Abstract
ZnO thin films were grown by the pulse laser deposition (PLD) method using Si (100) substrates at various substrate temperatures. The influence of the substrate temperature on the structural, optical, and electrical properties of the ZnO thin films was investigated. All of the thin films showed c-axis growth perpendicular to the substrate surface. At a substrate temperature of 500 °C, the ZnO thin film showed the highest (002) peak with a full width at half maximum (FWHM) of 0.39°. The X-ray Photoelectron Spectroscopy (XPS) study showed that Zn was in excess irrespective of the substrate temperature and that the thin film had a nearly stoichiometrical composition at a substrate temperature of 500 °C. The photoluminescence (PL) investigation showed that the narrowest UV FWHM of 15.8 nm and the largest ratio of the UV peak to the deep-level peak of 32.9 were observed at 500 °C. Hall effect measurement systems provided information about the carrier concentration, mobility and resistivity. At a substrate temperature of 500 °C, the Hall mobility was the value of 37.4 cm2/Vs with carrier concentration of 1.36 × 1018 cm−3 and resistivity of 2.08 × 10−1 Ω cm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Recently, there has been much interest in the study of new materials applicable to communication devices and optical components due to the rapid development of the information and communication technologies and the display industry. Especially, in the case of optical components, there have been many research activities regarding the development of new materials designed to replace GaN, which has been widely studied. Among these materials, ZnO has similar structural and optical properties to those of GaN and has thus gained the attention of researchers, because of its potential to be the next generation material for light emitting components. ZnO is a II–VI group based direct-transition-type compound semiconductor with a wide energy band gap of 3.3 eV and a hexagonal wurtzite structure [1]. ZnO has a high transmittance in the visible range and a large exciton binding energy of 60 meV at room temperature, as compared with 24 meV for GaN and 22 meV for ZnSe, making it a stable and highly efficient energy emitter [2]. Recently, there has been much interest in the application of ZnO to short-wavelength optical devices, such as blue light emitting diodes (LEDs) and laser diodes (LDs) and to transparent conductive films for display devices [3–5].
In order to apply ZnO thin films to optical components, it is important to grow high-quality films with good crystallinity and a low defect density [6]. To grow high-quality ZnO films, various deposition processes have been used, including the sol–gel [7], rf-sputtering [8], chemical vapor deposition (CVD) [9], pulsed laser deposition (PLD) [10], and molecular beam epitaxy (MBE) [11] methods. Among these different techniques, PLD is the most promising method for growing oxides such as high-quality ZnO, because of its being a high speed deposition process under a high partial pressure of oxygen [10]. The laser energy, target-substrate distance, substrate temperature and partial pressure of oxygen are the most important deposition factors that affect the quality of the ZnO thin films grown by PLD. Especially, the substrate temperature plays an important role in controlling the properties of the thin films by affecting their crystallinity and composition.
Presently, the deposition of ZnO thin films is performed on sapphire substrates, despite the large lattice mismatch (18.4%) between the ZnO thin film and sapphire substrate. However, sapphire substrates are expensive, hard to cut, and difficult to make integration of devices. On the other hand, Si substrates can overcome the weak points of sapphire substrates. Therefore, many studies should be performed using Si substrates to obtain ZnO thin films with high quality.
In this study, we systematically study the influence of the substrate temperature on the structural, optical, and electrical properties of ZnO thin films grown on Si (100) substrates by the PLD method at various substrate temperatures.
2 Experiments
ZnO thin films were grown by the PLD method using Si (100) substrates. Any impurity contamination on the surface of the substrates can adversely affect the deposition process. Thus, the substrates were cleaned and rinsed using acetone (15 min), alcohol (15 min), distilled water (10 min) and distilled water (10 min) in an ultrasonic cleaner, and dried using nitrogen gas prior to deposition.
In the PLD system used in our study, the initial vacuum pressure of the chamber was measured to be below 5 × 10−6 Torr. The target was ZnO with a diameter of one inch and purity of 99.99%. Oxygen (99.999%) was used as the atmospheric gas and its partial pressure was fixed at 250 mTorr. The distance between the target and substrate was maintained at 60 mm. A KrF excimer laser (Lambda Physics, Compex 205) with a wavelength of 248 nm was used as the laser source and ablated the target with an energy density of 2 J/cm2 and period of 5 Hz. The target was revolved to enable the laser to ablate a new target surface. In this experiment, we study the substrate temperature dependence of the properties of the ZnO thin film using various substrate temperatures (100, 300, 500 and 700 °C).
To analyze the structural properties of the ZnO thin films, X-ray diffraction (XRD, Philips, PW 3020) was used for the investigation of the crystallization and preferred orientation. X-ray photoelectron spectroscopy (XPS, VG, ESCALAB 220) measurements were performed to obtain the composition ratio of the ZnO thin films. The surface morphologies of the ZnO thin films were studied using atomic force microscopy (AFM, Seiko, SPA 400). The optical properties of the ZnO thin films were characterized by performing photoluminescence measurements (PL, SPEX, SPEX 1403) with an He–Cd laser (λ = 325 nm) as a light source at room temperature. Hall-effect measurements (Bio-Rad, HL 5500 PC) were performed using the van der Pauw method to investigate the electrical properties of the ZnO thin films.
3 Results and discussion
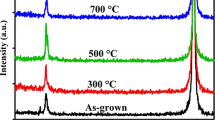
Figure 1 shows the XRD patterns of the ZnO films deposited at various substrate temperatures. As shown in Fig. 1, only the (002) diffraction peaks were observed. This means that the ZnO films showed a c-axis preferred orientation perpendicular to the substrate surface. This can be explained by the fact that in the case of ZnO films having a wurtzite hexagonal crystal structure, the (002) plane has the lowest surface energy density [12]. The ZnO thin film deposited at 100 °C showed a relatively low (002) diffraction peak. This is due to the low atomic mobility, which constrains the growth of the crystal during the crystallization process. As the substrate temperature increases up to 500 °C, enough thermal energy is supplied to the adatoms on the substrate and increases the surface mobility leading to an increase in the (002) plane orientation. However, there is a tendency for the intensity of the (002) diffraction peak to decrease for the thin film deposited at 700 °C. This is due to the degradation in the quality of the thin film resulting from the dissociation and desorption of adatoms at high temperature [13].
On the other hand, as the substrate temperature increases, the position of the (002) diffraction peak moves to a higher angle from 34.37 to 34.47° and the c-axis lattice constant, calculated from the XRD peak position for the ZnO thin films, decreases from 5.214 to 5.199Å. This indicates that the lattice constant approaches that of bulk ZnO (5.206 Å) with increasing substrate temperature up to 500 °C, however the lattice constant of the ZnO thin film grown at 700 °C deviates from that of bulk ZnO [14]. This is due to the difference in the thermal extension coefficients between the thin film (7 × 10−6/°C) and Si substrate (4.2 × 10−6/°C), and lattice imperfection [15, 16].
To investigate the effect of the substrate temperature on the stress of the ZnO thin films, we calculated the stress of the ZnO thin films using Eq. 1. The estimated data is presented in Table 1 [17].
where c 0 (5.206 Å) is the c-axis lattice constant of bulk ZnO and c is the c-axis lattice constant calculated from the XRD peak position. As shown in Table 1, the stress in the ZnO thin films grown at a relatively low temperature is mainly tensile stress. However, at 500 °C, we can confirm that the stress is changed to compressive stress as compared to the value of the bulk ZnO. This result indicates that the substrate temperature strongly affects the stress of the ZnO thin films.
Figure 2 shows (a) the FWHM and (b) the grain size of the ZnO thin films calculated from the XRD (002) peak. The value of the FWHM reveals the crystallinity of the ZnO thin films. As the substrate temperature is increased from 100 to 500 °C, the FWHM value is decreased from 0.5 to 0.39°, but increases again at 700 °C. The grain size is calculated using the Scherrer equation.
where λ, θ and β are the X-ray wavelength, the Bragg diffraction angle, and the FWHM of the (002) diffraction peak, respectively. The grain size is increased from 16.5 to 21.2 nm with increasing substrate temperature up to 500 °C. At 700 °C, the grain size is decreased to 17.5 nm. Thus, we find that the film deposited at 500 °C has the best crystallization result.
Figure 3 shows the X-ray photoelectron spectroscopy result of the O 1s spectra of the ZnO thin films deposited at various substrate temperatures. Only a slight shift of the O 1s binding energy was observed with increasing substrate temperature. However, as the substrate temperature is increased from 100 to 500 °C, a shift of the O 1s binding energy in the direction of the higher energy side was observed. This means that the number of oxygen atoms in the ZnO thin film has been increased. We suggest that the increase in the number of oxygen atoms will reduce the number of oxygen vacancies in the ZnO thin films, indicating an improvement of the stoichiometrical composition.
Figure 4 shows the X-ray photoelectron spectroscopy result of the Zn 2p Spectra of the ZnO thin films deposited at various substrate temperatures. The spectra shows two peaks for zinc corresponding to Zn 2p3/2 and Zn 2p1/2. The Zn 2p peak was sharp, demonstrating that only Zn2+ was present [18]. In the case of the thin films deposited at 100 °C, the Zn 2p3/2 peak was observed at 1022 eV. The binding energy of Zn 2p3/2 corresponds the bond of Zn and O in ZnO [19]. However, in the case of the thin films deposited at 500 °C, a shift of the Zn 2p3/2 peak in the direction of the higher energy side was observed. This indicates that more zinc and oxygen atoms bind together. We conclude that as the substrate temperature is increased from 100 to 500 °C, the binding ratio of the Zn atoms and O atoms is increased, corresponding to the formation of new ZnO molecules. Therefore, from the results of the O1s spectra and Zn2p spectra, it can be inferred that the ZnO thin film deposited at 500 °C has a composition closer to the stoichiometrical composition. However, in the case of the thin film deposited at 700 °C, the binding energy of the zinc shifted to the lower energy side, which may be due to the fact that the rapid dissociation of the thin films at high temperature would increase the number of oxygen vacancies and result in the reduction in the ratio of O–Zn.
Figure 5 shows the AFM images of the surface morphologies of the ZnO thin films deposited at various substrate temperatures. All of the thin films show nanometer-sized grains and a dense microstructure. In the case of the thin films deposited at 100 °C, a mixture of small grains and large grains was observed. As the substrate temperature is increased up to 500 °C, the surface morphology is observed to consist of larger and more uniform grains. On the other hand, the surface roughness is increased from 6.4 to 8.1 nm with increasing substrate temperature up to 500 °C. This increase of the surface roughness may be due to the increase of the grain size. At 700 °C, a decrease of the grain size and surface roughness (7.5 nm) is observed.
Figure 6 shows the PL spectra of the ZnO thin films deposited at various substrate temperatures. The ZnO emission is generally classified into two categories. One is the UV emission of the near band edge in the UV region related to free-exciton recombination and the other is the deep-level (DL) emission in the visible range. Especially, the deep-level emission in the ZnO thin films has been attributed to structural defects such as oxygen vacancies and interstitial zinc. Studeninkin’s research reported the deep-level emission of the ZnO thin films in the UV (380 nm), green (510 nm) and yellow–orange (640 nm) ranges [20].
In this work, all of the thin films show a strong UV emission peak situated at 382 nm and a relatively weak deep-level emission peak centered around 520 nm. It was observed that as the substrate temperature is increased, the intensity of the UV emission peak is increased. At 500 °C, the largest UV emission peak and the smallest deep-level peak were observed. It was reasoned that when enough thermal energy is supplied, more oxygen atoms would adsorb to the thin films, thereby reducing the number of oxygen vacancies induced by the structural defects of the thin films and thus improving the quality of the thin films. Therefore, it can be said that the use of a sufficient substrate temperature reduces the number of defects in the thin films and thus allows the growth of high quality thin films close to the stoichiometrical composition. However, in the case of the thin films deposited at 700 °C, it was observed that the UV emission peak was decreased and the deep-level peak was increased. It was reasoned that the rapid dissociation of the thin films at high temperature would deteriorate their quality and thus their emission properties. Meanwhile, the central peak of the deep-level emission was observed at 521 nm (2.38 eV) in the green region. This is consistent with Lin’s research which suggested that the green emission is related to the electron transitions from the bottom of the conduction band to the antisite oxygen defect level (2.38 eV) [21].
Figure 7 shows the FWHM of the UV emission peak and the calculation results for the ratio of the UV emission peak to the deep-level emission peak, which was obtained in order to easily infer the internal detects of the thin films at the various substrate temperatures. It was observed that as the substrate temperature increases from 100 to 500 °C, the FWHM of the UV emission peak decreases and the ratio of the UV emission peak to the deep-level emission peak increases. However, it was found that at 700 °C, the FWHM of the UV emission peak is increased and the ratio of the UV emission peak to the deep-level emission peak is decreased. It was observed that the thin films deposited at 500 °C have the narrowest FWHM of the UV emission peak (15.8 nm), the largest ratio of the UV emission peak to the deep-level emission peak (32.9) and, thus, the most desirable emission properties.
Figure 8 shows the Hall-effect measurements using the van der Pauw method were performed in order to investigate the electrical properties of the ZnO thin films. Generally, ZnO contains defects such as interstitial zinc atoms and oxygen vacancies. These defects can be easily ionized and the electrons induced by this process can contribute to the conduction of electricity, causing ZnO to act as an n-type semiconductor [22].
As shown in Fig. 8, We increase the substrate temperature from 100 to 500 °C, the carrier concentration decreases from 4.25 × 1018 to 1.36 × 1018 cm−3 while the resistivity increases from 1.23 × 10−1 to 2.08 × 10−1 Ω cm. It is reasoned that when the substrate temperature is increased, the number of oxygen atoms adsorbed on the thin film will be increased, causing a decrease of the defect density and resulting in a decrease of the carrier concentration. These results imply that as the substrate temperature is increased from 100 to 500 °C, the thin films come closer to the stoichiometrical composition, as observed in the PL and XPS measurements. The Hall mobility is increased from 17.5 to 37.4 cm2/Vs as the substrate temperature is increased from 100 to 500 °C and is decreased to 16.8 cm2/Vs at 700 °C. It is reasoned that Hall mobility is more affected by the quality enhancement of the thin film than by the change in its resistance. At a substrate temperature of 500 °C, the Hall mobility was a maximum of 37.4 cm2/Vs with a carrier concentration of 1.36 × 1018 cm−3 and resistivity of 2.08 × 10−1 Ω cm.
Meanwhile, in the case of the thin film deposited at 700 °C, it is reasoned that the volatilization of the oxygen atoms of the thin films at high temperature would increase the number of oxygen vacancies, which results in an increase in the carrier concentration and a decrease in the resistance.
4 Conclusion
In this study, the influence of the substrate temperature on the structural, optical, and electrical properties of ZnO thin films grown by the PLD method using Si (100) substrates was investigated. At a substrate temperature of 500 °C, the ZnO thin film was the highest (002) peak with an FWHM of 0.39°. The composition analysis result using XPS showed that the ZnO thin films came closer to the stoichiometrical composition as the substrate temperature was increased to 500 °C. The PL result showed that a strong UV emission peak and a deep level emission center were observed in the green region. At 500 °C, the narrowest UV FWHM (15.8 nm) and the largest ratio of the UV peak to the deep-level peak (32.9) were observed. The electrical properties of the ZnO thin films were investigated by measuring the Hall-effect using the van der Pauw method. As the substrate temperature is increased from 100 to 500 °C, the carrier concentration is decreased from 4.25 × 1018 to 1.36 × 1018 cm−3 while the resistivity is increased from 1.23 × 10−1 to 2.08 × 10−1 Ω cm. It is reasoned that an increase of the substrate temperature would cause a decrease of the defect density, thus resulting in a decrease of the carrier concentration and an increase of the resistivity. The Hall mobility showed the largest value of 37.4 cm2/Vs at 500 °C. It is reasoned that that Hall mobility is more affected by the quality enhancement of the thin film than by the change in its resistance.
Through this study, it was confirmed that ZnO thin films having highly desirable optical and electrical properties can be obtained and that ZnO thin films are promising materials for next generation optical component applications.
References
V. Srikant, D.R. Clarke, J. Appl. Phys. 83, 5447–5451 (1998)
A.S. Yamamoto, K.S. Miyajima, T. Goto, J. Appl. Phys. 90, 4973–4976 (2001)
T. Soki, Y. Hatanaka, D.C. Look, Appl. Phys. Lett. 76, 3257–3258 (2000)
X.W. Sun, H.S. Kwok, J. Appl. Phys. 86, 408–411 (1999)
T.L. Yang, D.H. Zhang, J. Ma, H.L. Ma, Y. Chen, Thin Solid Films 326, 60–62 (1998)
J. Zhao, L. Hu, Z. Wang, Z. Wang, H. Zhang, Y. Zhao, X. Liang, J. Cryst. Growth 280, 455–461 (2005)
J.H. Lee, B.O. Park, Thin Solid Films 426, 94–99 (2003)
M. Jung, J. Lee, S. Park, H. Kim, J. Chang, J. Cryst. Growth 283, 384–389 (2005)
K. Haga, T. Suzuki, Y. Kashiwaba, H. Watanabe, H.P. Zhang, Y. Segawa, Thin Solid Films 433, 131–134 (2005)
J.L. Zhao, X.M. Li, J.M. Bian, W.D. Yu, X.D. Gao, J. Cryst. Growth 276, 507–512 (2005)
P. Zu, Z.K. Tang, G.K.L. Wong, M. Kawasaki, A. Ohtomo, H. Koinuma, Y. Segawa, Solid State Commun. 103, 459–463 (1997)
N. Fujimura, T. Nishihara, S. Goto, J. Xu, T. Ito, J. Cryst. Growth 130, 269–279 (1993)
M. Liu, X.Q. Wei, Z.G. Zhang, G. Sun, C.S. Chen, C.S. Xue, H.Z. Zhuang, B.Y. Man, Appl. Surf. Sci. 252, 4321–4326 (2006)
JCPDS Card No. 74-0534
S.L. King, J.G.E. Gardeniers, I.W. Boyd, Appl. Surf. Sci. 96–98, 811–818 (1996)
Z.B. Fang, Z.I. Yan, Y.S. Tan, X.Q. Liu, Y.Y. Wang, Appl. Surf. Sci. 241, 303–308 (2005)
M.K. Puchert, P.Y. Timbrell, R.N. Lamb, J. Vac. Sci. Technol. A 14, 2220–2230 (1996)
L. Jing, Z. Xu, J. Shang, X. Sun, W. Cai, H. Guo, Mater. Sci. Eng. A 332, 356–361 (2002)
Y.S. Kim, W.P. Tai, S.J. Shu, Thin Solid Films 491, 153–160 (2005)
S.A. Studeninkin, N. Golego, M. Cocivera, J. Appl. Phys. 84, 2287–2294 (1998)
B. Lin, Z. Fu, Y. Jia, Appl. Phys. Lett. 79, 943–945 (2001)
H.S. Kang, J.S. Kang, J.W. Kim, S.Y. Lee, J. Appl. Phys. 95, 1246–1250 (2004)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, S.J., Joung, Y.H., Shin, H.H. et al. Effect of substrate temperature on structural, optical and electrical properties of ZnO thin films deposited by pulsed laser deposition. J Mater Sci: Mater Electron 19, 1073–1078 (2008). https://doi.org/10.1007/s10854-007-9469-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-007-9469-0