. 7 refers to the 968 cm−1 band of VO3. Figure 8 is in essence an alternative representation of Fig. 7. It shows the conversion ratio of the a VO3/a VO2 for various carbon concentrations, for each particular fluence used in this study. It is observed that this conversion ratio between VO2 and VO3 defects increases with the increase of the carbon concentration as opposed to the case between the VO and VO2 defects. Notably however, the VO3 defect formation occurs at temperatures above 450 °C through the reaction VO2 + Oi → VO3. At these temperatures oxygen impurity is already mobile [1], able to diffuse in the lattice and meet with the VO2 defect. In other words, in the case of the VO3 formation both partners, that is VO2 defect [24] and oxygen atoms are moving in the lattice attaching each other in a VO2 + Oi structure. Observations of an enhancement of oxygen diffusion in carbon containing Cz-Si, subjected to heat treatments at ~750 °C, has been attributed [50] to the formation of fast diffusing oxygen –carbon molecules. We can extend this idea in the case of irradiated Si and for anneals above 450 °C. In particular we envisage the formation and rapid dissociation of oxygen–carbon complexes, a process which promotes in essence the diffusion of the oxygen impurity in the lattice. More specifically, it has been suggested [1] that large clusters formed as a result of the irradiation can release self-interstitials with the increase of temperature. In that case carbon interstitials form (Cs + SiI → Ci), which are very mobile and they are captured by oxygen interstitial atoms to form CiOi defects. Above 450 °C these defects are unstable and they dissociate almost immediately only for the carbon atom to be trapped again by an oxygen atom and then again detrapping and so on. In other words, these oxygen-carbon complexes act as transient species and by their sequential formation and dissociation, in a self-sustaining loop at these temperatures, they could enhance the oxygen diffusion. Notably, a similar behavior has been suggested [51] by the interaction of oxygen atoms with intrinsic defects. In this case the formation of oxygen-vacancy transient pairs could enhance oxygen diffusivity as well. Going one step forward in our picture, the enhanced oxygen diffusivity due to the presence of carbon could result in an increase of the probability of encountering VO2 defects with Oi atoms, as can be inferred by simple reaction kinetics arguments, leading finally to an increase of the total number of the formed VO3 defects, that is to an enhancement of the a VO3 /a VO2 ratio.
Interestingly, the role of carbon on the sequential formation of VOn defects is different, depending on the temperature that determines the particular conversion. In the case of VO2 formation carbon affects reactions with self-interstitials, although in the case of VO3 defects we have considered the likelihood of the formation of transient species involving carbon in their structures, to understand the experimental observations. The present study is part of a concerted effort by the community to understand the impact of isovalent doping and/or localized strain on the defect processes of semiconductors [52–56].
4 Conclusions
We have investigated the effect of carbon on the properties of VO defect in electron-irradiated Cz-Si. In particular, we have focused our studies on the effect of carbon on the production of the VO defect and on its conversion to the VO2 defect, upon thermal annealing. It was found that when carbon concentration increases the production of VO defect is enhanced, although its conversion to the VO2 defect is suppressed. The phenomena were attributed to the ability of carbon to trap self-interstitials thus affecting the balance among the reactions that govern the VO production and its conversion to the VO2 defect. Additionally, the a VO3 /a VO2 ratio increases with the increase of the carbon content as a result of the enhanced effect of carbon on the oxygen diffusivity of Si.
References
R.C. Newman, R. Jones, in Oxygen in Silicon, vol. 42, ed. by F. Shimura, Semiconductors and Semimetals (Academic Press, Orlando, 1994), p. 289
C. Gao, X. Ma, J. Zhao, D. Yang, J. Appl. Phys. 113, 093511 (2013)
H. Wang, A. Chroneos, C.A. Londos, E.N. Sgourou, U. Schwingenschlögl, Appl. Phys. Lett. 103, 052101 (2013)
L.C. Kimerling, in Radiation Effects in Semiconductors, ed. by N.B. Urli, J. W. Corbett, IOP Conf. Ser. No. 31, (Bristol, London, 1977) p. 221
C.A. Londos, Phys. Stat. Solidi A 113, 503 (1989)
C.A. Londos, Phys. Stat. Solidi A 92, 609 (1985)
S.D. Brotherton, P. Bradley, J. Appl. Phys. 53, 5720 (1982)
A. Khan, M. Yamaguchi, Y. Ohshita, N. Dharmarasu, K. Araki, T. Abe, H. Itoh, T. Ohshima, M. Imaizumi, S. Matsuda, J. Appl. Phys. 90, 1170 (2001)
C.A. Londos, L.G. Fytros, G.J. Georgiou, Defect Diffus Forum 171–172, 1 (1999)
K. Gill, G. Hall, B. MacEvoy, J. Appl. Phys. 82, 126 (1997)
B.G. Svensson, J.L. Lindstrom, J.W. Corbett, Appl. Phys. Lett. 47, 841 (1985)
R.C. Newman, J. Phys. Condens. Matter 12, R335 (2000)
J.C. Mikkelsen, in Oxygen, Carbon, Hydrogen, and Nitrogen in Crystalline Silicon, MRS Symposia Proceedings No 59, ed. by J.C. Mikkelsen, Jn., S.J. Pearton, J.W. Corbett, P.W. Pennycook (Materials Research Society, Pittsburgh, 1986), p. 19
P. Wagner, J. Hage, J.M. Trombetta, G.D. Watkins, Mater. Sci. Forum 83–87, 401 (1992)
L.I. Murin, V.P. Markevich, I.F. Medvedeva, L. Dobaczewski, Semiconductors 40, 1282 (2006)
C.A. Londos, N. Sarlis, L.G. Fytros, K. Papastergiou, Phys. Rev. B 53, 6900 (1996)
V.V. Voronkov, R. Falster, J. Electrochem. Soc. 149, G167 (2002)
G. Kissinger, J. Dadrowski, C. Seuring, T. Muller, H. Richter, J. Electrochem. Soc. 154, H454 (2007)
G. Davies, R. C. Newman, in Handbook on Semiconductors, Materials Properties and Preparations, ed. by T.S. Moss, S. Mahajan (North Holland, Amsterdam 1994), p. 1557
S.G. Cloutier, P.A. Kossyrev, J. Xu, Nat. Mater. 4, 877 (2005)
E. Rotem, J.M. Shainline, J.M. Xu, Appl. Phys. Lett. 91, 051127 (2007)
D.D. Berhanuddin, M.A. Lourenço, R.M. Gwilliam, K.P. Homewood, Adv. Funct. Mater. 22, 2709 (2012)
C.A. Londos, A. Andrianakis, V. Emtsev, H. Ohyama, J. Appl. Phys. 105, 123508 (2009)
B.G. Svensson, J.L. Lindstrom, Phys. Stat. Sol. A 95, 537 (1986)
C.A. Londos, Jpn J. Appl. Phys. Part I 27, 2089 (1988)
C.A. Londos, Phys. Stat. Solidi A 102, 639 (1987)
J.W. Corbett, G.D. Watkins, R.S. McDonald, Phys. Rev. 135, A1381 (1964)
A.R. Bean, R.C. Newman, Solid State Commun. 9, 271 (1971)
J.L. Lindstrom, B.G. Svensson, in Oxygen, Carbon, Hydrogen, and Nitrogen in Crystalline Silicon, MRS Symposia Proceedings No 59, ed. by J.C. Mikkelsen, Jn., S.J. Pearton, J.W. Corbett, P.W. Pennycook (Materials Research Society, Pitttsburgh, 1986), p. 45
C.A. Londos, A. Andrianakis, V.V. Emtsev, H. Ohyama, Semicond. Sci. Technol. 24, 075002 (2009)
A. Chroneos, C.A. Londos, E.N. Sgourou, P. Pochet, Appl. Phys. Lett. 99, 241901 (2011)
A. Chroneos, C.A. Londos, E.N. Sgourou, J. Appl. Phys. 110, 093507 (2011)
G.D. Watkins, Mater. Sci. Semicond. Proc. 3, 227 (2000)
G.D. Watkins, in Symposium of Radiation effects on Semiconductors (Toulouse, Journees d Electronique, 1967), p. A1–1
J.W. Corbett, G.D. Watkins, Phys. Rev. 138, A555 (1965)
J.W. Corbett, G.D. Watkins, R.M. Chrenko, R.S. McDoland, Phys. Rev. 121, 1015 (1961)
A.R. Bean, R.C. Newman, R.S. Smith, J. Phys. Chem. Solids 31, 739 (1970)
C.A. Londos, N.V. Sarlis, L.G. Fytros, Phys. Stat. Solidi A 163, 325 (1997)
B.G. Svensson, J.L. Lindstrom, Phys. Rev. B 34, 8709 (1986)
C.A. Londos, M.S. Potsidi, G.D. Antonaras, A. Andrianakis, Phys. B 376–377, 165 (2006)
S.P. Chappel, R.C. Newman, Semicond. Sci. Technol. 2, 691 (1987)
O.O. Awadelkarim, H. Weman, B.G. Svensson, J.L. Lindstrom, J. Appl. Phys. 60, 1974 (1986)
L.F. Makarenko, Semicond. Sci. Technol. 8, 1692 (1993)
A. Chroneos, C.A. Londos, J. Appl. Phys. 107, 093518 (2010)
N. Inoue, H. Ohyama, Y. Goto, T. Suriyama, Phys. B 401–402, 477 (2007)
U. Gösele, Mat. Res. Symp. 610, B7.1.1 (2000)
S.S. Kapur, M. Prasad, T. Sinno, Phys. Rev. B 69, 155214 (2004)
R. Pinacho, P. Castrillo, M. Jaraiz, I. Martin-bragado, J. Barbolla, H.-J. Gossmann, G.-H. Gilmer, J.-L. Benton, J. Appl. Phys. 92, 1582 (2002)
J.L. Lindstrom, L.I. Murin, T. Hallberg, V.P. Markevich, B.G. Svensson, M. Kleverman, J. Hermansson, Nucl. Instr. Methods Phys. Res. B 186, 121 (2002)
F. Shimura, T. Higuchi, R.S. Hockett, Appl. Phys. Lett. 53, 69 (1988)
A.S. Oates, R.C. Newman, Appl. Phys. Lett. 49, 262 (1986)
C.N. Koumelis, G.E. Zardas, C.A. Londos, D.K. Leventouri, Acta Crystallogr. A 32, 306 (1976)
A. Chroneos, H. Bracht, R.W. Grimes, B.P. Uberuaga, Mater. Sci. Eng. B 154, 72 (2008)
A. Chroneos, E.N. Sgourou, C.A. Londos, J. Mater. Sci. Mater. Electron. 24, 2772 (2013)
H. Tahini, A. Chroneos, R.W. Grimes, U. Schwingenschlögl, A. Dimoulas, J. Phys. Condens. Matter 24, 195802 (2012)
A. Chroneos, J. Appl. Phys. 107, 076102 (2010)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Londos, C.A., Sgourou, E.N. & Chroneos, A. Oxygen-vacancy defects in electron-irradiated Si: the role of carbon in their behavior. J Mater Sci: Mater Electron 25, 914–921 (2014). https://doi.org/10.1007/s10854-013-1664-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-013-1664-6