Abstract
In this work, the functionalization of ZnS quantum dots using the thiol group of l-cysteine for different concentrations has been reported. Chemical precipitation method was used for the synthesis of nascent as well as l-cysteine functionalized ZnS quantum dots for optimized values of pH and molar concentrations of the precursors. Morphological studies were done by X-ray diffraction (XRD) and TEM. Optical measurements were done by UV–visible, Fourier transform infrared (FTIR) and energy resolved photoluminescence studies. Particle size was calculated by using Brus equation. Appreciable changes in morphological and optical properties of ZnS quantum dots were observed in few cases. XRD results shows that, the primary crystallite size decreases with increasing the capping concentration, however, the crystal structure remain same for all the used concentrations of l-cysteine. UV–visible analysis shows that band gap and particle size is also tunable with l-cysteine capping. FTIR studies confirmed l-cysteine capping on the surface of quantum dots. As l-cysteine is non toxic and stable compound, the surface modification of ZnS quantum dots with l-cysteine not only prevents the aggregation of quantum dots but also make them available for the interaction with the target materials and make them suitable for specific biomedical applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Synthesis of core–shell quantum dots is of great technological, biological and scientific interest due to their superior physical and optical properties. As we already know that most of the physical or chemical properties exhibited by the quantum dots are due to their crystallites and growth in their size is due to agglomeration of these crystallites to form primary particles. If this growth of particles is not controlled, then due to Ostwald ripening and Vander-Waals interactions between particles, they agglomerate and settle down [2]. This agglomeration can be arrested by either stabilizing them electrostatically or by inducing steric hindrance at appropriate stages to achieve size selective synthesis during precipitation reaction. In order to control the growth one can use different types of organic and inorganic capping agents to passivate the free QD’s [3, 4]. In addition, if one is interested in any kind of biomedical application using quantum dots, surface functionalization is also very important. For biomedical applications, the preference is always for nontoxic host as well as its surface group. As Zinc sulfide is an important wide band gap (3.68 eV) semiconductor and used as a key material for large range of applications [5–7], they are ideally suited for applications in biological systems [8–10] due to their less toxicity as compared to other II–VI nanoparticles such as CdS, CdTe etc. The differences in the nature and extent of surface functionalization make them most suitable for a particular type of applications. l-Cysteine is also a non-toxic material which contains thiol group as well as ionisable carboxyl and amine groups which can be used for surface binding with the quantum dot as well as with the desired moiety. Keeping all these things in mind, thorough review of literature has been carried out to see the ongoing work on l-cysteine capping. In 1998, Bae et al. [11] had synthesized Cysteine-capped ZnS nanocrystalline semiconductors by titrating sodium sulphide into preformed Zn-Cysteine complexes. The titration of inorganic sulphide into Zn-Cysteine led to the appearance of absorption shoulders in 260–275 nm range. The absorption shoulder red shifted as the quantity of sulphide was increased and the spectral changes became less pronounced when initial sulphide/Zn ratio exceeded 0.5. In 2002, Chen et al. [12] had studied the emission spectra l-Cysteine, thioglycerol, and polyphosphate capped CdS quantum dots. Emission maxima are observed at 460, 560, and 650 nm, respectively. The luminescence of l-Cysteine and thioglycerol-capped CdS QDs decreases when continuously irradiated by light with a wavelength from 300 to 800 nm (400 W/cm2). In 2006 Cai et al. [13] had studied the UV absorption spectra of l-Cysteine capped CdS nanoparticles and CdS nanoparticles assembly on the surface of aligned-Multiwalled carbon nanotubes (MWCNT). The CdS nanoparticles have an absorption peak at 350 nm. The attachment of CdS nanoparticles to the aligned-MWCNTs decreases the absorption band of the CdS at 190–270 nm, and creates a new absorption peak at 200 nm. Significant fluorescence quenching and 25 nm blue shift of the characteristic peak of CdS nanoparticles (from 500 to 475 nm) due to increase of the concentration of aligned-MWCNTs were observed, indicating a strong interaction between the aligned-MWCNTs and CdS nanoparticles. In 2007 Chatterjee et al. [14] had studied the UV–visible absorption spectra of l-Cysteine capped ZnS nanoparticles. With increasing time of reflux, the absorption onset of the nanoparticles gradually shifts to longer wavelength suggesting increase in particle size. Photoluminescence spectra show longer wavelength emission around 428 nm. Size of the nanoparticles increases from 2.4 to 3.2 nm with increasing time of reflux. In 2008 He et al. [15] had studied the Phosphorescence Characteristics of the Mn-Doped ZnS quantum dots. The fluorescence spectra of the Mn-doped ZnS quantum dots show two well-defined emission bands, one at 435 nm and the other at 590 nm, while the phosphorescence spectra exhibit only a single emission peak at 590 nm. Without Mn doping, the ZnS quantum dots show no phosphorescence peaks. In 2009 Koneswaran et al. [16] had studied the absorption and fluorescence spectra of l-cysteine-capped ZnS. The absorption peak occurs at 288 nm and emission maxima obtained at 390 nm with the excitation of 290 nm. The optimum fluorescence intensity was obtained in the pH range between 4.9 and 5.5. In 2009 Liang et al. [17] had studied the effect of pH value of the precursor solution on the fluorescence efficiency and emission wavelength l-Cysteine capped CdSeTe quantum dots. The fluorescence intensity at a given reflux time increased with the increase in pH value, and no emission spectra were observed at pH values below 8.0 or above 12.5. In 2010 Liang et al. [18] had studied the absorption spectra and fluorescence spectra of l-Cysteine capped CdSeTe quantum dots. The maximal fluorescence wavelength was 685 nm. The full width at half-maximum (FWHM) fluorescence intensity is about 60 nm and symmetric, showing that the CdSeTe quantum dots were monodisperse and homogeneous. The fluorescence Intensity of these quantum dots was also pH-dependent.
From the literature survey, it is clear that very less work is done on l-cysteine capped ZnS quantum dots. In this work, l-Cysteine capped ZnS quantum dots have been synthesized directly in aqueous solution using chemical precipitation method. l-Cysteine is attached on the surface of ZnS quantum dots through the thiol groups as capping agents, As ZnS is non-toxic compound and l-Cysteine is also nontoxic and very important capping agent due to vast applications so in this work, l-Cysteine capped ZnS QDs have been synthesized directly in aqueous solution by chemical precipitation method. Effect of changing capping concentration on the optical and morphological properties of ZnS quantum dots has been thoroughly investigated.
2 Experimental
The ZnS nanoparticles were synthesized by chemical precipitation method. Homogeneous Solutions of 0.5 M (Zn(CH3COO)2·H2O) and 0.5 M Na2S solutions were used for the synthesis of ZnS nanoparticles. l-Cysteine was added in zinc acetate solution as a capping agent to the reaction medium for controlling the particle size. First of all the nascent quantum dots were allowed to interact freely in the aqueous medium without any capping agent. In the second attempt l-Cysteine (0.01, 0.1, 1.0 and 2.0 % at. wt) was added in 0.5 M zinc acetate and then 0.5 M sodium sulphide was added drop wise. The white precipitate appears soon after the addition of sodium sulphide (Na2S). The stirring was allowed for 15 min at room temperature using a magnetic stirrer. Then the particles were centrifuged at 4,000 rpm for 5 min. The precipitated particles were filtered using Whatman 40 filter paper. The particles were washed several times using double distilled water to remove the last traces of adhered impurities. The washed particles were dried at 60 °C in vacuum oven.
3 Morphological and optical characterization
X-ray diffraction (XRD) studies were done by using Rigaku, model D–maxIIIC diffractometer with Cu Kα radiation. TEM studies were conducted using a Transmission Electron Microscope, Hitachi (H-7500) 120 kV equipped with CCD Camera, having a resolution of 0.36 nm (point to point) with 40–120 kV operating voltage and magnification capacity of 6 lakh times in high resolution mode. For TEM studies, the powder was ultrasonicated in ethanol. A drop of this solution was dropped on carbon coated grid. Ethanol was allowed to evaporate and powder remains on grid. Optical absorbance of the ZnS particles were recorded with a double beam UV–Visible spectrophotometer (Model: Hitachi—330) in the range of 200–800 nm. Fourier transform infrared (FTIR) spectra were recorded in range of 4,000–400 cm−1 with Perkin Spectrum RXI (40). Energy resolved photoluminescence studies were done with Perkin Elmer LS (50B) fluorescence spectrometer.
4 Results and discussion
The nascent ZnS QDs and l-cysteine capped (0.01, 0.1, 1.0, and 2.0 %) ZnS quantum dots were characterized by XRD, TEM, FTIR, UV–visible spectroscopy and energy resolved Photoluminescence spectroscopy.
4.1 X-ray diffraction results
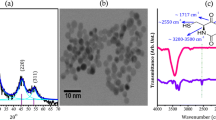
The X-ray diffractograms of uncapped ZnS and l-Cysteine capped (0.01 and 2.0 At.wt%) ZnS quantum dots are shown in Fig. 1.
All the samples show pure zinc blende crystal structure and three broad peaks corresponding to the (111), (220) and (311) planes. It is to be noted that the three peaks observed in the XRD pattern match well with those of the β-ZnS (cubic) reported in the JCPDS Powder Diffraction (File No: 5-0566). Broadening of XRD peaks shows the formation of nanocrystals of ZnS. These peaks are matched with a cubic (zinc blende) phase. The shape and location of peaks did not change with the input amount of l-cysteine which signifies that no new phase is formed and nor the crystalline structure changed with the addition of l-cysteine. The average crystallite size of all the samples is estimated from the half width of diffraction peaks using Debye–Scherrer equation, which is tabulated in the Table 1. It can be concluded that with increasing capping concentration of l-cysteine, the crystallite size of ZnS nanoparticles decreases with the broadening of XRD peaks.
4.2 Transmission electron microscopy results
Figure 2a–c shows the TEM images of uncapped ZnS QDs and the l-cysteine capped ZnS QDs. From the TEM micrographs it is clearly observed that uncapped ZnS nanoparticles are agglomerated; this is due to high surface to volume ratio. On the other hand l-Cysteine capped ZnS QDs are monodispersed in nature. Hence presence of l-Cysteine capping on ZnS nanoparticles can prevent their agglomeration. The average size of QDs observed through TEM studies ranges from 2 to 3 nm which is in conformance to the XRD results. The selected area diffraction pattern presented, in Fig. 2d shows poly-crystallanity of the fabricated quantum dots.
4.3 Fourier transform infra-red spectroscopy results
The FTIR scans of uncapped ZnS, l-cysteine capped ZnS quantum dots and pure l-cysteine are shown in Fig. 3.
In uncapped ZnS, the bands at 1,406 and 1,561 cm−1 can be assigned to the symmetric and asymmetric stretching of COO− respectively. This shows that the acetate group from the zinc acetate precursor used for zinc ions, have been retained in the synthesized quantum dots. The broad band at 3,393 cm−1 is due to O–H stretching and band at 930 cm−1 due to O–H out of plane bending. Whereas, the band having moderate intensity at 1,339 cm−1, may be due to the in-plane C–O–H bending. A moderate intensity band at 1,020 cm−1 may be due to S–O–C stretching. In the FTIR spectra of l-cysteine capped ZnS quantum dots, absorption band around 1,600 cm−1 (COO−), 1,350 cm−1 (COO−), 3,500–3,000 cm−1 (OH, COOH), indicate the –COO− group and the peak at 2,900–3,420 cm−1 indicates –NH2 group and 600–800 cm−1 indicate the C–S group. There are coexisting IR absorption bands of –COO−, –NH2 observed on both l-cysteine and l-cysteine-capped ZnS QDs. Therefore carboxylic acid and amino group are present on the surface of the ZnS QDs, while the S–H group vibration (2,550–2,750 cm−1) is absent on the surface of the l-cysteine-capped ZnS QDs. This may be due to the formation of covalent bonds between thiols and the surface of ZnS. Thus FTIR studies strongly support the formation of l-cysteine capped ZnS quantum dots.
4.4 UV–visible absorption spectroscopy results
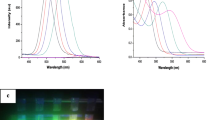
Figure 4 shows the absorption spectra of l-cysteine encapsulated and uncapped ZnS quantum dots. The absorption peaks are observed at 292, 300, 313, 304 and 297 nm for uncapped, 0.01, 0.1, 1.0 and for 2.0 % l-cysteine capped ZnS quantum dots respectively. For calculating the direct band gap value (αhν)2 versus hν is depicted in Fig. 5, and by extrapolating the straight portion of the graph on hν axis at α = 0, the optical band gaps are calculated which are 3.85, 3.74, 3.77, 3.82 and 3.91 eV for uncapped, 0.01 % capped, 0.1 % capped, 1.0 % capped and 2.0 % l-cysteine capped ZnS QDs respectively. From the absorption spectra of all samples, we have found that 2 % l-cysteine capped ZnS have maximum band gap i.e., 3.91 eV, which shows better confinement effect compared to other l-cysteine capped and uncapped samples while strong quantum confinement effect is observed in all the samples in comparison to the bulk ZnS which has a band gap of 3.68 eV.
4.5 Particle size studies
The particle size of l-cysteine capped QDs was computed using the Brus equation [1]. The average particle size comes out to be 2.72 nm, 3.92 nm, and 3.41 nm, 2.93 nm and 2.43 nm for uncapped, 0.01 % capped, 0.1 % capped, 1.0 % capped and for 2.0 % l-cysteine capped ZnS QDs. As the Bohr exciton radius in case of ZnS is 5 nm and we are getting average particle size less than 5 nm. Thus, from our theoretical calculations, we suggest the observation of quantum confinement effect in case of ZnS. It means with the slight change in size of quantum dot, band gap will change.
4.6 Photoluminescence studies
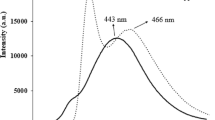
Energy resolved photoluminescence studies were conducted to study the tunability in emission wavelength and emission intensity. The room temperature photoluminescence emission spectra are recorded at fixed excitation wavelengths, taken from the absorption peaks of UV–visible spectra as shown in Fig. 6. The emission peak at 422.6 nm for uncapped quantum dots is blue shifted with l-cysteine capping. The emission spectra shows that for 0.01, 0.1 and 2 % l-cysteine capped ZnS, the fluorescence intensity decreases as compared to the uncapped ZnS while for 1.0 % l-cysteine capped ZnS fluorescence intensity increases about two times of that of uncapped ZnS. Increase in emission intensity indicates that the defects are better passivated for 1.0 % l-cysteine capping. Hence it is clear that emission intensity is tunable with l-cysteine capping which is very good for biomedical applications.
Table 2 shows the emission data for the uncapped and l-cysteine capped ZnS quantum dots.
Further extension of this work which is also being done is regarding the synthesis and characterization of Mn2+ doped & organically capped highly fluorescent ZnS QDs for directly using them for specific biomedical applications like targeted drug delivery and monitoring of controlled drug release rate.
5 Conclusion
Nascent and l-Cysteine capped ZnS quantum dots were synthesized with varying capping concentrations through a wet chemical method. The XRD patterns exhibited a zinc blende structure for all the samples. There was no crystalline phase change with the increasing l-Cysteine concentration but the grain size varies appreciably in case of 2 % capping concentration of l-Cystiene. Variation of grain size leads to the changes in band gap also as seen from UV–visible studies. FTIR studies confirm the capping of ZnS quantum dots with the thiol group of l-Cysteine. Photoluminescence studies reveal that in l-Cysteine capped quantum dots, both emission wavelength and emission intensity is tunable which is very novel for biomedical applications which further opens a road to synthesize highly fluorescent ZnS QDs for desired biomedical applications.
References
L.E. Brus, J. Chem. Phys. 80, 4403–4409 (1984)
H.C. Warad, S.C. Ghosh, B. Hemtanon, C. Thanachayanont, J. Duttaa, Sci. Technol. Adv. Mater. 6, 296–301 (2005)
C. Torres-Marty′nez, L. Nguyen, R. Kho, W. Bae, K. Bozhilov, V. Klimov, R.K. Mehra, Nanotechnology 10, 340–354 (1999)
G. Ghosh, N.M. Kanti, A. Patra, M. Chatterjee, Opt. Mater. 28, 1047–1053 (2006)
H. Yang, P.H. Holloway, J. Phys. Chem. B 107, 9705 (2003)
N. Karar, S. Raj, F. Singh, J. Cryst. Growth 268, 585 (2004)
W. Chen, A.G. Joly, J.O. Malam, J.O. Bovin, J. Appl. Phys. 95, 667 (2004)
J.P. Li, Y. Xu, Y. Liu, D. Wu, Y. Sun, China Particuol. 2, 266 (2004)
M. Labrenz et al., Science 290, 1744 (2000)
Y. Li, J. Chen, C. Zhu, L. Wang, D. Zhao, S. Zhao, Y. Wu, Spectrochim. Acta, Part A 60, 1719 (2004)
W. Bai, R.K. Mehra, J. Inorg. Biochem. 70, 125–135 (1998)
Y. Chen, Z. Rosenzweig, Anal. Chem. 74, 5132–5138 (2002)
Z.X. Cai, X.P. Yan, Nanotechnology 17, 4212–4216 (2006)
A. Chatterjee, A. Priyam, S. Bhattacharya, A. Saha, Colloids and surfaces a: physicochem. Eng. Aspects. 297, 258–266 (2007)
Y. He, H.F. Wang, X.P. Yan, Anal. Chem. 80, 3832–3837 (2008)
M. Koneswaran, R. Narayanaswamy, Sens. Actuators, B 139, 104–109 (2009)
G.X. Liang, M.M. Gu, J.R. Zhang, J.J. Zhu, Nanotechnology 20, 1–9 (2009)
G.X. Liang, H.Y. Liu, J.R. Zhang, J.J. Zhu, Talanta 80, 2172–2176 (2010)
Acknowledgments
Corresponding author is very thankful to the Department of Science and Technology (DST), New Delhi, India for supporting this research work (vide Project No-SR/FTP/PS-69/2008, dated-15/1/2010).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, S., Singhal, M. & Sharma, J.K. Functionalization and characterization of ZnS quantum dots using biocompatible l-cysteine. J Mater Sci: Mater Electron 24, 3875–3880 (2013). https://doi.org/10.1007/s10854-013-1332-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-013-1332-x