Abstract
The composite ceramics (NiMn2O4)0.50(La1−x Ca x MnO3)0.50 (0 ≤ x ≤ 0.3) consisting of spinel-structured NiMn2O4 and perovskite-structured CaO-doped LaMnO3 were prepared by classical solid state reaction. The X-ray diffraction (XRD) patterns have shown that the major phases presented in the sintered samples are NiMn2O4 compounds with a spinel structure, La1−x Ca x MnO3 with a perovskite structure. The Scanning Electron Microscope (SEM) pictures have exhibited that the grain size of the composite ceramics decreases from ca. 6.5 to 2.0 μm as the mole fraction of CaO increases from 0 to 0.3. The ρ 25 °C and B 25/50 constants of the composite samples are in the range of 0.234–8.61 Ω cm and 2,600–2,962 K, respectively. In particular, CaO-doped leads to a decrease in the resistance drift of the (NiMn2O4)0.50(La1−x Ca x MnO3)0.50 composite NTC (negative temperature coefficient) ceramics after aging test. This indicates that the CaO-doped (NiMn2O4)0.50(La1−x Ca x MnO3)0.50 NTC ceramics display high electrical stability in comparison with the Ca-free (NiMn2O4)0.50(LaMnO3)0.50 ceramics. The X-ray photoelectron spectroscopy (XPS) analysis verifies that the valence states of the manganese ions have a highly mixed state of Mn2+, Mn3+ and Mn4+ at B site. And the electrical conduction of the composite ceramics can be elaborated by the ions migration mechanism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The NTC thermistors (TRs) based on mixed transition-metal and rare earth metal manganite electroceramics have been widely used for temperature measurement, control, compensation and suppression of inrush current, due to their high stability and low cost [1, 2]. In practice, the NTC thermistors are generally characterized by two parameters: B, the thermal constant which indicates a sensitivity to temperature excursions (B = Ea/k, unit in Kelvin, in which Ea is the electronic activation energy and k is the Boltzmann constant) and ρ 25 °C, the resistivity at 25 °C. By compounding spinel and perovskite structure oxides, varying their relative proportions, doping with Alkalierdelement and controlling the processing conditions, a right combination of the resistivity and B value can be obtained [3].
The popular NTC materials are Ni- and Mn- based spinel oxides of general formula AB2O4, such as Mn–Ni–Co–O, Ni–Cu–Mn–O, Fe–Mn–Ni–O, Mn–Ni–Co–Zn–O [4–7]. For those spinel oxides, a high B value generally couples with high electrical resistivity and vice versa. However, some applications such as suppressing the inrush current require NTC materials with a low electrical resistivity (≤10 Ω cm) while possessing a large B (≥2,600 K) and sufficient stability. Therefore, there is a need for the development of new materials that have good electrical characteristics in suppressing the inrush current. Traditional methods to design a new material are composite and doping. Zhao et al. [8] have studied the composites made of spinel-structured (Ni, Mn)3O4 and perovskite-structured La(Mn, Ni)O3, and found that the electrical resistivity of the composite decreased depending on the amount of the perovskite phase. Luo et al. [9] have prepared a composite ceramics material with high electrical resistivity and low B by introducing yttrium-stabilized zirconia as the second phase into Fe0.5Cu0.2Ni0.66Mn1.64O4 spinel matrix, the former is of high electrical resistivity and the latter of low electrical resistivity and low B. In principle, the material with low electrical resistivity and high B may be obtained through introducing a second phase with low electrical resistivity into a spinel oxide of high B. What’s more, the composite can make full use of favorable condition and promote complementarity in each component due to the adjustability of its multiplicity and binding form [10], and the outstanding and attractive properties that the single component couldn’t achieve may be obtained by complex method. Therefore, we propose an alternative approach by introducing a highly conductive component doped by a certain element into the spinel oxide. Perovskite structure LaMnO3 is a good electronic conductor with the electrical resistivity of <than 1 Ω cm at 25 °C [11]. The Ca ion has a stable valence state bivalence and the Ca2+ has a approximate ionic radii with La3+(the radius of Ca2+, La3+ are 0.1000 and 0.1032 nm, respectively). Proper substitution of La for Ca may also decrease the resistivity of LaMnO3 [12]. In the present work, the effects of CaO-doped in NiMn2O4–LaMnO3 composite ceramics on microstructure and electrical properties are investigated.
2 Experimental procedure
The NiMn2O4 and La1−x Ca x MnO3 (0 ≤ x ≤ 0.3) powder was synthesized using the conventional solid-state reaction method. The raw materials were analytical reagent La2O3, CaO, MnO2 and Ni2O3. With appropriate amounts of those oxides, NiMn2O4 and La1−x Ca x MnO3 powder was ball-milled at room temperature for 8 h separately in agate containers using agate balls as the milling medium and deionized water as a dispersant. The two kinds of ball milled slurry were then dried at 80 °C and calcined for 2 h at 1,000 °C respectively. The powder was ball-milled for 8 h again to break agglomerates.
With a mass ratio of the as-prepared NiMn2O4 powder to La1−x Ca x MnO3 powder of 1:1, the mixture was grinded and then sieved. Disk-shaped compacts with a diameter of 10 mm and a thickness of 2 mm were formed by uniaxial pressing the powder at 60 MPa followed by isostatic cool pressing at 350 MPa. These green compacts were sintered at 1,300 °C for 4 h under air atmosphere.
The phase composition of the composite was determined by X-ray diffraction (XRD: BRUKER D8-ADVANCE, Germany) with Cu Kα radiation (λ = 1.5406 Å). The microstructure and element compositions of sintered samples were observed by the Scanning Electron Microscope (SEM; LEO1430VP, Germany) in combination with energy dispersive spectroscopy (EDS; OXFORD INCA200, England). The X-ray photoelectron spectroscopy (XPS: Kratos Amicus, England) analysis was performed on the compacts in an ultrahigh vacuum and the spectra were recorded using an Mg Kα X-ray source (hν = 1,256.6 eV). For electrical properties measurements, two opposite sides of the sintered compacts were coated with silver-palladium conductive paste, heated at 835 °C for metallization. The resistances of the composite were measured with disk-shaped samples using the Agilent 34401A digital multimeter. To ensure the temperature deviation from the designated value to be <±0.05 °C, samples were immersed in silicon oil. The thermal constant B was calculated according to the formula B = 3,853.89·ln (R 25 /R 50), in which R 25 and R 50 are the resistances at 25 and 50 °C, respectively. To obtain aging properties, these samples were aged by heat treatment at 125 °C in air for 500 h, and the aging is characterized by ΔR/R 0 = (R − R 0)/R 0, in which R 0 and R are the resistivity at 25 °C before and after the heat treatment.
3 Results and discussions
3.1 Phase composition and microstructure
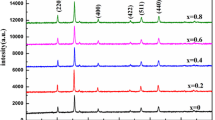
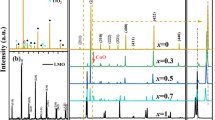
Figure 1 shows the XRD patterns of NiMn2O4 (NiMn2O4)0.50 (La1−x Ca x MnO3)0.50(0 ≤ x ≤ 0.3) and La0.9Ca0.1MnO3 samples sintered at 1,300 °C. It can be seen that the composite ceramics are consisted of cubic structure spinel phase and orthorhombic structure perovskite phase. Before compositing, there is a little amount of monoclinic structure rock-salt NiO phase separated out from the spinel phase NiMn2O4 matrix and then disappeared in composites. It may be that introducing LaMnO3 doped by CaO into NiMn2O4 retarded the separating process of rock-salt NiO phase. In addition, the substitution of La for Ca doesn’t change the perovskite structure of LaMnO3. Compared to the single phase without compounding, the interplanar spacing of (111) in the spinel phase increases from 4.8561 to 4.8704 Å, while the interplanar spacing of (101) in the perovskite phase decreases from 3.8914 to 3.8824 Å in (NiMn2O4)0.50 (La0.9Ca0.1MnO3)0.50 sample.
The observed changes of the interplanar spacing can be explained in terms of valence and size of the cations occupying the octahedral sites. The Mn-rich spinel induced by the separation of the NiO is known to possess a cationic distribution [Mn2+]\( \left[ {{\text{Ni}}_{\alpha }^{2 + } {\text{Mn}}_{ 2- 2\alpha }^{3 + } {\text{Mn}}_{\alpha }^{4 + } } \right] \)O4 [13]. According to this result, when a Ni2+ ion is replaced by a Mn3+ ion, to maintain the electrical neutrality of the lattice, a Mn4+ ion has to convert to Mn3+. In other words, annihilation of one Ni2+ ion is accompanied by the annihilation of one Mn4+ ion and generation of two Mn3+ ions. Accordingly, the sum of these two Mn3+ ions in size is larger than that of one Ni2+ ion and one Mn4+ ion based on Table 1 [14], and it explains the larger interplanar spacing observed from the Mn-rich spinel phase. As to the perovskite phase, both the migration of Ni2+ ions and the substitution of Ca2+ for La3+ make the change of ions valence state toward the opposite direction. The Mn3+ at B site in the perovskite structure is replaced by Ni2+ and this process leads to annihilation of two Mn3+ ions accompanying by generation of one Ni3+ ion and one Mn4+ ion which induces decrease of the interplanar spacing. Moreover, the radius of Ca2+ is smaller than La3+ and then the substitution of Ca2+ for La3+ also results in the decrease in the interplanar spacing of the perovskite phase.
Figure 2 shows the SEM images of the composite samples (NiMn2O4)0.50(La1−x Ca x MnO3)0.50 sintered at 1,300 °C. From these SEM figures, it is immediately seen that the average grain size decreases from 6.5 to 2.0 μm with x increasing from 0 to 0.3, crystalline grains become integrated and uniform and the grain boundary turns more distinguishable. Obviously, the presence of the perovskite phase doped by CaO suppresses the grain growth and produces great influence on the microstructure of the composites. It is known that La can scatter electrons much more effectively than Mn, Ni and Ca, for the atomic number of the former is much larger than those of the latter. A reasonable conclusion can be drawn that the brighter area corresponds to the perovskite phase, while the darker area the spinel phase which is verified by the EDS spectra in Fig. 3. Based on the SEM/EDS results, Table 2 shows the values of weight and atomic percentages obtained from different spots in Fig. 2a4. The EDS spectra of spot A refers to the brighter area where is La-rich and Ni-poor area, while the EDS spectra of spot B referring to darker area is the contrary. From Table 2, it also can be seen that there is no pure La1−x Ca x MnO3 or NiMn2O4 existed in the sintered body, which may indicate that the perovskite phase and the spinel phase formed solid solution during sintering process and ions migration occurred between two phases.
Representative EDS spectra of different spots in Fig. 2a4
3.2 Electrical properties
Figure 4 shows the Arrhenius plot of the resistivity of the composites in the temperature ranging from −35 to 50 °C. It can be seen that the composites exhibit typical NTC effect. Thermal constant B and ρ 25 °C of (NiMn2O4)0.50(La1−x Ca x MnO3)0.50 as a function of doping CaO content are presented in Fig. 5. The resistivity of the composites first decreases and then increases as x value increases from 0 to 0.3. When x = 0.3, the value of resistivity is even larger than the CaO-free samples. In contrast, the thermal constant B increases sharply from 1,639 to 2,962 K with x increasing from 0 to 0.3. Therefore, a small quantity (≤0.2) of CaO-doped can produce a composite which possessed a right combination of resistivity and thermal constant (ρ 25 °C ≤ 8.61 Ω cm, B ≥ 2,690 K) required for the application of suppressing the inrush current. The aging coefficient (ΔR/R 0) for the composites is shown in Fig. 6. It can be seen that the values of ΔR/R 0 decreases with an increase in x. For x = 0.3, the resistivity of the composite was increased by 0.34% after aging at 125 °C in air for 500 h, while 5.02% for the samples without doping CaO. It is likely that the presence of CaO provides a physical barrier to the aging of the composite [15].
As to the electrical conduction of the composite ceramics, it can be explained well by the ions migration mechanism and the ions migration mainly happens at B sites between the spinel structure NiMn2O4 and perovskite structure La1−x Ca x MnO3 as the followed Eq. 1.
The electrical conduction of the composite is mainly determined by the amount of the carriers and their mobility, especially for manganite, the ion pairs of different valence states, Mn3+/Mn4+, for example, influence the electronic transport behavior markedly [16]. CaO-doped changes the ratio of Mn3+/Mn4+ in LaMnO3 which effects the ion migration in the composite ceramics. The relationship between resistivity and x value in the composite ceramics can be explained as follows. For undoped LaMnO3 where all the Mn ions are nominally in the tervalence state, predominant carriers are holes at room temperature. When La3+ is substituted by a small amount of Ca2+, some of the Mn3+ ions shift their valence state from tervalence to tetravalence, which contributes to the enhancement of hole concentration. Consequently, the drop in the resistivity of x = 0.1 composite sample is considered to be associated with the increase of the hole concentration in the perovskite phase. Due to the radius of Ca2+ is a little smaller than La3+, the increase of x induces the enhancement of lattice distortion and further decreases of interplanar spacing, which bates the Ni2+ ions migration between two phases because it also reduces the interplanar spacing of the perovskite structure La1−x Ca x MnO3 with x = 0.2, 0.3. Therefore, the ion pair number of different valence states (Mn2+/Mn3+, Mn3+/Mn4+) decrease, then the electrical conduction of the composite weakens. Therefore, as the amount of CaO increases, the resistivity of the composite ceramics decreases to a minimum value and then increases, which is shown in Fig. 4.
The change of the composite electrical conductivity above can be confirmed further by the XPS results. Figure 7 shows the XPS spectra for the Mn 2p level of the samples NiMn2O4, La0.9Ca0.1MnO3 and (NiMn2O4)0.50(La0.9Ca0.1MnO3)0.50. In Table 3 it is summarized the binding energy and peak intensity value obtained from the XPS experiments using the approximate relationship: \( \frac{{n_{1} }}{{n_{2} }} = \frac{{I_{1} }}{{I_{2} }} \cdot \frac{{\sigma_{1} }}{{\sigma_{2} }}\left[ {\frac{{Ek_{2} }}{{Ek_{1} }}} \right]^{\frac{1}{2}} \)where n 1, Ek 1, and σ 1 are the number of atoms of element I, the kinetic energy value corresponding to the line considered, and the cross-section calculated theoretically, respectively[17]. From Fig. 6 and Table 3, it can be seen that the valence states of the manganese ions have a highly mixed state of Mn2+, Mn3+ and Mn4+ at B site. Before compositing, the substitution of Ca2+ for La3+ in LaMnO3 leads to the Mn3+ ions translating into Mn4+, which produces more ion pair Mn3+/Mn4+ and contribution to the high conductivity of the perovskite structure [16]. In the composite, in order to keep charge conservation, the migration of Ni2+ ions between two phases results in Mn4+ ions translating into Mn3+ in the spinel phase and Mn3+ ions translating into Mn4+ in the perovskite phase, and the latter is predominant which is in good agreement with the previously analysis and the peak intensity data in Table 3.
4 Conclusion
The composite ceramics (NiMn2O4)0.50(La1−x Ca x MnO3)0.50 (x = 0, 0.1, 0.2, 0.3) consisted of spinel structure NiMn2O4 and perovskite structure La1−x Ca x MnO3 were prepared by the conventional solid state reaction. Introduction of low-resistivity perovskite CaO-doped LaMnO3 into the spinel oxide NiMn2O4 results in a decrease of the grain size from 6.5 to 2.0 μm, a large increase in B from 1,639 to 2,962 K as x increases from 0 to 0.3. The resistivity firstly decreases slightly with increasing CaO content from x = 0 to 0.1, and then in the range of x ≥ 0.1, the resistivity increases as the amount of Ca content increases, however, the ρ 25 °C is always <8.61 Ω cm. Doping CaO in LaMnO3 make the composite NTC thermistors have better electrical stability (ΔR/R 0 = 0.34% when x = 0.3) in comparison with CaO-free thermistors (ΔR/R 0 = 5.02% when x = 0). The electrical conduction of the two phase composite can be well described using the ion migration mechanism.
References
A. Feteira, J. Am. Ceram. Soc. 92, 967 (2009)
M.M. Vakiv, O.I. Shpotyuk, V.O. Balitska, B. Butkiewicz, L.I. Shpotyuk, J. Eur. Ceram. Soc. 24, 1243 (2004)
T. Yokoyama, T. Meguro, Y. Shimada, J. Tatami, K. Komeya, Y. Abe, J. Mater. Sci. 42, 5860 (2007)
T. Yokoyama, T. Meguro, Y. Shimada, J. Tatami, K. Komeya, Y. Abe, J. Mater. Sci. 42, 5860 (2007)
R.N. Jadhav, V. Puri, J. Alloy Compd. 507, 151 (2010)
Z.B. Wang, C.H. Zhao, C.S. Chen, A.J.A. Winnubst, J. Eur. Ceram. Soc. 26, 2833 (2006)
K. Park, J.K. Lee, S.J. Kim, W.S. Seo, W.S. Cho, C.W. Lee, S. Nahm, J. Alloy Compd. 467, 310 (2009)
C.H. Zhao, Z.B. Wang, S.M. Wang, P.H. Yang, C.S. Chen, J. Electroceram. 20, 113 (2008)
W. Luo, H.M. Yao, P.H. Yang, C.S. Chen, J. Am. Ceram. Soc. 92, 2682 (2009)
M.L. Singla, S. Awasthi, A. Srivastava, D.V.S. Jain, Sens. Actuators A 136, 604 (2007)
I. Maurin, P. Barboux, Y. Lassailly, J.P. Boilot, J. Solid State Chem. 160, 123 (2001)
Z. Branković, K. Ðuriš, A. Radojković, S. Bernik, Z. Jaglićić, M. Jagodić, K. Vojisavljevic, J. Sol–Gel Sci. Technol. 55, 311 (2010)
S. Guillemet-Fritsch, J.L. Baudour, C. Chanel, F. Bouree, A. Rousset, Solid State Ion. 132, 63 (2000)
R.D. Shannon, Acta Cryst. A 32, 751 (1976)
K. Park, I.H. Han, J. Electroceram. 17, 1079 (2006)
P.N. Lisboa-Filho, M. Bahout, P. Barahona, C. Moure, O. Pena, J. Phys. Chem. Solids 66, 1206 (2005)
Y. Boudeville, F. Fiqueras, M. Forissier, J.L. Portefaix, J.C. Vedrine, J. Catal. 58, 52 (1979)
Acknowledgments
The authors are thankful to the National Natural Science Foundation of China (Grant No. 50902148) and the “Western Light Joint Scholar Foundation” Program of Chinese Academy of Sciences (No. RCPY200901) for providing the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guan, F., Zhang, H., Chang, A. et al. Effect of CaO-doped in NiMn2O4–LaMnO3 composite ceramics on microstructure and electrical properties. J Mater Sci: Mater Electron 23, 1728–1733 (2012). https://doi.org/10.1007/s10854-012-0654-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-012-0654-4