Abstract
We have successfully deposited cadmium selenide (CdSe) thin films by simple dip method using ascorbic acid as complexing agent. Variation of thickness with time and temperature were studied. Deposited samples were characterized by X-ray diffraction, scanning electron microscopy. The absorption, electrical and photoelectrochemical properties are also studied. The X-ray diffraction analysis shows that the film samples are in hexagonal structure. The optical band gap energy was found to be 1.70 eV. Activation energy was found to be 0.447 and 0.034 eV for higher temperature and lower temperature respectively. For CdSe photoelectrode, the open circuit voltage and short circuit current are found to be 267 mV and 175 mA respectively. The calculation shows the fill factor is 28.67 %. The power conversion efficiency is found to be 1.01 %.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent past years, much importance has given in the field of II–VI class of semiconducting compounds because of their optoelectronic properties and applications [1]. CdSe is a direct band gap semiconductor. It is used as an n-type window layer material in thin film solar cells [2]. It has high absorption coefficient near the band edge, which allow using in thin film devices, it is especially interesting for applications in solar hybrid system [3]. It is also applicable in laser diodes, nanosensors and bio medical imaging devices [4–6]. The CdSe energy band gap is about 1.8 eV, which divides the solar spectrum in two parts, namely, the “thermal” part with hv < Eg and the “optical” part with hv > Eg [7, 8]. Electrochemical photovoltaic cells are very attractive for the production of electricity, because they use free and renewable solar energy and present number of advantages over p–n junction solar cell [9]. The deposition parameters such as pH, temperature, concentration of the solution and deposition time influence of the formation of thin film and crystallite size, which in turn engineer the band gap of the material [10]. Several ligands have been utilizing in the deposition of CdSe, such as triethanolamine [11], nitrilotriacetate [12], tartaric acid [13], malonic acid [14], potassium nitrilotriacetate [15]. Pramanik and colleagues show variation of thickness with different bath parameters has been studied to obtain thickest deposition possible [16]. Murali et al. deposited CdSe thin films in the temperature range 5–30 °C from the precursors. The films exhibited hexagonal structure. Optical band gap was found to vary in the range of 1.65–2.1 eV as the substrate temperature is decreased from 30 to 5 °C [17]. Cerderia et al. deposited cadmium selenide thin film on Ti substrate using chemical bath deposition. The photoluminescence and Raman spectra were studied [18].
Keeping in view all aspects an attempt has been made to deposit CdSe thin films at room temperature. Structural, optical, morphological, electrical and electrochemical photovoltaic properties of the deposited sample have been studied.
2 Experimental details
2.1 Substrate cleaning
The deposition was done on glass slides of 26 mm × 76 mm × 2 mm dimensions and good quality polished stainless steel strips. The glass substrates were cleaned by boiling them in chromic acid for 1 h, followed by washing successively with detergent and alcohol. They were finally stored in double distilled water before use. The stainless strips were polished using emery cloth and zero number polishing paper.
2.2 Reagents and preparation of solution
All reagents used for the deposition were analytical grade. It contains cadmium sulphate octahydrate, nickel sulphate octahydrate, ascorbic acid, hydrazine hydrate, sodium sulphite, selenium powder and ammonia. All the solutions were prepared in double distilled water. Sodium selenosulphate (~0.25 M) solution was prepared by refluxing 5 g of selenium powder with 15 g sodium sulphite in 200 mL double distilled water for 9 h at 363 K. The solution was cooled, filtered to remove undissolved selenium and stored in an airtight container [19]. All the films were deposited using dip method.
2.3 Deposition of CdSe thin films
To prepare the bath, 10 mL (0.25 M) cadmium sulphate octahydrate was poured in 100 mL beaker; other chemicals were used in the following sequence: 2.5 mL (1 M) Ascorbic acid, 10 mL (2.8 M) ammonia, 10 mL (0.25 M) sodium selenosulphate. The pH of the reactive mixture is 10.53. The total volume was made 50 mL with double distilled water. The temperature of the bath was maintained at 278 K using ice bath. The solution was stirred vigorously before dipping non-conducting glass substrates. The substrates kept vertically slightly tilted in a reactive bath. The temperature of the bath was then allowed to increases up to 298 K very slowly. After 5 h, the slides were removed washed several times with double distilled water. The film was dried naturally preserved in dark desiccators over anhydrous CaCl2. The film was deposited on both sides of slides.
3 Results and discussions
3.1 Growth mechanism
Thin films were deposited by decomposition of sodium selenosulphate in alkaline solution containing cadmium sulphate and ascorbic acid as complexing agent. Ascorbic acid controls the metal ion concentration in the reaction vessel. As the temperature increases slowly, the decomposition of sodium selenosulphate and metal complex take place in alkaline medium favors the formation of thin film. For cadmium chalcogenides films obtained from alkaline medium, Cd(OH)2 is known to acts as seeding nucleus [20].
The deposition process is based on the slow release of Cd2+ ions and Se2− ions in the solution by ion-by-ion basis [Ksp = 10−33 (CdSe)]. The growth mechanism of film can be understood from the following reaction:
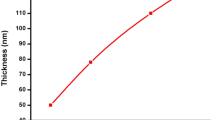
The nucleation is not observed within the first 30 min and hence the process require an induction time for the nucleation on substrate. This suggests the ion-by-ion growth mechanism instead of cluster-by-cluster. The thickness was measured every 60 min and plotted against time as shown in Fig. 1. From the figure, the thickness varies linearly with time, but above certain time the concentration of the reactive species decreases. Thickness remains the constant. All the films were homogenous well adherent to the substrate. Growth kinetics for the development of thin films is shown in Fig. 2. The figure shows that, in early stages of growth the deposition varies linearly with deposition temp and then decreases after typical temperature. At higher temperature the rate of release of Cd2+ and Se2− ions is faster, so precipitation occurs instead of film formation. Terminal thickness was found to be 0.65 μm.
3.2 X-ray diffraction
The X-ray diffraction pattern of the films were recorded by using Philips-PW-1710, X-ray diffractometer, in 2θ range from 10° to 80° using Cu Kα1 line (λ = 1.54056 Å). The crystal structure, grain size and lattice parameters were determined from X-ray diffraction pattern. The chalcogenide of cadmium normally show the duality in the crystal structure. They can be formed with either sphalerite (cubic, zinc blend type) or wurtzite (hexagonal type) structure [21, 22]. The spectra for CdSe [JCPDF Card No. 08-0459] were used for identification purpose.
The X-ray diffraction (XRD) spectra of CdSe, films deposited on glass substrate are shown in Fig. 3. The XRD pattern shows a large number of peaks indicating that the films are polycrystalline in nature. The analysis of spectrum indicated that the films are having hexagonal structure. The analysis of XRD patterns in terms of hkl planes, interplanar distances, crystallite size, lattice parameters have been carried out by considering hexagonal structure and is given in Table 1.
The diffused background is due to amorphous glass substrate and also to some amorphous phase present in CdSe thin films. (100) (102) (110) (103) (112) planes of a hexagonal CdSe were observed. The highest intense reflection at d = 3.715 Å which can be normally indexed as hexagonal (100). The average crystallite size was determined by using Scherrer’s formula. The average crystallite size was calculated by resolving the highest intensity peak. The average crystallite size for the thin films falls of 244 nm.
3.3 Morphological characterization
The scanning electron micrograph of film is shown in Fig. 4. It revealed homogenous film growth with some cracks. The micrographs also showed a compact structure composed of single type of densely packed microcrystal spherical in shape with well defined grains and almost similar in size.
3.4 Optical characterizations
The optical density of the thin film samples were measured at a room temperature as function of wavelength. Hitachi-330 (Japan) UV–VIS–NIR Double beam spectrophotometer was used for this purpose. The absorption spectra recorded from 400 to 800 nm. The absorption spectra were used to calculate band gap, type of optical transition and absorption coefficient, etc.
The optical absorption spectra of CdSe film are shown in Fig. 5. The study show that the presences of absorption edge of exponential shape. It is due to homogeneity of the films and normal band structure. The spectra shows two regions, one for higher wavelength with practically lower absorption and other for lower wavelength in which absorption increases steeply. The optical studies revealed that the films are highly absorptive with direct type of transition (α × 104 cm−1). The value of absorption coefficient depends upon radiation energy as well as composition of film. The data were systematically studied in the vicinity of the absorption edge on the basis of three-dimensional model. The interpretation of the results can be easily drawn with the help of formula derived for three-dimensional crystal. The simplest forms of equations obeyed near and above absorption edge are [23];
where α is absorption coefficient (cm−1), hν the photon energy (eV). A and n are constants. A is complex parameter, which depends on temperature, photon energy, phonon energies etc. The n values are 0.5, 1.5, 2 and 3 for allowed direct, forbidden direct, allowed indirect and forbidden indirect transition respectively. Eg is the direct band gap energy. A plot of (α)2 versus hν should be a straight line whose intercept to the x-axis gives the optical band gap. The graph of (αhv)2 versus hν for ‘as deposited’ samples are shown in Fig. 6. The band gap of CdSe was found to be 1.70 eV [24].
3.5 Electrical properties
The electrical transport properties play key role in deciding the quality as well as applications of the semiconductor devices. These properties are mainly influenced by their structural characteristics, purity, nature, concentration of the impurities [25–27].
The dark D.C. conductivity of all samples was measured by using two probe method, in temperature range of 300–525 K for heating and cooling cycles. The electrical conductivity variation with temperatures during heating and cooling cycles was found to be different and this shows that the ‘as-deposited’ film undergoes irreversible changes due to annealing out of non-equilibrium defects during first heating. The specific conductance at room temperature of CdSe were found to be of the order of 10−7 (Ω cm)−1 [28].
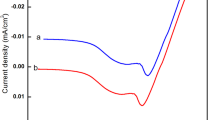
The values of specific conductance of different composition at 300 and 525 K are listed in Table 2. The conductivity of the samples increases with increase in temperature, showing semiconducting behavior of the films. The plots of log δ versus 1,000/T are shown in Fig. 7 for the films. There are two distinct linear regions, indicating the presence of two-conduction mechanism, the low temperature intrinsic and high temperature extrinsic. In the lower temperature range (300–375 K) is characterized by small slope. In the higher temperature range, the curve is characterized by large slope [29–31].
The activation energy is calculated using Arrehenius equation. The activation energies obtained from the slope of straight lines of log δ versus 1,000/T plots are included in Table 2.
3.6 Photoelectrochemical characterization
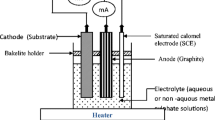
For fabrication of photoelectrochemical cell we used an H-shaped glass tube. One of the arm of the tube was made from hard glass having diameter of size 2.7 cm and length 7 cm and other is ordinary test tube of inner diameter 1.5 cm and length 7 cm. The cell can be represented as
Counter electrode is constructed by using a graphite rod sensitized in a medium containing concentrated CoS solution for 24 h. The power output characteristic has been obtained for a photoelectrochemical cell at a constant illumination of 30 mW/cm2. Films were deposited onto the smooth polished stainless steel substrate, which acts as photoelectrode.
Figure 8 shows the photovoltaic power output characteristics of various cells were recorded under 30 mW/cm2 illumination intensity using sulphide–polysulphide electrode. The various cell parameters like open circuit voltage (Voc), short-circuit current (Isc), fill factor (ff), series resistance (Rs), shunt resistance (Rsh) and conversion efficiency (η) was determined. The maximum power output of the cell is given by the largest rectangle that can be drawn inside the curve.
For CdSe photoelectrode, the open circuit voltage and short circuit current are found to be 267 mV and 175 mA respectively. The calculation shows the fill factor is 28.67 %. The power conversion efficiency is found to be 1.01 %. The value of series resistance and shunt resistance were found to be 830 and 525 Ω respectively. The main drawback in utilizing PEC cell is the absence of space change region at the photoelectrode-electrolyte interface. In this situation, the photogenerated charge carriers can move in both the direction. Lu and Kamat [32] reported that the photogenerated electrons in n-type material either recombine readily with holes or leak out into the electrolyte, instead of flowing through external circuit.
4 Conclusions
CdSe thin film can be deposited by using ascorbic acid as a chelating agent. This controls the deposition of thin film. Terminal thickness was found to be 0.65 μm. The XRD pattern shows a large number of peaks indicating that the films are polycrystalline in nature. The analysis of spectrum indicated that the films are having hexagonal structure. The homogenous formation of crystalline grains was observed from scanning electron microscope. The optical study reveals the direct transition band gap for CdSe thin film was found to be 1.70 eV. The specific conductance at room temperature of CdSe were found to be of the order of 10−7 (Ω cm)−1. The power conversion efficiency is found to be 1.01 %. This shows CdSe is a potential candidate for solar cell fabrication.
References
A.A. Yadav, M.A. Barote, E.U. Masumdar, Mater. Chem. Phys. 121, 53 (2009)
T.S. Shyju, S. Anandhi, R. Indirajith, R. Gopalakrishan, Alloys Compd. 506, 892 (2010)
Y. Vorobiev, J. Hernadez, P. Vorobiev, L. Bulat, Sol. Energy 80, 170 (2006)
M. Califano, A. Zunger, A. Franceschettl, Appl. Phys. Lett. 84, 2409 (2004)
R.D. Schaller, M.A. Petruska, V.I. Klimov, Appl. Phys. Lett. 87, 3102 (2005)
E. Hendry, M. Koeberg, F. Wang, H. Zhang, C.M. Donega, D.V. Bergh, M. Bonn, Phys. Rev. Lett. 96, 7408 (2006)
H. Ponce, J. Hernandez Borja, A. Rojas, M. Sabchez, Y. Vorobiev, R. Bon, J. Robles, J. Hernandez, Mater. Chem. Phys. 113, 824 (2009)
M. Christopher, J. Am. Chem. Soc. 126, 708 (2004)
Y. Gudage, R. Sharma, Curr. Appl. Phys. 10, 1062 (2010)
B.K. Rai, H.D. Bist, R.S. Katiyar, M.T.S. Nair, P.K. Nair, A. Mannivannan, J. Appl. Phys. 82, 1310 (1997)
E. Masumdar, L. Deshmukh, Turk J. Phys. 27, 271 (2003)
G. Hodes, A. Yaron, F. Mostisuke, D. Mostisuke, Phys. Rev. B 36, 4215 (1987)
H. Pathan, B. Sankapal, J. Desai, C. Lokhande, Mater. Chem. Phys. 78, 11 (2002)
A. Khomane, P. Hankare, Alloys Compd. 489, 605 (2010)
M. Simurda, P. Nemec, P. Formanek, I. Nemec, Y. Nemcova, P. Maly, Thin Solid Films 511, 71 (2006)
A. Mondal, T.K. Chaudhuri, P. Pramanik, Sol. Energy Mater. 7, 431 (1983)
K.R. Murali, P. Elango, P. Gopalakrishnan, Mater. Chem. Phys. 96, 103 (2006)
F. Cerderia, I. Torriani, P. Mostisuke, V. Lemos, F. Decker, Appl. Phys. 46, 107 (1988)
P. Hankare, A. Manikshete, D. Sathe, P. Chate, Mater. Sci. Mater. Electron. 21, 698 (2010)
P.P. Hankare, P.A. Chate, M.R. Asabe, S.D. Delekar, I.S. Mulla, K.M. Garadkar, J. Mater. Sci.: Mater. Electron. 17, 1055 (2006)
P.P. Hankare, P.A. Chate, D.J. Sathe, B.V. Jadhav, J. Alloys Compd. 503, 220 (2010)
R.B. Kale, S.D. Sartale, B.K. Chougule, C.D. Lokhande, Semicond. Sci. Technol. 19, 980 (2004)
J. Bardeen, F.J. Blutt, L.H. Hall, in Proceedings of the Photoconductivity Conference, ed. by R. Brechepride, B. Russel, E. Hahn (Wiley, NY, 1975)
A. West, Solid State and Its Applications (Wiley, New York, 1989)
K.L. Chopra, Thin Film Phenomenon (McGraw-Hill, New York, 1969)
G. Harbeke (ed.), Polycrystalline Semiconductors: Physical Properties and Applications (Springer, Berlin, 1985)
G.I. Rusu, M.E. Popa, G.G. Rusu, I. Salaoru, Appl. Surf. Sci. 218, 222 (2003)
P.A. Chate, D.J. Sathe, P.P. Hankare, S.D. Lakade, V.D. Bhabad, J. Alloys Compd. 552, 40 (2013)
E.U. Masumdar, V.B. Gaikwad, V.B. Pujari, P.D. More, L.P. Deshmukh, Mater. Chem. Phys. 77, 669 (2002)
S. Erat, H. Metina, M. Arı, Mater. Chem. Phys. 111, 114 (2008)
A.S. Khomane, Alloys Compd. 506, 849 (2010)
D. Lue, P. Kamat, J. Phys. Chem. 97, 1073 (1993)
Acknowledgments
One of the Authors S.L. Hake is grateful to University of Mumbai, India for the financial assistance through minor research project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hake, S.L., Chate, P.A., Sathe, D.J. et al. Studies on hexagonal cadmium selenide thin film deposited by chemical route using ascorbic acid. J Mater Sci: Mater Electron 25, 811–816 (2014). https://doi.org/10.1007/s10854-013-1650-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-013-1650-z