Abstract
ZnO nanoparticles were synthesized by precipitation method. To reduce the agglomeration among small ZnO nanoparticles, an efficient surface modification method was proposed using 2-aminothiols as a capping agent. The effect of capping reagent is investigated on optoelectronics properties of ZnO. The capping of ZnO with 2-aminothiol leads to the shift in fluorescence intensity and also effected the UV–vis spectra of ZnO. The strategy exposed new dimensions to fine tune the fluorescence signatures of the ZnO.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
From last few decades wide band gap semiconductors have gained considerable interest because of their potential application as optoelectronic devices in the ultraviolet region of the electromagnetic spectrum [1]. Zinc oxide belongs to family of Group II–VI semiconductors and is highly transparent semiconducting oxide with a direct band gap of 3.37 eV [2] with high exciton binding energy (60 meV) [3], which enable its use even at room temperature i.e., at room temperature it can exhibit ultraviolet luminescence. The outstanding properties of ZnO have triggered an incredible inspiration among the researchers to investigate the possibilities of using them in varieties of applications like transparent electronics, laser diode, ultraviolet light emitters, photonic devices like solar cell and also in the field of spin electronics [4, 5]. ZnO based devices exhibit various excellent properties like near UV emission, transparency, conductivity, color tunability and can carry high current without material breakdown. The most significant advantage of ZnO is its high exciton binding energy (60 meV) which leads to have efficient excitonic emission at room temperature [6, 7] i.e., lasing action can takes place at room temperature. It is worth mentioning that outstanding and unusual properties of nanoparticles occur due to quantum size effect and large surface area of nano-particles. The luminescent emission efficiency of these ZnO based devices change with varying the size of nanoparticles. Due to size dependent properties of ZnO particles, we can use zinc oxide for broad range of applications in optoelectronic industry, such as optical filters, memories and in various optical switching devices etc. [8–12]. A variety of techniques are used for the synthesis of nanoparticles e.g. Sol–gel method, sputtering, laser ablation, plasma jet, ion-implantation, evaporative decomposition of solution and wet chemical route. Wet chemical route is considered as simple and cost effective method for synthesis of ZnO nanoparticles. However, the synthetic nanoparticles must address the issue of high purity, uniform size and shape of nanoparticles, surface defects and superior optical properties. Hence after preparation of the desired samples, the structural and optical properties have to characterized for understanding the mechanisms behind unusual properties possessed by nanoparticles in comparison to their bulk counterpart [13]. Owing to their remarkable electrical and optical properties, ZnO find great technical potential in the optoelectronic applications. Nanoparticles due to smaller size have high surface to volume ratio and high density of surface defects. It is due to small size of particles and high surface defects, sample particles tends to agglomerate and result to the formation of large clusters or secondary phase. Hence it is necessary to decorate the surface of ZnO nanoparticles, so that no agglomeration of particle can occur at nanoscale.
Upon excitation at suitable wavelength (around 325 nm), the photoluminescence spectrum of ZnO nanoparticles shows a near-band-edge UV emission (around 386 nm) and strong defect related visible emission (around 560 nm). This defect related visible emission shows green luminescence, with emission of blue and yellow bands. This is due to these defects, the emission at UV devices get quenched. Hence for high efficiency of UV light emitting devices, it is mandatory to suppress the defect related green visible region of the spectrum [14]. However the origin of green luminescence is still very controversial and in this regard too many theories have been proposed. Most of theories proposed that green luminescence or defect related visible region is result due to number of defects such as zinc vacancies [15], oxygen vacancies, zinc interstitial [16] and due to large numbers of donor and acceptor pairs [17–19]. Generally green luminescence is due to ionized oxygen vacancy in ZnO which is produced by recombination of photo generated hole with ionized charge state of that specific defect. Strong green luminescence means more ionized oxygen vacancies and hence more and more defect may be there in sample. Hence, in order to reduce the densities of these defects and to prevent the agglomeration of small ZnO nanoparticles, we are using different capping agent to modify the surface of ZnO nanoparticles. These capping agents effectively control the particles size by limiting the growth of particle after nucleation stage. When chemical reaction occurs between modifier and nanoparticles surface, it results in change the surface structure of nanoparticles. Due to bonding of modifier on the surface of nanoparticles, which decrease the surface force of hydroxyl, hydrogen bonding between particles gets eliminated and hence formation of oxygen bride bonding is prevented. Under present work, 2-aminothiols are decorated on surface of ZnO nanoparticles through colloidal synthesis. The main objective of the present study is to obtain how much improvement we can expect in optoelectronics properties of ZnO nanoparticles by the surface modification using 2-aminothiols as a capping agent.
2 General information of synthesis and measurements
All the chemicals and solvents were purchased from Aldrich and were used without further purifications. Zinc nitrate and NaOH are used as starting material for precipitation of zinc oxide nanoparticles while 2-aminothiols as capping agents are added for the purpose of surface modification in the reaction stage. Finally surface modified prepared ZnO samples were annealed at different time i.e., annealing time was varied for 6 to 10 h in steps in colloidal solution. Size and crystalline quality of prepared modified ZnO nanoparticles were analyzed by XRD. Optical properties of the modified ZnO samples were analyzed by photoluminescence (PL) and UV–visible spectroscopy. Emission and excitation spectra of ZnO particles were obtained and recorded by using a Perkin–Elmer luminescence spectrophotometer (Model-LS55). The absorption spectrum was obtained by using Perkin–Elmer Lambda 45 UV/VIS spectrophotometer. The attachment of 2-aminothiols ligands on ZnO nanoparticles surface was characterized through FTIR spectroscopy. FTIR spectra were obtained and acquired by using Perkin–Elmer spectrum spectrometer.
3 Results and discussion
3.1 Structure and particle size of uncapped and capped ZnO nanoparticles
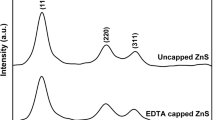
The XRD analysis was obtained by using Riga-Ku Miniflex X-ray diffractometer and purity of phase was examined by using Cu-Kα radiation, 45 kV and 40 mA, time constant of 0.5 s and crystal graphite monochromator. Figure 1 shows the XRD spectra for commercially prepared ZnO nanoparticles. XRD pattern of commercially prepared ZnO shows three distinct ZnO diffraction peaks (100), (002) and (101) which appear at 2θ = 31.73°, 34.37° and 36.21°, respectively. Here in the Fig. 1 all the diffraction peaks can be indexed to wurtzite structure of ZnO. No other peaks of impurities were detected which indicates high purity of ZnO crystal structure. According to position of diffractions peak and width at the half maxima, size of the ZnO nanoparticles can be estimated. The crystallite size of commercially prepared ZnO nano particles which was deduced from Scherrer’s formula, gave the values of about 32 nm.
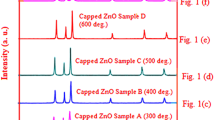
Figure 2 shows the XRD pattern for one of case of annealing time (10 h) in colloidal solution for modified ZnO nanoparticles. Modified ZnO nanoparticles were synthesized using capping agent 2-aminothiols as a capping agent. On synthesis we get white powder from the colloidal solution, which was further annealed at 6, 7, 8, 9, 10 h respectively, analyzed by XRD. The crystallite size of modified ZnO nano particles which was deduced from Scherrer’s formula, gave the values of about 24 nm for one of case of annealing time (10 h) is as shown in Fig. 2. In Fig. 2 broadening of peaks occurs, indicating smaller size of modified ZnO nanoparticles. We can conclude that, the ZnO nanoparticles sample synthesized commercially were comparatively bigger than capped ZnO nanoparticles or modified ZnO nanoparticles. This will be due to the fact that commercially prepared ZnO nanoparticles were collected after some crystal growth without using any capping agent. In the present work crystal growth can be limited by using 2-aminothiol as a capping agent, which results in smaller size of modified ZnO nanoparticle as confirmed by XRD pattern.
3.2 Photoluminescence spectrum analysis of modified ZnO nanoparticles
The fluorescence signatures of ZnO are shown in Fig. 3, revealing the PL spectrum of surface coated (with 2-aminothiols) ZnO nanoparticles. Actually when excited by UV light of suitable wavelength of 320 nm by a xenon lamp, it shows the high emission intensity of visible region and low intensity of UV region. Generally speaking the PL spectra of pure ZnO showed green luminescence around 560 nm. Here in present work PL spectra of modified ZnO is investigated by varying the annealing time from 6, 7, 8, 9 and 10 h in colloidal synthesis using 2-aminothiols. Here in all ZnO modified samples, a blue-green luminescence peak (around 495 nm) and UV luminescence peak (355 nm) were graphically observed in PL spectra. Hence 2-aminothiols results in blue shift in spectra of both UV and visible region of the spectrum. Also in modified ZnO the intensity of UV region is more than visible region. PL spectra of modified ZnO showed blue shift in individual spectra as we are varying the annealing time in colloidal solution from 6 to 10 h in successive steps. It is evident that UV luminescence will be due to recombination of exciton of ZnO nanoparticles. The origin of blue-green luminescence is rather controversial and it has been studied extensively [20–22]. Most of the researcher believes that singly ionized vacancy is responsible to have green luminescence in ZnO material and it results due to recombination of photo-generated hole with a singly ionized charge state in valence band [23]. Dangling bond on the surface is responsible to have nonradiative recombination centre for all photogenerated charge carriers. Hence the passivation of ZnO nanoparticle surface is main concern to all researchers in these days. The high ratio of UV to visible emission shows a good quality of ZnO nanoparticles and hence surface defect will be low. It also indicates that Dangling bond and number of defect on surface of ZnO particles will be less. PL spectra also showed that there is hardly any shift in UV emission peak by capping agent, which imply that the capping agent did not result in change the size of ZnO nanoparticles and hence no increase in surface defects. In this regard Dijiken et al. [24] proposed that nanoparticles surface plays a vital role in deciding the visible emission. Hole of valence band can be trapped by surface states and tunnel back into the oxygen vacancy which contain one electron (Vo*), and it results in the formation of recombination centre (Vo**). Due to recombination of a shallowly trapped electron with deeply trapped hole in Vo** center, it leads to have visible emission. While in uncapped ZnO nanoparticles, due to presence of many surface defects strong visible emission will occur. At the grain boundaries, there is presence of many zinc and oxygen defects. Here zinc defects at the interface adsorb oxygen, which can combine with imine linkage of capping agent 2-aminothiols. Hence as a result, interface defects start to decrease, and probability of surface trapped hole is decreased. At the same time capping agent 2-aminothiols molecule spatially obstruct the process of surface trapped hole tunneling back into the particles to form the Vo** centre. Both these effect results in decreasing the probability of Vo** recombination (visible emission). So, in comparison with the uncapped ZnO nanoparticles, the visible emission of ZnO nanoparticles capped with 2-aminothiols becomes weak. Decreasing the probability of these two process leads to increasing the intensity of UV PL peak when the ZnO nanoparticles are capped by molecules 2-aminothiols.
3.3 UV–visible spectra of modified ZnO nanoparticles
The optical properties of ZnO nanoparticle were become significant as size of particles is decreased to nanoscale level. Here we have investigated the radiative absorption in modified ZnO nanoparticles with respect to commercially pure ZnO nanoparticles counterpart by employing UV–visible spectroscopy. Here in this discussion, UV spectra of uncapped & capped ZnO was explained in term and UV absorption curve at different annealing time (from 6 to 10 h) in the colloidal solution.
Figure 4a, b shows the UV–visible absorption spectra of uncapped or commercially prepared ZnO nanoparticle sample. It reports that the optical absorption peak value of 378 nm (3.27 eV) for commercially prepared sample. For ZnO nanostructure 380 nm wavelengths corresponds to bulk value of band edge at 3.26 eV. Absorption around 378 nm means that optical absorption spectrum is slightly blue shifted with respect to bulk ZnO absorption value of 380 nm. It is evident that absorption edge is slightly blue shifted, which is due to the smaller size of ZnO nanoparticles prepared commercially. This apparent blue shift in absorption edge is due to quantum confinement effect. Figure 5 showed the optical absorption spectra of capped (2-aminothiols) ZnO nanoparticles in colloidal solution for different annealing time. One can observe from the optical absorption spectra that optical absorption region due to modified ZnO nanoparticles appearing in the range of 315–325 nm when sample were annealed from 6 to 10 h in colloidal solution. For ZnO nanoparticles 380 nm wavelengths corresponds to bulk value [25] of band edge at 3.27 eV, but the absorption in wavelength range from 315 to 325 nm indicates that there is blue shift, which was due to quantum confinement effect from small crystallite size of individual ZnO nanoparticles. As the annealing time in colloidal solution is increased the absorbance will start shifting to higher wavelength, suggesting that there is an increase in size of modified ZnO nanoparticles in colloidal solution. Wide region of the peaks indicates the size distribution of modified ZnO nanoparticles, as evident by XRD results as shown in Fig. 2 also. Optical Absorption spectra obtained in Fig. 5 giving all the absorption peak value (band gap) in case of for 6, 7, 8, 9 h except in case of 10 h. In case of 10 h where peak is concentrated in region ranges from 320 to 325 nm. For measurement of band gap of this region, peak position is ascertained & determined by taking [26] the derivative d (abs)/dE of the experimental plot in the range of 300–600 nm to arrive at peak position at 323 nm, which correspond to band gap of 3.83 eV.
Hence it can be concluded that capping agent 2-aminothiols effectively can effectively control the growth of individual ZnO nanoparticles. While uncapped ZnO nanoparticles can grow rapidly in colloidal solution, this phenomenon was noticed by the formation of residue at bottom of container. Here in the present work 2-aminothiols is used as capping agent which will slow down the growth process of individual ZnO nanoparticles in colloidal solution. When added in a controlled quantity, the amine groups are partially hydrolyzed by available water molecule in the solution and one group would react with OH− group on the ZnO surface to form capping layer, with unreacted side group on the surface making whole surface as a stable one.
3.4 FT-IR spectrum analysis of modified ZnO nanoparticles
The wurtzite structure of commercially prepared ZnO was again further supported by FTIR spectrum as shown in Fig. 6. Generally in FTIR spectrum IR transmittance signifies the characteristics of Zn–O vibration and it is dependent on the particle size & morphology, which can be confirmed through small size spherical aggregates formation in XRD or TEM analysis of the samples. Series of absorption peaks which ranges from 500 to 4,000 cm−1 can be found, which corresponds to presence of organic groups and chemisorbed species in commercially prepared ZnO, surface modified ZnO nanoparticles samples, respectively. Figure 7a shows the FTIR spectrum of commercial prepared ZnO nanoparticles. It shows presence of various absorption band at ~3,500, ~2,344, ~1,630 and around 1,385 cm−1, which signifies O–H mode, CO2 mode and symmetric and asymmetric C = O stretching modes, respectively [27–29]. Peaks around 2,830 and 3,000 cm−1 are due to C–H stretching of alkane groups. Presence of two absorption bands at 1,095 and 890 cm−1 are due to C–OH bond in stretching and deformation modes, respectively. The band around 510 cm−1 in FTIR spectra will be associated with oxygen vacancy defect in ZnO. The presence of CO2 mode probably results from re-active carbon-containing ion species during synthesis steps and O–H mode results due to hygroscopic nature of ZnO nanoparticles.
Figure 7 shows the FTIR spectra of samples of modified ZnO annealed at different annealing time from 6 to 10 h in colloidal solution. Figure 7 shows the broad peak region between 3,228 and 3,415 cm−1 was due to presence of stretching vibration of –OH group and amine group (N–H) on the surface of ZnO nanoparticles. The peak observed at 1,300 and 1,488 cm−1 will corresponds to stretching vibration of C–H group. The presence of peaks at 820 and 1,097 cm−1 will be due to stretching vibration of N–H group. While the band around 2,240–2,260 showing the presence of C = N group in stretching vibration mode. It is clear from various Fig. 7a–e that with the increase in annealing time the size of modified ZnO nanoparticles increases, the FTIR signatures of various impurities such as hydroxyl, CO2 mode and alkane will start to decrease.
4 Conclusions
ZnO nanoparticles capped with 2-aminothiols are prepared by precipitation method and particles are obtained in the white powered form. The influence of capping agent 2-aminothiol on optical properties of ZnO nanoparticles has been investigated. When ZnO nanoparticles are capped with 2-aminothiols molecules, its molecules perform two functions. (1) It modify the surface of ZnO nanoparticle by compensating all the dangling bonds and defects. (2) It block the process of surface-trapped hole tunneling back to form Vo** recombination centre. PL spectra of modified ZnO showed high intensity of UV emission and less intensity in visible emission region, which further indicates good quality of ZnO nanoparticles with less surface defects. The photoluminescence spectra of modified ZnO showed that surface modification by capping agent 2-aminothiol is an efficient method to improve the optoelectronic properties of ZnO nanoparticles. While the optical absorption spectra showed that the capped ZnO colloids absorbed at lesser wavelength than the uncapped ZnO nanoparticles due to their smaller size. This blue shift in optical absorption edge was due to quantum confinement effect.
References
J. Pal, S.G. Kwon, J.H. Yu, T. Hyeon, Adv. Mater. 15, 1873 (2005)
B.K. Meyer, H. Alves, D.M. Hofmann, W. Kriegseis, D. Forster, F. Bertram, J. Christen, A. Hoffmann, M. Strasburg, M. Dworzak, U. Haboeck, A.V. Rodina, Physica Status Solidi (b) 241(2), 231 (2004)
O. Madelung (ed.), Data in Science and Technology: Semiconductors (Springer, Berlin, 1992)
A. Mendoza-Galvan, C. Trejo-Cruz, J. Lee, D. Bhattacharyya, J. Metson, P.J. Evans, U. Pala, J. Appl. Phys. 99, 14306 (2006)
Y.H. Ni, X.W. Wei, J.M. Hong, Y. Ye, Mater. Sci. Eng. B 121, 42 (2005)
J.E. Nause, III-Vs Review. 12, 28 (1999)
Y. Chen, D. Bagnall, T. Yao, Mater. Sci. Eng. B 75, 190 (2000)
S.N. Bai, T.Y. Tseng, J. Appl. Phys. 74, 695 (1993)
M. Aslam, V.A. Chaudhary, I.S. Mulla, S.R. Sainkar, A.B. Mandale, A.A. Belhekar, K. Vijayamohanan, Sens. Actuators A Phys. 75, 162 (1999)
O. Kluth, B. Rech, L. Houben, S. Wieder, G. Schope, C. Beneking, H. Wagner, A. Loffl, H.W. Schock, Thin Solid Films 351, 247 (1999)
N.K. Zayer, R. Greef, K. Roger, A.J.C. Grellier, C.N. Pannell, Thin Solid Films 352, 179 (1999)
S.J. Pearton, C.R. Abernathy, D.P. Norton, A.F. Hebard, Y.D. Park, L.A. Boatner, J.D. Budai, Mater. Sci. Eng. R 40, 137 (2003)
A.P. Alivisatos, Science 271, 933 (1996)
H.S. Kang, J.S. Kang, J.W. Kim, S.Y. Lee, J. Appl. Phys. 95(3), 1246 (2004)
S.A. Studenikin, N. Golego, M. Cocivera, J. Appl. Phys. 84(4), 2287 (1998)
K. Vanheusden, C.H. Seager, W.L. Warren, D.R. Tallant, J.A. Voigt, Appl. Phys. Lett. 68(3), 403 (1996)
L. Wu, Y. Wu, X. Pan, F. Kong, Opt. Mater. 28(4), 418 (2006)
S.B. Zhang, S.H. Wei, A. Zunger, Phys Rev B 63(7), 075205 (2001)
F. Tuomisto, K. Saarinen, D.C. Look, G.C. Farlow, Phys. Rev. B Condens. Matter Mater. Phys. 72(8), 085206 (2005)
H.S. Kang, J.S. Kang, J.W. Kim, S.Y. Lee, J. Appl. Phys. 95(3), 1246 (2004)
S.A. Studenikin, N. Golego, M. Cocivera, J. Appl. Phys. 84(4), 2287 (1998)
K. Vanheusden, C.H. Seager, W.L. Warren, D.R. Tallant, J.A. Voigt, Appl. Phys. Lett. 68(3), 403 (1996)
S. Sakohara, M. Ishida, J. Phys. Chem. B 102, 10169–10175 (1998)
A. Van Dijkan, E.A. Meulenkamp, D. Vanmaekelbergh, A. Meijerink, J. Phys. Chem. B 104, 1715 (2000)
Y. Hu, H.J. Chen, J. Nanopart. Res. 10, 401 (2008)
Navndu Goswami, Dhirendera Kumar Sharma, Physica E 42, 1675 (2010)
S.C. Liufu, H.N. Xiao, Y.P. Li, Polm. Degrad. Stab. 87, 103 (2005)
Y.J. Kwon, K.H. Kim, C.S. Lim, K.B. Shim, J. Ceram. Proc. Res. 3, 146 (2002)
R.F. Silva, M.E.D. Zaniquelli, Colloids Surf. A 198–200, 551 (2002)
Acknowledgments
The authors would like to acknowledge the Instrumentation facilities provided by IIT Ropar (Punjab) for investigating the samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rana, S.B., Bhardwaj, V.K., Singh, S. et al. Synthesis and optical characterization of ZnO nanoparticles capped with 2-aminothiols. J Mater Sci: Mater Electron 24, 20–26 (2013). https://doi.org/10.1007/s10854-012-0767-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-012-0767-9