Abstract
CoLa0.1Fe1.9O4 ferrite nanocrystal was synthesized under an induced AC magnetic field by the emulsion method. From XRD pattern, it is shown that all the samples are cubic structure of the spinel ferrite. The morphology of samples synthesized under an induced AC magnetic field was flake-like in shape from TEM image. Both the strength and acting time of external magnetic field influence the crystallite sizes of samples. Magnetic properties of samples were measured by vibrating samples magnetometer (VSM). The changing morphology of sample when an external magnetic field was applied, which might be responsible for the low magnetic properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Development of morphology-controlled synthesis methodologies is of great interest in materials science [1–3]. Ferrites are important materials, which are broadly used in magnetic fields, including ferrofluid technology [4], contrast enhancement of magnetic resonance imaging [5], magnetically guided site-specific drug delivery [6] and data storage [7]. Among ferrites, spinel ferrites are the most important materials and have been widely applied [8]. In recent years, fabrication and characterization of low-dimensional nanostructures have attracted considerable attention, not only because they have many special potential applications [9, 10], but also because they contribute to the functional understanding of physical and chemical properties of materials. Spinel ferrites nanoparticles provide opportunities to understand magnetic properties at the atom level without the interference from complicated domain wall movement, especially for studying the size-dependent magnetic properties. Up to now, a number of methods are used to prepare spinel nano-ferrites. These methods can usually be used to produce spherical or irregular-shape nanoparticles. In this paper, flake-like CoLa0.1Fe1.9O4 nanoparticles were synthesized under an induced AC magnetic field by emulsion method.
2 Experimental
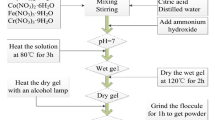
Nanocrystalline CoLa0.1Fe1.9O4 ferrites were prepared by the emulsion method. The chemical reagents for this experiment are all analytical grade of Co(NO3)2 6H2O, Fe(NO3)3 9H2O, La2O3 and polyethylene glycol-20000. For sample preparation, raw materials were first of all made to weigh according to prescribed compound radios, and then mixed solutions were prepared with distilled water. Secondly, while agitating well, ammonia solution (2 mol/L) was added to these mixed solutions, adjusting the pH, and the solutions were co-precipitated in a range of pH 9.0–9.5. The suspension was placed into the external magnetic field immediately and aged at 100 °C for different time, then centrifuged, washed with distilled water for three times and then dried to prepare precursors. The precursors were calcined at 600 °C at air atmosphere for 2 h.
The structure and the crystallite sizes were tested by X-ray diffractometer in the 2θ range 25–65° using CuKα radiation (λ = 0.15,405 nm). The type of X-ray diffractometer is SHIMADZU Co. Tokyo Japan. The crystallite sizes are calculated using Scherrer’s relationship D = kλ/Bcosθ, where ‘D’ is the average diameter in nm, ‘k’ is the shape factor, ‘B’ is the broadening of the diffraction line measured half of its maximum intensity in ‘radians’, ‘λ’ is the wave length of X-ray and ‘θ’ is the Bragg’s diffraction angle. The crystallite sizes of the samples are estimated from the line width of the (311) XRD peaks.
The morphology and the particle sizes of the samples were observed from the Hitactli H-800 TEM. For this measurement, the sample was deposited on copper grids and the microscope was operated at an accelerating potential of 175 KV.
Magnetic measurements are carried out at room temperature using a vibrating samples magnetometer (VSM) (Digital Measurement System JDM-13) with a maximum magnetic field of 10000Oe.
3 Results and discussion
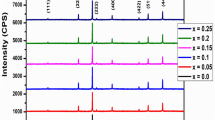
As shown in Fig. 1, the sample formed under a magnetic field is cubic structure of spinel ferrite without any impurity phases. Moreover, from the broadening of diffraction peaks of XRD, it is indicated there is the nature of small crystallite sizes for the sample.
Flake-like nanoparticles are observed in the sample prepared when a 0.125T magnetic was applied (Fig. 2). A magnetic field applied, CoLa0.1Fe1.9O4 flake-like nanoparticles (13 nm) are hexagonal in shape.
From Table 1, it is shown that the crystallite sizes of samples are increased with the intensity of external magnetic field. This may be due to the magnetic effects. The Lorentz force increases with the intensity of external magnetic field. It seems that Lorentz force plays a role at the level of individual ions in the aqueous solution, inducing the preferential nucleation and growth of CoLa0.1Fe1.9O4 nanoparticles.
Under the same intensity of external magnetic field, the crystallite sizes are decreased with the increase of acting time (Table 2). It is in favor of uniform nucleation to prolong the acting time properly. That is to say uniform nucleation conduces to the decrease of crystallite sizes.
From Figs. 3 and 4, the magnetic parameters of the samples are listed in Tables 3 and 4, respectively. Interestingly, the magnetic properties of samples are changed drastically when an external magnetic field was applied. From Tables 3 and 4, we can observe that all magnetic parameters are decreased with both the increasing intensity and acting time of external magnetic field. The increase of strength and acting time of external magnetic field leads to the change of morphology. Shape anisotropy are enhanced, simultaneously macroscopically magnetic properties are influenced. The magnetic domain in the flake-like nanoparticles may be oriented parallel, perpendicular, and even antiparallel to the measured magnetic field. The antiparallel magnetic domain, for example, must overcome much higher energy barriers to align in the direction relative to the measured magnetic field. In addition, the high shape anisotropy of the flake-like naoparticles, preventing them magnetizing in directions other than along their easy magnetic axes, might be a major reason. With a random orientation of flake-like nanoparticles, the projection of the magnetization vectors along the field direction will be lower than that for a collection of nanoparticles without the large shape anisotropy effect. All of above mention lead to the decrease of saturation magnetization.
Hi = He − Hd, Hi is the true magnetic field for magnetizing materials, He is the applying magnetic field for magnetizing materials, Hd is receding magnetic field. The energy of receding magnetic field is the energy of shape anisotropy in nature. The flake-like shape makes the increase of shape anisotropy, so the energy of receding magnetic field is increased. According to the above equation, the increase of Hd leads to the decrease of Hi. So the change of morphology might be responsible for the low coercivity.
4 Conclusions
With flake-like shape, nanocrystalline CoLa0.1Fe1.9O4 ferrite are synthesized under an induced AC magnetic field by the emulsion method. From XRD pattern, it is shown that all the samples synthesized under external magnetic field are still cubic structure of spinel ferrite. The crystallite size of sample is increased with the increasing intensity of induced AC magnetic field, but decreased with the increase of acing time. The magnetic properties are decreased with both the intensity of external magnetic field and acting time.
References
J.Wang, C. Zeng, J. Cryst. Growth 270, 729 (2004)
D.E. Zhang, X.J. Zhang, X.M. Ni, H.g. Zheng, D.D. Yang, J. Magn. Magn. Mater. 292, 79 (2005)
G.B. Ji, S.L. Tang, S.K. Ren, F.M. Zhang, B.X. Gu, Y.W. Du, J. Cryst. Growth 270, 156 (2004)
(a) C. Liu, B.S. Zou, A.J. Rondinone, Z.J. Zhang, J. Phys. Chem. B 104, 1141 (2000); (b) A. Cabanas, M. Poliakoff, J. Mater. Chem. 11, 1408 (2001)
M. Sugimoto, J. Am. Ceram. Soc. 82, 269 (1999)
U. Hafeli, W. Schutt, J. Teller, M. Zborowski (eds.), Scientific and Clinical Applications of Magnetic Carriers (Plenum Press, New York, 1997)
S. Sun, C.B. Murray, D. Weller, L. Folks, A. Moser, Science 287, 1989 (2000)
A.K.M. Akther Hossain, M. Seki, T. Kawai, H. Tabata, J. Appl. Phys. 96(2), 1273 (2004)
E. Rezlescu, L. Sachelarie, P.D. Popa, N. Rezlescu, IEEE Trans. Magn. 36(6), 3962 (2000)
S.M. Hoque, Md.A. Choudhury, Md.F. Islam, J. Magn. Magn. Mater. 251, 292 (2002)
Acknowledgement
This work is supported by National Natural Science Foundation of China (NSFC) (Grant No. 50572033).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, L., Yang, H. & Lu, L. The magnetic properties of nanocrystalline CoLa0.1Fe1.9O4 ferrite under an external AC magnetic field. J Mater Sci: Mater Electron 19, 992–995 (2008). https://doi.org/10.1007/s10854-007-9436-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-007-9436-9