Abstract
BaTi1−x Co x O3−δ (0.01 ≤ x ≤ 0.4) ceramics were prepared by a wet chemical process polymerized with polyvinyl alcohol. The phases and related electrical properties of the ceramics were investigated. The phase component of the ceramics changes from a tetragonal phase to a hexagonal one with the Co concentration increase. A pure hexagonal phase formed in the ceramic with x = 0.2. The measurement of the temperature dependence of resistances revealed that the ceramic resistivities increase with temperature rising at the temperatures (T) lower than half of the related Debye temperature (ΘD), and the ceramics show a negative temperature coefficient (NTC) effect at T > ΘD/2. The material constants B 50/120 of the BaTi1−x Co x O3−δ NTC thermistors were calculated to be 3,187, 2,968 and 2,648 K for x = 0.2, 0.3 and 0.4, respectively. Narrow-band conduction and non-adiabatic hopping models are proposed for the conduction mechanisms at T < ΘD/2 and T > ΘD/2, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The thermistors with a characteristic of negative temperature coefficient (NTC) of resistivity are widely used in various industrial and domestic applications. Traditionally, most of the NTC thermistors are based on the solid solutions of transition metal oxides with a spinel structure, such as Mn–Ni–O, Mn–Ni–Cu–O and Mn–Co–Ni–O systems [1–3]. For the interesting electrical property, lots of scientists and engineers have also focused on the doping effect of the spinel type NTC thermistors, e.g., MgO, Al2O3 and Cr2O3 doped NTC thermistors [4–6], Cu and/or Zn doped Ni0.5Mn2.5O4 or Mn1.17−x Ni0.93Co0.9Zn x O4 [7, 8], and the doping effect of Fe in Cu0.10Ni0.66Mn2.24O4 thermistors [9]. However, the applications of NTC thermistors based on the spinel oxides are commonly limited to the temperatures below 200 °C for the relaxation of the crystal structure at high temperature [10]. For the different applications, some new type of NTC oxides have been developed, such as Bi2O3 doped with TiO2, Ta2O5 and WO3 [11], Bi3Zn2Sb3O14 ceramics [12], BaTiO3 co-doped with BaBiO3 and Y2O3 [13], and composite of polyaniline and Mn3O4 [14], etc.
BaTiO3-based ceramics have been widely studied and applied as the electronic ceramics such as capacitors for their dielectrical properties and thermistors for the effect of positive temperature coefficient (PTC) of resistivity. Meanwhile, it is reported that a hexagonal structure BaTiO3 (h-BaTiO3) can be stabilized at room temperature by rapid quenching from temperatures higher than 1,460 °C [15], firing in reducing atmospheres [16–18], or doping with some acceptor-type ions (e.g., Mg, Al, Cr, Mn, Cu, Fe, Co, Zn, Ga, Ni and In) on Ti site [19–22]. Most of the previous researches on h-BaTiO3 focused on the phase transformation. However, as our knowledge, there has not been any report on the NTC effect of h-BaTiO3 ceramics so far. During investigating the influence of Co doping on the crystal structure and electrical property of the BaTi1−x Co x O3−δ ceramics, the authors found that the BaTi1−x Co x O3−δ ceramics can not only transform from a tetragonal structure to a hexagonal one when the Co concentration increases, but also, more interesting, display an excellent NTC effect. The investigation results about the phase transformation and temperature dependence of resistivity in the BaTi1−x Co x O3−δ ceramics are reported in present paper.
2 Experimental
Appropriate quantities of barium carbonate (BaCO3,), tetra-n-butyl titanate (TBT) and cobalt oxide (Co2O3) were weighed according to the given formula BaTi1−x Co x O3−δ (x = 0.01, 0.05, 0.1, 0.2, 0.3, 0.4), respectively. TBT was dissolved in anhydrous alcohol, BaCO3 and Co2O3 were dissolved in dilute nitric acid, respectively. And then the above solutions were mixed together. Proper quantity of polyvinyl alcohol was employed as polymeric carrier in the solution mixture [23]. The obtained solution was heated and dried under magnetically stirring to form precursor. The precursors were calcined at 850 °C for 2 h, and then were pressed into pellets in about 15 mm in diameter and 3.5 mm in thickness. The glue pellets were sintered at 1,300 °C for 2 h in air. The sintered pellets were ground to a thickness of about 2 mm and painted with silver paste on both parallel sides of the samples as the electrode.
The temperature dependence of resistance (R–T) of the samples was measured by an R–T measurement system (ZWX-C, HuaZhong University of Science and Technology, China), and the temperature dependence of resistivity (ρ–T) was calculated according to the related sample size. The test temperature ranges from 26 to 300 °C. The phase components of the prepared ceramics were examined by an X-ray diffractometer (D/MAX 2550 PC) with Cu Kα radiation. The microstructures of the BaTi0.8Co0.2O3-δ ceramics were investigated with a scanning electron microscope (SEM) (JEOL, JSM-6360LV) and a transmission electron microscope (TEM) (FEI Tecnai G2 F20), respectively.
3 Results and discussion
3.1 Phase analysis
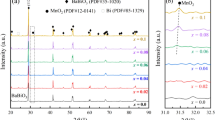
Figure 1 shows the XRD patterns of the as-sintered ceramics with different Co concentrations. For x = 0.01, a pure tetragonal phase (marked by ‘▼’) can only be detected in the ceramic. It indicates that the BaTi0.99Co0.01O3 ceramic has the similar phase composition as that of BaTiO3. The lattice parameter was determined to be a = 0.3996 nm and c = 0.4019 nm. As the Co concentration increases, e.g. x = 0.05, some diffraction peaks belonging to a hexagonal phase, \( \left( {10\overline{1} 3} \right),\;\,\left( {20\overline{2} 3} \right) \) and \( \left( {10\overline{1} 7} \right), \) can be detected besides the ones from the tetragonal phase. These indicate that a hexagonal phase formed and the ceramics is composed of the tetragonal and hexagonal phases. The peak intensity from the hexagonal phase increases, while those from the tetragonal phase decreases, with the Co concentration increases. It is found from Fig. 1 that the x = 0.2 ceramic has a single phase of a hexagonal polymorph with the space group of P63/mmc. The lattice parameter can be determined to be a = 0.5708 nm and c = 1.3987 nm. In the ceramics with x = 0.3 and x = 0.4, two extra diffraction peaks as marked by ‘◆’ from an impurity can be detected at 2θ = 28.5°–30°. The impurities were determined to be Ba2TiO4.
Figure 2a is a SEM observation of the as-sintered BaTi0.8Co0.2O3-δ ceramic. The ceramic has the grain size about 5 μm. Some pores, which should result from the volatilization of cobalt atoms during sintering, can be seen in the ceramic. The analysis of X-ray energy dispersive (EDX) shown that all the grains have the similar composition with Ba, Ti, Co and O. The EDX is not shown here. During TEM investigation, only hexagonal phase was observed. Figure 2b is a TEM image of the BaTi0.8Co0.2O3-δ ceramic. A selected-area-electron diffraction (SAED) pattern taken from the center grain, in Fig. 2b, along \( \left[ {2\overline{1} \overline{1} 0} \right] \) direction, is shown in Fig. 2c. Two diffraction points marked by arrows were indexed in Fig. 2c, indicating the hexagonal structure as described in Fig. 1.
It is commonly accepted that the oxygen deficiency plays a key role in stabilizing the h-BaTiO3 at room temperature. Both the Rietveld refinement of neutron diffraction data and study of density functional theory suggested that oxygen nonstoichiometry occurs by removal of O(1) from Ba(1)O(1)3 layers which separate the pairs of occupied face-sharing Ti2O9 octahedra (see in Fig. 3) [24, 25]. In undoped case, the oxygen-loss, which is chargedly compensated by partial reduction of Ti4+ to Ti3+, plays an important role in the reduction of the Gibbs energy and in the retention of the h-BaTiO3. The hexagonal phase increased proportionally to the amount of Ti3+ ions by the reduction of Ti4+ ions, and the minimal concentration of Ti3+ for stabilizing the hexagonal BaTiO3 at room temperature was 0.3% of the total Ti ions [17]. On the other hand, the transformation temperature from cubic phase to hexagonal one is also related to the concentration of equilibrium oxygen deficiency or Jahn–Teller effect induced by accepter dopants. Co atom has an electronic configuration of 3d 74s 2 and may lose two or three electrons in compounds. Co2+ and Co3+ ions are easy to substitute for Ti4+ site because of that the radius of either Co2+ or Co3+ (rCo2+= 79 pm,rCo3+= 68.5 pm) is much less than that of Ba2+ (149 pm) and is close to that of Ti4+ (74.5 pm) (www.webelements.com). Both Co2+ and Co3+ ions always substitute for Ti(2) site and have the lowest energy when they are located within the same Ti2O9 octahedra [25]. The BaTi1−x Co x O3−δ ceramics are expected to lose a slight amount of oxygen by the doping of Co ions in accordance with the following reaction:
where \( {\text{O}}_{\text{O}}^{ \times } \) is neutral oxygen, \( {\text{V}}_{\text{O}}^{ \bullet \bullet } \) is the doubly ionized oxygen vacancy, \( e^{'} \) is the electron.
The BaTi1−x Co x O3−δ ceramics with a low level of Co concentration (e.g. x < 0.05) do not have much enough oxygen vacancies for the full transition from tetragonal phase to hexagonal one, and still contain the tetragonal phase. With the Co concentration increase, the amount of oxygen vacancies increases. More and more octahedrons convert from the corner-sharing structure to the face-sharing one, and the amount of the hexagonal phase increases. As a result, a single hexagonal phase can be obtained when the concentration of Co dopant is high enough, in present experiment, x ≥ 0.2. On the other hand, because of the volatilization of Co element during the high temperature treatment such as calcining and sintering, the Ba-rich region could form. As a result, the Ba2TiO4 phase as showed in Fig. 1 might occur in the ceramics with x > 0.2.
3.2 Electrical property
Figure 4 shows the plots of the temperature dependence of resisitivity, by ln(ρ/T 1.5) versus 1000/T, of the BaTi1−x Co x O3−δ ceramics with various Co concentrations after sintering at 1,300 °C. Where, ρ is the ceramic resistivity and T is the temperature in Kelvin degree. For the samples x = 0.01, 0.05 and 0.1, when the measurement temperature increases, the resistivities increase at the temperature lower than the related transition temperature ΘD/2, and then decrease at the temperatures higher than the ΘD/2. The ΘD is the Debye temperature [26]. It can be seen that ΘD decreases with cobalt-concentration rising (see in Table 1). Meanwhile, the ln(ρ/T 1.5) behaves linear dependence of 1000/T at T > ΘD/2. The nonlinear variation of ln(ρ/T 1.5) versus 1000/T plots at ΘD/2 often shows evidence of small polaron hopping conduction [27].
However, in the samples of x ≥ 0.2, the resistivities monotonously decrease with the temperature increase in all the test temperature range, meaning that these samples show the pure NTC effect. It is assumed that ΘD/2 in these samples is less than the measurement temperatures, 26 °C. The material constants B 50/120 of the ceramics showing pure NTC effect in the test temperature range can be expressed by:
where ρ1 and ρ2 are the resistivities at temperatures T 1 and T 2, respectively. So the B 50/120 constants of the samples with x = 0.2, 0.3 and 0.4 can be obtained to be 3,187, 2,968 and 2,648 K, respectively.
Charge transport in transition metal oxides usually occurs through the partially filled 3d orbitals. The 3d overlap is too small to form a metallic band, but it is sufficient for the charge carrier to migrate through the crystal by electron exchange. If the interaction between the lattice vibrations and the carrier is sufficiently strong, the carrier bandwidth can be small enough to form small polaron. At low temperatures, the polaron tunnels through the crystal in a narrow band, the bandwidth and mobility decrease with temperature rising until the mean free path becomes equal to the lattice spacing. The band model then breaks down and the polaron hops between ions, by either thermal activation over the barrier in a classical sense or phonon-assisted tunneling through the barrier. The former is dominant if the motion of the charge carrier and lattice are coupled adiabatically. Here the small polaron theory is proposed to discuss the conductivity mechanism in BaTi1−x Co x O3−δ ceramics.
For x < 0.2, one can see from Fig. 4 that the resistivities increase with temperature rising at the temperatures T < ΘD/2. This can be explained by the polaron conduction in a narrow-band [28, 29]. At low temperature, the polaron moves in Bloch-type bands, the width W of which is determined by the product of the electronic overlap integral J and a vibrational-overlap integral. Thus
where \(s = \gamma (2n + 1),\quad n = 1/\exp (\hbar \omega _{0} /2k_{{\text{B}}} T) \), γ is the coupling parameter, \( \hbar \) is the Planck constant, k B is the Boltzmann’s constant and ω 0 is the optical phonon frequency. Here, ω 0 is replaced by the Debye frequency ω D which stands for the maximum of vibration frequency. The values of ω D of the samples can be obtained as following relation and are presented in Table 1:
The bandwidth, which is determined by electronic overlap, tends to a limiting value of \( J \approx 3\hbar \omega _{0} \) at low temperatures (T < ΘD/2), and the electron–phonon (e–p) interaction can be regarded as a perturbation. Then the bandwidth decreases exponentially with temperature rising due to increasing vibrational overlap. As the bandwidth is reduced, the mobility of charge carrier is assumed thermal degenerate thus the resistivity increase. At higher temperature range (T > ΘD/2), non-diagonal transitions play a dominant role [28, 29], giving rise to a thermally activated hopping mobility. Thus the resistivity decreases exponentially with the rising temperature, which shows the NTC behavior.
The substitution of Co3+ at Ti4+ site leads to the formation of vacancies in the oxygen sublattice in order to maintain charge neutrality. Electrons release in this process may be captured by Ti4+ or Co3+. Since Ti4+ has the inert gas (Ar) configuration and has lower Sanderson electronegativity (1.50) than that of Co3+ (2.56) [30], the possibility of generation of Ti3+ is very small. Therefore, these materials are expected to contain Co2+ states. The NTC behavior at T > ΘD/2 occurs due to the thermal activated hopping of electrons between Co3+/Co2+ pairs:
The expression of conduction at T > ΘD/2 can be given as: for non-adiabatic hopping [31]
and for adiabatic hopping
where E a and E b is the activation energy, ρ0 and ρ1 is the pre-exponential factor, \( \hbar \) is the Planck constant, k B is the Boltzmann’s constant, n is the density of charge carrier, a is the jump distance.
From Eqs. 6 and 7, the resistivity increases exponentially with temperature rising in a wide temperature range. The pre-exponential factor that is proportional to either T (for adiabatic hopping) or T 1.5 (for non-adiabatic hopping) only acts a key role when k B T is approximate to E a or E b. The activation energy E a (or E b) can be expressed by [32]:
where W h is the hopping energy, W d is the disorder energy rising due to the energy difference of the neighboring sites and is significant at very low temperature (T < ΘD/4). At high temperature (T > ΘD/2), W d is small and the activation energy E a (or E b) is approximate to W h, that is E a ≈ W h. As shown in Table 1, E a decreases with cobalt-concentration rising. This can be explained by considering that the increasing of cobalt concentration causes stronger electron–electron (e–e) interaction determining the bandwidth between Co ions, and causes the less e–p interaction corresponding to hopping process. As a result, the energy required to liberate a free charge carrier is reduced. Thus the activation energy E a decreases with cobalt-concentration rising
It is suggested that a value of γ > 4 usually indicates strong e–p interaction, where γ is the coupling parameter and can be estimated from the relation [27]:
The large values of γ as presented in Table 1 indicate that the e–p interaction is dominant in those ceramics and is large enough to form polarons.
Emin and Holstein give the condition for non-adiabatic by [28]:
In order to give the overlap integral J, Holstein uses the relation:
and J < W h/3 is given for small polaron model [33]. The values of J max is presented in Table 1 when T/ΘD is selected a representative value of 3.
According to the equations from 8 to 11, one can get
as a condition for non-adiabatic (E a ≈ W h), Δ = 5.384 × 10−4ΘD. As shown in Table 1, all the samples accomplish the condition E a > Δ and J max < E a/3. So it can be concluded that the conduction mechanism in those samples follows the non-adiabatic small polaron hopping model for T > ΘD/2 and the narrow-band conduction model for T < ΘD/2. The ceramics showed excellent NTC effect at T > ΘD/2.
4 Conclusions
The phase transforms from a tetragonal one to a hexagonal one in the BaTi1−x Co x O3−δ ceramics with the Co dopant concentration rising, and a pure hexagonal phase formed when x = 0.2. With the Co concentration higher than x = 0.2, a small amount of the Ba2TiO4 impurity formed besides the hexagonal phase in the BaTi1−x Co x O3−δ ceramics. The resistivity-temperature characteristic of the BaTi1−x Co x O3−δ ceramics depends on the related Debye temperatures (ΘD). The resistivity increases with temperature rising at the temperature lower than ΘD/2, and decreases with temperature rising at the temperature higher than ΘD/2. The pure NTC effect exists in the ceramics of x ≥ 0.2 at the temperatures from 26 to 300 °C, and the material constants B 50/120 are 3,187, 2,968 and 2,648 for x = 0.2, 0.3 and 0.4, respectively. The narrow-band conduction and non-adiabatic hopping conduction are proposed for the conduction mechanisms at the temperature ranges T < ΘD/2 and T > ΘD/2, respectively.
References
G.D.C. Csete de Györgyfalva, I.M. Reaney, J. Eur. Ceram. Soc. 21, 2145–2148 (2001)
J.F. Gao, D.L. Fang, Z.B. Wang, P.H. Yang, C.S. Chen, Sens. Actuators A Phys. 135, 472–475 (2007)
W.M. Wang, X.C. Liu, F. Gao, C.S. Tian, Ceram. Int. 33, 459–462 (2007)
K. Park, S.J. Kim, J.-G. Kim, S. Nahm, J. Eur. Ceram. Soc. 21, 2009–2016 (2007)
K. Park, I.H. Han, Mater. Sci. Eng. B 119, 55–60 (2005)
K. Park, J.K. Lee, J.-G. Kim, S. Nahm, J. Alloy. Compd. 437, 211–214 (2007)
C.H. Zhao, B.Y. Wang, P.H. Yang, L. Winnubst, C.S. Chen, J. Eur. Ceram. Soc. 28, 35–40 (2008)
K. Park, J.K. Lee, S.-J. Kim, W.-S. Seo, J. Alloy. Compd. 467, 310–316 (2009)
D.L. Fang, C.S. Chen, A.J.A. Winnubst, J. Alloy. Compd. 454, 286–291 (2008)
A. Feltz, W. Polzl, J. Eur. Ceram. Soc. 20, 2353–2366 (2000)
A. Basu, A.W. Brinkman, T. Hashemi, Int. J. Inorg. Mater. 3, 1219–1221 (2001)
M.A.L. Nobre, S. Lanfredi, Appl. Phys. Lett. 82, 2284–2286 (2003)
Y. Luo, X.Y. Liu, G.H. Chen, J. Alloy. Compd. 429, 335–337 (2007)
K. Majid, S. Awasthi, M.L. Singla, Sens. Actuators A Phys. 135, 113–118 (2007)
F.D. Morrison, D.C. Sinclair, J.M. Skakle, A.R. West, J. Am. Ceram. Soc. 81, 1957–1960 (1999)
R.M. Glaister, H.F. Kay, Proc. Phys. Soc. 76, 763–771 (1960)
M. Wakamatsu, N. Takeuchi, G.C. Lai, I. Shingo, Yogyo Kyokaishi 95, 1181–1185 (1987)
H. Arend, L. Kihlborg, J. Am. Ceram. Soc. 52, 63–65 (1969)
G.M. Keith, M.J. Rampling, K. Sarma, N.M. Alford, D.C. Sinclair, J. Eur. Ceram. Soc. 24, 1721–1724 (2004)
H.T. Langhammer, T. Muller, R. Bottcher, V. Mueller, H.-P. Abicht, J. Eur. Ceram. Soc. 24, 1489–1492 (2004)
I.E. Grey, C. Li, L.M.D. Cranswick, R.S. Roth, T.A. Vanderah, J. Solid State Chem. 135, 312–321 (1998)
A. Jana, T.K. Kundu, Mater. Lett. 61, 1544–1548 (2007)
Z.C. Li, H. Zhang, B. Bergman, X.D. Zou, J. Eur. Ceram. Soc. 26, 2357–2364 (2006)
A. Feteira, G.M. Keith, M.J. Rampling, C.A. Kirk et al., Cryst. Eng. 5, 439–448 (2002)
T.A. Colson, M.J.S. Spencer, I. Yarovsky, Comput. Mater. Sci. 34, 157–165 (2005)
I.G. Austin, N.F. Mott, Adv. Phys. 18, 41–102 (1969)
A. Banerjee, S. Pal, E. Rozenberg, B.K. Chaudhuri, J. Phys. Condens. Matter. 13, 9489–9504 (2001)
D. Emin, T. Holstein, Ann. Phys. 53, 439–520 (1969)
I.G. Austein, A.J. Springthorpe, B.A. Smith, C.E. Turner, Proc. Phys. Soc. 90, 157–174 (1967)
R.T. Sanderson, Inorg. Chem. 25, 3518–3522 (1986)
M.H. Brodsky, Amorphous semiconductors (Springer, Berlin, NY, 1979)
N.F. Mott, E.A. Davis, Electronic processes in non-crystalline materials, 2nd edn. (Clarendon, Oxford, 1979)
T. Holstein, Ann. Phys. 8, 343–391 (1959)
Acknowledgement
The authors acknowledge the support of the National Nature Science Foundation of China (No. 50872155).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, J., Zhang, H., Li, Y. et al. Phase component and conductivities of Co-doped BaTiO3 thermistors. J Mater Sci: Mater Electron 21, 811–816 (2010). https://doi.org/10.1007/s10854-009-9997-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-009-9997-x