Abstract
In this work, poly(3-hexylthiophene) (P3HT) film was synthesized electrochemically in non-aqueous media through the oxidation of the 3-hexylthiophene monomer using a standard three-electrode cell in acetonitrile with 0.100 mol L−1 LiClO4. The polymeric thick film was deposited on platinum plates for best quality control of the process. Cyclic voltammetry of this material showed two bands of oxidation and two bands of reduction attributed to radical cation and dication segments in the polymer chain. These were characterized by reflectance and Raman spectra, of the reduced (chemically de-doped) and oxidized (electrochemically doped) films. The generated film was subjected to anodic potentials of 1.00 and 1.45 V and characterized by an in situ Raman technique, which indicated the stabilization of the radical cation segments. In addition Raman ex situ spectra of as-prepared film was obtained. The increase in the irradiation time of the sample was enough to alter the nature of the constituent species of the oxidized film, preferably favoring the aromatic chains, together with the dication segments. Photoluminescence spectra showed a larger contribution of dication than radical cation segments for the chemically de-doped sample and under this condition showed good stability, even with variation in laser power. SEM images of P3HT film with indication of the thickness and spherical shape of the sample studied were obtained. Aided by these data it was possible to verify the Stokes shift and ionization potential (I p), electron affinity (E.A.) and energy gap (E g) parameters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
One of most promising conducting polymers of poly(3-alkylthiophenes) compounds [1, 2], the poly(3-hexylthiophene) (P3HT), has become the focus of recent researchers because of its processability, environmental stability, and easily tunable electronic and optical properties [3, 4]. Potential applications are electronic conductors, organic light-emitting diodes (OLED’s) [1, 5], polymer photovoltaic cells, rechargeable batteries [6] and organic transistors [7, 8].
The study of conducting polymers, like P3HT, should include seeking structural alterations in the polymeric matrix and identification of the resulting properties for these materials. A goal is to identify synthesis processes that should be promising for optical–electronics applications [9, 10].
Raman spectroscopy is very useful because it can be used to identify chemical composition and it greatly aids in the diagnostics of subtle structural changes in the electronically conducting polymeric chains [11].
The objective of this work was to characterize the influence of the radical cation and dication segments, together with thiophenic ring structure, in the properties of the electrochemically generated film. Techniques used for analysis in conjunction with cyclic voltammetry consisted of reflectance, in situ and ex situ Raman and photoluminescence (PL) spectroscopies. These allowed defining more accurately the different parameters such as ionization potential (I p), electron affinity (E.A.) and energy band gap (E g), which are important to understand to control electrical and optical properties, as well the doping process in semiconductors [12, 13].
2 Experimental section
2.1 Chemicals
The 3-hexylthiophene (C10H16S) monomers was purchased from Acros-Organics—99.0 %; lithium perchlorate (LiClO4) from Acros-Organics—99.0 % was used as salt for the supporting electrolyte. All these reagents were used without further purification. The acetonitrile (CH3CN), solvent, was obtained from J. T. Baker with 99.5 % purity and also used as supplied.
2.2 Electrochemical polymerization and samples
A standard three-electrode cell was used for the synthesis. A platinum plate (25 × 30 × 0.5 mm) acted as the working electrode, and a platinum plate (10 × 10 × 0.5 mm) was used as the auxiliary electrode. The area of the working electrode covered with the film was maintained constant at 3.0 cm2. All potentials were determined with reference to Ag/AgCl in a Luggin-Haber capillary. The supporting electrolyte was a solution 0.100 mol L−1 LiClO4 in acetonitrile.
The best conditions for formation of thin films on the substrate surface with homogeneity were established. The polymerization of the 3-hexylthiophene (0.040 mol L−1) was carried out by applying a fixed potential (1.70 V) at 18 °C for 60 s with a current density similarly used by Louarn et al. [14].
The current versus time curve and cyclic voltammograms were obtained with a MQPG-01: Microquimica potentiostat–galvanostat controlled by a personal computer.
The polymeric film synthesized on the electrode surface was obtained from the working electrode after removing it from the reaction cell, and is referred to as-prepared. The films labeled chemically de-doped were obtained by submitting them to a concentrated basic solution (ammonium hydroxide).
2.3 Spectroscopic characterization
The reflectance spectra of films were obtained in a fiber optic spectrophotometer from Ocean Optics, model S2000, from the reflection of polychromatic light from a tungsten-halogen lamp operating at a range of 400–950 nm on the sample beam. For equipment calibration the WS-1 Ocean Optics standard reflectance was used.
The Raman spectra were obtained from films samples, using a portable Raman Advantage532® Spectrometer from Delta Nu with a 532 nm laser line, the spectral resolution was 8 cm−1. DeltaNu’s software, using baseline features, was used to remove background fluorescence.
Raman in situ experiments were carried out by oxidizing the polymer film synthesized on the electrode surface at 1.00 or 1.45 V in a solution of 0.100 mol L−1 Lithium perchlorate (LiClO4) in acetonitrile.
The (PL) spectra were recorded using a USB4000 Ocean Optics Mini spectrometer. The excitation source was a diode laser emitting at 405 nm, with power set at 2 or 17 mW, using neutral density filters.
2.4 Scanning electron microscopy (SEM)
SEM images were taken on Philips model Quanta 200 (FEI), in Scanning Electron Microscope and Microanalysis laboratory of UEL, equipped with an energy dispersive X-ray (EDX) model INCA 200 at 30 keV. The samples were fixed on ‘stubs’ using carbon adhesive tape and then coated with a layer of gold.
3 Results and discussion
Figure 1 shows the cyclic voltammogram (CV) of polymeric film on platinum in a 0.100 mol L−1 solution of LiClO4 in acetonitrile, synthesized by applying 1.70 V for 60 s at 18 °C.
In the CV of the film were observed two oxidation peaks at 0.89 and 1.23 V, and two reductions peaks at 0.67 and 1.00 V. This result proves that two species were generated during the oxidation of P3HT and stabilized by the anodic potential, that later are reduced in an inverse process. This result was considered important, because literature work states for this polymer only one oxidation peak and another of reduction [15–17]. This behavior indicates that segments containing radical cation species were more stable than with the dication during the film oxidation, since dication is probably a soluble oxidation product, one would expect that once it is formed it would diffuse to the bulk of the solution.
Reflectance and Raman spectra were obtained to identify and characterize these possible species in the polymeric backbone, initially of the reduced (chemically de-doped) film and then of the oxidized (electrochemically doped) film.
Figure 2 shows (a) the reflectance and (b) ex situ resonance Raman spectra of the chemically de-doped films of P3HT. Observed in the deconvoluted reflectance spectrum were bands at 447 and 677 nm. The band at 447 nm is assigned to the absorption band characteristic of polythiophene—neutral state—and was close to the excitation radiation at 532 nm, favoring the resonance enhancement of the Raman spectrum. The other band can be related to the radical cation that remains in the polymer backbone after the chemical de-doping [18]. Figure 2b shows the frequencies obtained in the Raman spectrum of the chemically de-doped P3HT observed at 1089, 1167/1204, 1374, 1452, 1522 cm−1. These are attributed to the C–Csubs stretching; C–C inter-ring stretching, C–C stretching, C=C symmetrical stretching and C=C asymmetric stretching, respectively, of the thiophene rings [14, 19]. However, previous results demonstrated that the band asymmetry at 1452 cm−1 is due to radical cation and dication contribution, persistent in the polymeric matrix.
In addition, the ex situ reflectance spectra of the P3HT films were obtained just after applying a constant potential at 1.00 and 1.45 V for 60 s and asymmetric bands were observed. In the spectrum obtained at 1.00 V, after the deconvolution, a band centered at 695 nm and a component with the maximum centered around 900 nm were observed. These structures were related to the radical cation and dication segments, respectively. In the spectrum of the sample generated at the second oxidation peak of the polymer an accentuated conversion of the radical cation to dication species was expected. However, an increase in the relative intensity and a small bathochromic displacement from 695 to 707 nm was observed, indicating a stabilization of radical cation segments and a decrease of relative intensity of the characteristic dication band at 900 nm.
For this last sample another spectrum was obtained after 4 h, with the intention of evaluating the stability of the two species. This experiment was performed considering for poly(alkylthiophenes), like poly(3-octylthiophene), that a natural de-doping happens in time. This is caused by the instability of the material due to the presence of the alkyl side chain and other factors, such as: the doping nature, the length of the alkyl side chain, temperature, humidity and the atmospheric conditions [20]. Observed in this spectrum was a reduction of the relative intensity of the band at 707 nm and the presence of the band centered at 870 nm. This effect can be attributed to a natural stabilization of the dication species with time, in detriment to radical cation species, which are still persistent in the polymer.
The results discussed previously for the oxidized film were also studied using the in situ and ex situ Raman techniques.
Figure 3 shows the in situ Raman spectra of the films of P3HT at 1.00 and 1.45 V. The film oxidized at 1.00 V demonstrated bands at 1227, 1448 and 1520 cm−1 and when oxidized at 1.45 V demonstrated bands at 1225, 1463 and 1508 cm−1. The frequencies at 1225/1227 and 1508/1520 cm−1 were related to the C–C inter-ring stretching and C=C asymmetric stretching of the aromatic ring [21], respectively. Based on observed shifts and of relative intensity increase the bands at 1225, 1463 and 1508 cm−1 were sensitive to the variation of the potential.
The band at 1448 cm−1 was attributed to symmetrical stretching of the thiophene ring and the shift to 1463 cm−1 demonstrates the contribution of the radical cation segments at 1.45 V [22]. This effect can be understood because in exciting the Raman spectrum with the laser line at 532 nm, there is pre-resonance Raman for the radical cation species, as verified in the reflectance spectrum for the band at 695 nm, near the excitement value. This was observed because with the increase of the potential, it formed a larger amount of radical cation segments.
Figure 4 shows the ex situ Raman spectra of the P3HT film as-prepared registered with (a) 24 s and (b) 120 s of integration time.
Bands at frequencies of 1089, 1167, 1204, 1374, 1460 and 1502 cm−1 observed in the spectrum obtained after 24 s, all stayed unchanged after 120 s of irradiation, except the one at 1460 cm−1 that shifted to 1445 cm−1 with a significant intensity increase, and a decrease of the band at 1502 cm−1. The increase in the irradiation time of the sample was enough to modify the nature of the constituent species of the oxidized film, probably favoring the aromatic chains together with the dication segments. This behavior was also observed for diphenylbenzidine radical cation (DPB+.), which suffered the disproportionation reaction: DPB+. + H+ → DPB2+ + DPBH+, during the diphenylamine oxidation [23].
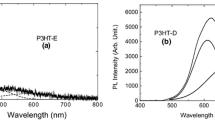
Figure 5 shows the (PL) spectra of chemically de-doped P3HT, obtained with laser intensity of 2 and 17 mW. The PL study for this sample can be justified, because in previous results the chemically de-doped samples presented greater intensity than the electrochemically doped samples.
In Fig. 5 can be observed that the relative intensity between the bands at 611/673 and 610/670 nm stayed constant even with the increase of the irradiation intensity from 2 to 17 mW. It was proposed that the contribution centered at 670–673 nm is exclusively related to the emission of pristine chains [24, 25], which presents a stable structure and longer polymeric chains. On the other hand, the emission centered at 610–611 nm is attributed to the formation of mixed chains, composed of non-pristine structures, in chains of the P3HT polymeric film synthesized by the electrochemical technique. From these results it can be concluded that the band at 610 nm has a larger contribution of dication rather than radical cation segments for the chemically de-doped samples, not being possible to verify by PL spectra the conversions between them.
Another important factor verified with the PL results and reflectance was the accentuated Stokes shift which was evaluated at 164 nm. This consists of the difference of the intensity maxima between PL and the reflectance absorption. These results indicate that in an application, a device containing the P3HT film electrochemically synthesized and chemically de-doped, would not have the issues of self-absorption of the emission light [26].
Figure 6a, b shows SEM images of P3HT film with indication of the thickness of the samples studied, that varied of 3.344–3.803 μm, and a spherical shape for films synthesized were obtained, respectively. This effect can be understood taking into consideration the electrolyte function as doping, which reorganizes the chemical structure of the film on platinum electrode and the doping, stimulated by the applied potential, likely leads to the formation of structured polymer layers [27].
Table 1 shows the results of gap energies (E g) for P3HT film. These values were obtained considering the maximum absorption wavelengths in the reflectance spectra of the chemically de-doped P3HT, since if the cut wavelength was used there would be substantial deviations in the observed values [12]. This is due to the fact that the asymmetric bands have contributions of the radical cation segments. The importance of defining the E g parameter is that the value of this indicates the optical properties related to the polymer. Also obtained was the electron affinity (E.A.), which was defined by subtracting the ionization potential (I p) from the value for E g.
The results observed in Table 1 were very close to those found in the literature [15, 18, 28, 29], indicating that in spite of different synthesis methods that have been used there is little influence in the optical and electronic properties of these materials. Besides, the film synthesized here presented a high ionization potential (5.05 eV), indicating a significant potential in the application of this film as an electron donor in bulk heterojunctions [18, 30, 31].
4 Conclusions
In this work it was observed by cyclic voltammetry the presence of two oxidation peaks for P3HT film and the oxidation species formed. The radical cation and dication, were characterized by ex situ reflectance and Raman spectroscopies. From the in situ Raman spectra it was possible to observe that the radical cation species were stabilized by the applied potential and displayed by our research was the absence of accentuated conversion expected to dication segments. Besides, with the ex situ Raman spectroscopy, a conversion of the radical cation was observed for aromatic and dication species with an increase of the irradiation time. The PL spectra were not sensitive to this conversion probably due to the largest contributor in the spectrum being the dication form in the chemically de-doped samples.
References
E. López-Elvira, E. Escasaín, A. Baró, J. Colchero, E. Palacios-Lidón, Polym. Degrad. Stab. 96, 1279 (2011)
Q.M. Jia, J.B. Li, L.F. Wang, J.W. Zhu, M. Zheng, Mater. Sci. Eng. A. 448, 356 (2007)
Y.G. Seol, J.G. Lee, N.-E. Lee, S.S. Lee, J. Ahn, Thin Solid Films 515, 5065 (2007)
R. Singh, R.J. Singh, J. Kumar, R. Kant, V. Kumar, J. Polym. Sci. Part B Polym. Phys. 48, 1047 (2010)
Y. Yamashita, Sci Technol. Adv. Mater. 10, 024313 (2009)
M.E. Nicho, H. Hub, C. Lopez-Matab, J. Escalante, Sol. Energy Mater. Sol. Cells. 82, 105 (2004)
Y. Pang, X. Li, H. Ding, G. Shi, L. Jin, Electrochim. Acta. 52, 6172 (2007)
M.T. Khan, R. Bhargav, A. Kaur, S.K. Dhawan, S. Chand, Thin Solid Films 519, 1007 (2010)
F. Liesa, C. Ocampo, C. Alemán, E. Armelin, R. Oliver, J. Appl. Polym. Sci. 102, 1592 (2006)
M. Fall, A.A. Diagne, M.M. Dieng, F. Deflorian, S. Rossi, P.L. Bonora, C.D. Volpe, J.J. Aaron, Synth. Met. 155, 569 (2005)
H.-J. Kim, D.-C. Kim, R. Kim, J. Kim, D.-H. Park, H.-S. Kim, J. Joo, Y.D. Suh, J. Appl. Phys. 101, 53514 (2007)
L. Micaroni, F.C. Nart, I.A. Hummelgen, J. Solid State Electrochem. 7, 55 (2002)
F.M. Nodari, R.L. Patyk, L.S. Roman, A.R.V. Benvenho, I.A. Hummelgen, E.K.C. Yoshikawa, J. Gruber, J. Mater. Sci. Mater. Electron. 21, 1235 (2010)
G. Louarn, J.Y. Mevellec, J.P. Buisson, S. Lefrant, J. Chim. Phys. 89, 987 (1992)
C.C. Ho, Y.C. Liu, S.H. Lin, W.F. Su, Macromolecules 45, 813 (2012)
R.A. Kruger, T.J. Gordon, T. Baumgartner, T.C. Sutherland, ACS Appl. Mater. Interfaces 3, 2031 (2011)
G.A. dos Reis, I.F.L. Dias, H. de Santana, J.L. Duarte, E. Laureto, E. di Mauro, M.A.T. da Silva, Synth. Met. 161, 340 (2011)
D. Izuhura, T.M. Swager, Macromolecules 44, 2678 (2011)
X. Feng, X. Wang, Thin Solid Films 519, 5700 (2011)
H. Koizumi, H. Dougauchi, T. Ichikawa, J. Phys. Chem. B. 109, 15288 (2005)
S. Lefrant, I. Baltog, M.L. de la Chapelle, M. Baibarac, G. Louarn, C. Journet, P. Bernier, Synth. Met. 100, 13 (1999)
M. Baibarac, M. Lapkowski, A. Pron, S. Lefrant, I. Baltog, J. Raman Spectrosc. 29, 825 (1998)
H. de Santana, M.L.A. Temperini, J.C. Rubim, J. Electroanal Chem. 356, 145 (1993)
E.M. Therézio, J.L. Duarte, E. Laureto, E. Di Mauro, I.L. Dias, A. Marletta, H. de Santana, J. Phys. Org. Chem. 24, 640 (2011)
K. Yamamoto, S. Ochiai, X. Wang, Y. Uchida, K. Konjima, A. Ohashe, T. Mitzutani, Thin Solid Films 516, 2695 (2008)
Z. Peng, M.E. Galvin, Chem. Mater. 10, 1785 (1998)
M. Lapkowski, J. Zak, M. Kolodziej-Sadlok, S. Guillerez, G. Bidan, Synth. Met. 119, 417 (2001)
S. Hotta, S.D.D.V. Rughooputh, A.J. Heeger, F. Wudl, Macromolecules 20, 212 (1987)
B. Thémans, W.R. Salaneck, J.L. Brédas, Synth. Met. 28, 359 (1989)
P. Bolond, S.S. Sunkavalli, S. Chennuri, K. Foe, T.A. Fattah, G. Namkoong, Thin Solid Films 518, 1728 (2010)
K. Sugiyama, T. Kojima, H. Fukuda, H. Yashiro, T. Matsuura, Y. Shimoyama, Thin Solid Films 516, 2691 (2008)
Acknowledgments
This work was supported by Araucaria Foundation (processes no. 18.575 and 20.380) and CNPq (processes no. 470533/2009-9 and 301980/2011-0), and Danielly and Elaine are indebted to CAPES for the fellowship grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cervantes, T.N.M., Bento, D.C., Maia, E.C.R. et al. In situ and ex situ spectroscopic study of poly(3-hexylthiophene) electrochemically synthesized. J Mater Sci: Mater Electron 23, 1916–1921 (2012). https://doi.org/10.1007/s10854-012-0880-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-012-0880-9