Abstract
Multi-layered TiO2 nanostructured films were fabricated to improve the light harvest efficiency of the dye-adsorbed TiO2 electrode in dye-sensitized solar cells (DSSCs) by light scattering. Three different structures of TiO2 electrodes, with layers consisting of TiO2 pastes with average diameters of 9, 20, and 300 nm, respectively, were fabricated and their photovoltaic effects on the DSSC devices were investigated. By utilizing the multi-layered TiO2 electrode constructed using the three different TiO2 pastes, the overall power conversion efficiency of the DSSC devices in the PEG-based electrolyte was increased to 5.24% under irradiation of 100 mW/cm2 at AM 1.5.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Dye-Sensitized Solar Cells (DSSCs) consisting of dye molecules, nanocrystalline metal oxides and organic liquid electrolytes have attractive features of high-energy conversion efficiency and low production cost [1, 2]. The main difference between DSSCs and organic solar cells is that the functional element in DSSCs, which is responsible for light absorption (the dye), is separated from the charge carrier transport. Such a feature makes it possible for DSSCs to improve the overall power conversion efficiency by the development of functional elements. Many research groups have focused on improving the photocurrent and photovoltage by developing new dye-sensitizers, suppressing the charge recombination, improving the interfacial interaction and/or modifying the electrolyte components [3]. Several studies on the improvement of the light harvest efficiency of dye-adsorbed TiO2 electrodes by light scattering have recently been reported [4–6]. Using a TiO2 layer with a higher surface area increases the dye adsorption, and such a higher surface area is usually obtained by using smaller particle sizes. The usual consequence is films which are relatively transparent, but which exhibit poor light scattering. Light scattering can be achieved by the presence of additional scattering layers in the TiO2 layer [7]. The addition of scattering layers consisting of large particles ensures adequate light trapping in the device [8]. The optical modeling described by Ferber et al. suggested the use of larger TiO2 particles having a radial dimension of 125–150 nm in a TiO2 matrix of particles with a diameter of 20 nm [9].
In this work, multi-layered TiO2 nanostructured films for DSSCs were fabricated to improve the light harvest efficiency of the dye-adsorbed TiO2 electrode by light scattering. Three kinds of TiO2 pastes were used for the electrode of the DSSC device, which were composed of TiO2 particles with average diameters of 9, 20, and 300 nm, respectively. The bottom 9 nm-TiO2 layer is dense and transparent, while the top 20 nm- or 300 nm-TiO2 layer can be a porous and opaque layer. Multi-layered TiO2 nanostructured films were prepared by layer-by-layer deposition and their effect on the photovoltaic performances of the DSSCs was examined.
2 Experimental details
2.1 Materials
TiO2 pastes, viz. Ti-Nanoxide HT/SP (particle size: 9 nm) and Ti-Nanoxide 300 (containing about 18% in weight of titanium oxide with a particle size of 300 nm), cis-bis(isothiocyanato)bis(2,2′-bipyridyl-4,4′-dicarboxylato)-ruthenium(II) dye (N3 dye), 1-propyl-3-methylimidazolium iodide (PMImI) as an ionic liquid, F-doped SnO2 glass (SnO2:F glass, 15 Ω/square) and Pt paste (Pt catalyst T/SP) were purchased from Solaronix SA. TiO2 powders (P-25, particle size: 20 nm) purchased from Degussa AG, Germany. Iodine (I2), propylene carbonate (PC), ethylene carbonate (EC), and tetrabutylammonium iodide (TBAI) were purchased from Aldrich Co. and used without purification. Polyethyleneglycol (PEG, Mw = 20,000) was purchased from Fluka Co.
2.2 Fabrication of the multi-layered TiO2 films
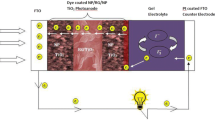
Three different kinds of TiO2 pastes were used for the electrode of the DSSC device, which were composed of TiO2 particles with average diameters of 9, 20, and 300 nm, respectively. The 9 nm-TiO2 pastes and 300 nm-TiO2 pastes were used as-received, and the 20 nm-TiO2 pastes were prepared as a reference [10]. As shown in Fig. 1, three types of TiO2 electrode were deposited onto SnO2:F glass by the layer-by-layer deposition of multi-layered TiO2 particles by repeated use of the doctor-blade method and subsequent calcination process at 500 °C. Structures A and B, consisting of two different layers, are regarded as a double-layer, whereas structure C consisting of three layers is referred to as a multi-layer.
2.3 Fabrication of DSSC devices
We prepared the DSSC devices, using N3 dye as a photosensitizer, sandwiched between the TiO2 electrode and Pt-coated electrode. The TiO2 electrodes used were of three different types, viz. structures A, B, and C. To evaluate the effect of light scattering, a single-layered TiO2 electrode without a light scattering layer was fabricated using only 9 nm-TiO2 paste. Before assembling the two electrodes, the polymer electrolyte was cast onto the TiO2 electrode, impregnated with N3 dye, and dried at 50 °C for 2 h in an oven to evaporate the solvent. The polymer electrolyte contains I2, TBAI, PMImI as an ionic liquid, EC/PC (EC: PC = 4:1 v/v), and PEG as a polymer matrix in acetonitrile.
2.4 Measurements
The single-, double-, and multi-layered TiO2 films were characterized by means of a Scanning Electron Microscope (SEM) to investigate their surface and thickness. The transmittance and absorbance of the TiO2 films were measured using a UV–Vis-NIR spectrophotometer (Varian, Cary 5000). The Haze (%) of the TiO2 films was measured using a hazemeter (BYK Gardner, model 4725) to investigate the light scattering. The photovoltaic characteristics of the DSSC devices were measured using a Solar Simulator (150 W simulator, PEC-L11/PECCELL) under simulated solar light with an ARC Lamp power supply (AM 1.5, 100 mW/cm2). The solar simulator was calibrated to a verified Si reference cell. The active area of the DSSC device measured using a black mask was 0.25 cm2.
3 Results and discussion
Figure 2 shows the SEM images of the double- and multi-layered TiO2 films with the scattering layers and single-layered TiO2 film without the scattering layers on the SnO2:F substrate. Small grains are observed within the dense 9 nm-TiO2 layer structure, and relatively large grains within the porous 20 nm- and 300 nm-TiO2 layer structures. The thicknesses of the TiO2 films measured by SEM were about 12 um (9 nm), 16 um (A type), 16 um (B type), and 21 um (C type). For the A and B type double-layered TiO2 films, the light scattering layers comprising 20 or 300 nm TiO2 pastes were observed to have a thickness of about 5 um, which is sufficient for the effect of light scattering to be observed in the double- or multi-layered TiO2 films.
The UV–Vis absorbance and transmittance spectra of the single-, double- and multi-layered TiO2 films are shown in Fig. 3. Figure 3a illustrates the absorbance spectra of the TiO2 films on the SnO2:F glass, and Fig. 3b illustrates that of the TiO2 films containing adsorbed N3 dye on the SnO2:F glass. The absorbance of the TiO2 films is remarkably increased by the introduction of the light scattering layers. The high absorbance of the multi-layered TiO2 films in the region of 650–750 nm is apparent in the absorbance spectra, because of the light scattering by the additional scattering layers comprising 20 nm- and 300 nm-TiO2 layers.
Absorbance and transmittance spectra of the various TiO2 film such as structure A, B and C; (a) absorbance spectra of the TiO2 films on the SnO2:F glass, (b) absorbance spectra of the TiO2 films adsorbed N3 dyes on the SnO2:F glass, and (c) transmittance spectra of the TiO2 films on the SnO2:F glass, (d) transmittance spectra of the TiO2 films adsorbed N3 dyes on the SnO2:F glass
Figure 3c and d show the transmittances of the double- and multi-layered TiO2 films with the light scattering layers and the single-TiO2 film without the light scattering layers. The transmittance of the films is decreased by the addition of the light scattering layers. Particularly, the B and C type films comprising 300 nm-TiO2 layers showed a transmittance of nearly 0% in the visible and near-IR region. In all representations, the light scattering layers comprising larger TiO2 particles, such as those with a diameter of 300 nm, appear to be promising candidates for light collection in the device.
In addition, in order to investigate the effect of the light scattering layer, the haze (%) of the TiO2 films on the SnO2:F glass after the calcination process was measured by the hazemeter and the results are summarized in Table 1. Haze is the cloudiness of a material that is caused by the scattering of light [11–13]. This is an important appearance attribute, which can be quantified and then used to assess the quality of objects such as liquids, glass, plastics, painted panels, and even metals. Therefore, measurement using a hazemeter can be a useful method of estimating the light scattering of TiO2 electrodes in DSSCs. In this work, the haze of the TiO2 electrodes was defined as the ratio of diffused light to the total light transmitted through the electrodes. With the addition of the scattering layers, the haze of the TiO2 films increased enormously from 46.5% to 100%. Hence J sc can be improved by using a multi-layered TiO2 electrode with a large haze value, due to the increase in the optical path length in the cells. The optical path length is increased by the addition of relatively large particles to small particles to induce the light scattering of the TiO2 electrodes.
The I–V curves of the DSSC devices constructed with the various TiO2 electrodes and PEG-based electrolytes under AM 1.5 illumination are shown in Fig. 4 and their photovoltaic characteristics are summarized in Table 1. With the addition of the scattering layers to the multi-layered TiO2 films, the photocurrents of the DSSC devices were increased by more than 45%, compared to those of the devices without the scattering layers. Remarkably high power conversion efficiencies of the DSSC devices of 5.24% were observed, despite the application to the polymer electrolytes. This result was mainly attributed to the scattering properties of the multi-layered TiO2 films capturing light inside the device. Furthermore, the larger pore diameter and volume of the TiO2 film surface resulting from the use of large particles, such as those with a diameter of 300 nm, allows the polymer electrolyte to permeate into the pores and it may be helpful in improving the J sc value and efficiency in DSSC devices using polymer electrolytes.
4 Conclusion
In conclusion, we investigated the improvement of the DSSC performance afforded by using a PEG-based electrolyte and multi-layered TiO2 electrodes with high haze caused by light scattering. The use of the light scattering layers resulted in an increase in both the J sc value and fill factor, thus increasing the overall power conversion efficiency of the DSSC devices by 64%.
References
B. O’regen, M. Grätzel, Nature 353, 737 (1991)
M.K. Nazeeruddin, A. Kay, I. Rodicio, R. Humphry-Baker, E. Muller, P. Liska, N. Valchopoulos, M. Grätzel, J. Am. Chem. Soc. 115, 6382 (1993)
F.C. Krebs, M. Biancardo, Sol. Energ. Mat. Sol. Cells 90, 142 (2006)
S. Hore, P. Nitz, C. Vetter, C. Prahl, M. Niggemann, R. Kern, Chem. Commun. 15, 2011 (2005)
Z.S. Wang, H. Kawauchi, T. Kashima, H. Arakawa, Coordinat. Chem. Rev. 248, 1381 (2004)
G. Rothenberger, P. Comte, M. Grätzel, Sol. Energ. Mat. Sol. Cells 58, 321 (1999)
J.M. Kroon, N.J. Baker, H.J.P. Smit, P. Liska, K.R. Thampi, M. Grätzel, A. Hinsch, S. Hore, J.R. Durrant, E. Palomares, H. Pettersson, T. Gruszecki, J. Walter, K. Skupien, G. Tulloch, in Proceedings of the 19th European Photovoltaic Solar Energy Conference, Paris, 2004
S. Hore, C. Vetter, R. Kern, H. Smit, A. Hinsch, Sol. Energ. Mat. Sol. Cells 90, 1176 (2006)
J. Ferber, J. Luther, Sol. Energ. Mat. Sol. Cells 54, 265 (1998)
I. Zumeta, R. Espinosa, J.A. Ayllon, X. Domenech, R. Rodriguez-Clemente, E. Vigil, Sol. Energ. Mat. Sol. Cells 76, 15 (2003)
A. Usami, Sol. Energ. Mat. Sol. Cells 64, 73 (2000)
P. Wang, S.M. Zakkeruddin, P. Comte, R. Charvet, R. H-Baker, M. Grätzel, J. Phys. Chem. B 107, 14336 (2003)
N. Koide, A. Islam, Y. Chiba, L. Han, J. Photochem. Photobiol. A 182, 296 (2006)
Acknowledgment
This work was supported by the Ministry of Information & Communications, Korea, under the Information Technology Research Center (ITRC) Support Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, JK., Jeong, BH., Jang, SI. et al. Multi-layered TiO2 nanostructured films for dye-sensitized solar cells. J Mater Sci: Mater Electron 20 (Suppl 1), 446–450 (2009). https://doi.org/10.1007/s10854-008-9665-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-008-9665-6