Abstract
Presence of the hydroxyls in the lattice is believed to be the major cause of the reduced tetragonality in the barium titanate ceramic powder. Commercial barium titanate that is known to be cubic in nature has been used in this study. This sub-micron powder is treated with N-Methyl-2-Pyrrolidinon (NMP) to obtain a tetragonal powder as confirmed by X-ray diffraction analysis, differential scanning calorimetry and the c/a ratio. The dielectric constant of a single particle of this NMP treated cubic powder is reported to be around 64% higher than the as-received cubic powder. To add weight to the hypothesis mentioned hitherto, simulation experiments have been performed by aging in acidic water, with a pH ∼ 3–4 and in basic water, with a pH ∼ 12–13. The as-received cubic barium titanate powder, calcined at different temperatures, has been aged in different pH conditions, acid and basic waters. Then the powder is further used for the characterization of electrical properties. The dielectric properties of the barium titante ceramic powder that is determined does depend inversely on the lattice OH content as confirmed by FT-IR spectroscopic analysis and TGA results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The miniaturization of the electronic devices and electronic components has received phenomenal interest in the prospects of future technology [1–5]. The perovskite type structured barium titanate which is a metal oxide exhibits outstanding chemical and physical properties, such as catalysis, oxygen-transport, ferroelectric, piezoelectric and dielectric behavior [6–8]. High values of the dielectric constant and low loss factor of barium titanate make it a particularly desired material from which capacitors, condensers, resistors, insulators and other electronic components can be fabricated [9–15]. The high value of dielectric constant of barium titanate particle arises due to the high polarizability of its relatively simple lattice structure in which small Ti+4 ions have relatively more space within the oxygen octahedra [16]. It was well known that the stability of the unit cell strongly depends on the size of the crystals and the Curie temperature, 130 °C. Below the curie temperature, the Ti+4 ions occupy off-center positions, which results in the change of crystal structure from cubic to tetragonal [17–19]. This displacement is the origin of the room-temperature ferroelectricity and piezoelectricity of barium titanate and other perovskite oxides. The values of the dielectric constant of the barium titanate also change accordingly depending on the size and the crystal phase. The dependence of dielectric constant on the particle size of barium titanate has been already reported [10, 11, 20]. The synthesis of barium titanate via hydrothermal process is carried out in excess water content at very high OH concentration [21, 22]. Therefore, it is evident that some OH groups may be entrapped in the crystal lattice. The adverse effects of the entrapped OH groups have been well documented [23–28]. No method other than heating has been reported so far, which can effectively extract the lattice hydroxyls. Also, experimental evidence of the lattice impurity effect on the cubic–tetragonal transition has been reported [29–31]. However, a clear understanding of the nature of the incorporation and mechanism of removal of these species is still lacking. Lattice hydroxyl groups are the most well-known lattice impurity in barium titanate, which cause cationic vacancies, enlargement of unit cell and sintering difficulty. Our previous study showed that OH extraction could be achieved by treating the hydrothermal barium titanate powder in Dimethyl Formamide [32]. The dielectric properties were significantly impressive. By changing the reaction media from a water-providing type to a water-extracting one, the water molecules can be extracted from the crystallites by diffusing and dissolving in a highly polar, high boiling point organic liquid.
It has been reported that OH groups adsorbed on the surface of the barium titanate powder exhibit an infrared absorption because of the availability of many different surface adsorption sites [33]. However, in the case of hydrothermal barium titanate powders, a relatively sharp peak occurring at a similar wave number 3496 cm−1 was observed by Noma et al. [34] and attributed to the absorbance of OH groups incorporated into the barium titanate ceramic lattice. The broad OH resonance was almost eliminated only after calcining the barium titanate powder for 1 h at 600–800 °C, as observed in previous studies. It is also observed that there will be a band height increase at 600 °C which is explained by the diffusion of the incorporated lattice hydroxyl ion to the surface of the barium titanate particles [12, 34–36]. There is an appreciable amount of lattice incorporated protons and hydroxyl ions present in hydrothermally synthesized barium titanate powder and it is found that this lattice OH diffuses to the surface by the process of annealing [25, 32].
Some information on the presence and location of OH groups has been obtained using a variety of techniques, including infrared analysis, FTIR and TGA which have been used to further elucidate the nature of lattice hydroxyl groups in hydrothermal barium titanate powder ceramics. The formation mechanism and the effect of hydroxyl ion on the dielectric properties of the barium titanate nano-crystals were not explored till date. Therefore, our present investigation involves the reporting the chemical treatment of converting nanocrystalline cubic barium titanate to tetragonal phase without particle growth. The results are then related to dielectric properties.
2 Materials and procedure
A commercial grade barium titanate, Cabot BT-8, (hydrothermal powder with a mean particle size of 0.2 μm obtained from Cabot Performance Materials, Boyertown, PA), was used in the present work. A highly polar solvent N-Methyl-2-pyrrolidinon (NMP), (bearing a density of 1.03 g/cc obtained from Acros Organics), was used to extract OH ions, Castor oil, (Eur. Pharm. grade, having a density of 0.957 g/cc obtained from Acros Organics), acetic acid, CH3COOH (glacial with 99.7% purity obtained from Fisher products) and potassium hydroxide, KOH (flakes obtained from Aldrich). The solvent was used without any prior treatment and purification. The commercial BT that retains a cubic phase was initially estimated for a definite amount of 5 g in a closed teflon jar. A pre-determined amount, 75 mL of NMP was then added to the teflon jar. The mixture or the suspension was thoroughly agitated at a temperature of 200 °C for 24 h on a magnetic stirring hot plate. Then the obtained sol was centrifuged to remove the solvent, washed with diluted CH3COOH and then with water and ethyl alcohol alternately. The washing process was repeated for three times. The collected powder was then dried under vacuum at 90 °C only to remove the excess surface water and solvent present leaving a pure barium titanate powder behind. The crystallinity and the tetragonal phase transformation were confirmed by the X-ray diffractometry.
As discussed, the major constituent in this type of cubic-to-tetragonal phase transformation is due to the elimination of OH ions present in the lattice. Simulation experiments have been done to elaborate the concept. 200 mL of deionized water was taken in two different beakers each of 100 mL. We prepared the water in one beaker with CH3COOH maintaining a pH around 3–4 and naming it as acidic water whereas the other beaker of water with KOH maintaining a pH around 12–13, naming it as basic water. The commercial cubic phase barium titanate was introduced as three different samples. The first sample—as-received and at room temperature, the second sample—as-received and calcined at 200 °C, and the third sample—as-received and calcined at 1100 °C. Each sample was exactly estimated to 10 g, 5 g for acidic water and 5 g for basic water respectively. Once the samples were ready, they were aged for 24 h in the different pH waters at room temerature. Then the samples were dried under vacuum at 90 °C only to remove the excess surface water content.
Initially, castor oil and the NMP treated barium titanate ceramic powder was mixed in different proportions. These mixtures had a variable ceramic content of 10–50% by volume on the dry basis. Then castor oil was mixed with the differently aged barium titanate ceramic powder samples one-by-one. These mixtures had a variable ceramic content by volume on the dry basis. All these samples were ready for the characterization. After the X-ray diffraction analysis and other thermal analysis methods, the samples were used for the determination of the dielectric properties using the capacitor technique. The capacitor was fabricated using the same procedure that was followed in our earlier work [37, R. Kota, A.F. Ali, B.I. Lee, M.M. Sychov, Microelectron. Eng. (submitted)] and the samples were characterized for capacitance and loss factor. Dielectric constants of all the ceramic powders were determined by preparing a slurry/paste form free from pores composed of different volume fractions of barium titanate particles and castor oil followed by filling the teflon cell with aluminum plate electrodes. The capacitance was measured at 1 MHz using HP 4284A Precision LCR Meter. The dielectric constant values (Ks) were calculated from the measured capacitance data using the equation
where ε0 = dielectric permittivity of the free space, 8.854 × 10−12 F/m; A = area of the electrode and ceramic contact area, 1 cm2; t = thickness of the ceramic specimen, 0.4 cm
The dielectric constant of all the samples was determined using the capacitance values.
3 Results and discussion
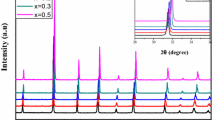
This method is a low-temperature solvothermal treatment of hydrothermal commercial barium titanate powders in a polar solvent, NMP in a sealed teflon jar. Figure 1 shows the XRD pattern, in the 2θ range of 35–55 for the NMP treated barium titanate particles at 200 °C for 24 h. The tetragonality of barium titanate crystal is characterized by measuring the broadening of the {200} peaks. Before the NMP treatment, there was no appreciable broadening of the {200} peaks. When the samples were treated for 24 h, a noticeable peak splitting is clearly observed at 2θ = 45 °.
The peak splitting value and the c/a ratio were determined by Rietveld refinement of the {200} peaks through the single peak deconvolution approach. The c/a ratio changed from 1.001 (as-received) to about 1.0078 after samples were treated in NMP at 200 °C for 24 h. Considering the theoretical tetragonal asymmetry (c/a = 1.01) [38] and the fact that tetragonal and cubic phases always co-exist in barium titanate crystals, it is very much obvious to conclude that the NMP-treated barium titanate particles were tetragonal. The enhanced tetragonality was also confirmed by the DSC results, a curie transition at 133 °C, as shown in Fig. 2. Before the NMP treatment, the barium titanate particles were cubic at room temperature and showed no phase transformation in DSC upon heating up to 200 °C.
The hydroxyl content in the as-received, aged and NMP treated barium titanate particles was determined by FTIR analysis and TGA. Figure 3 shows the FTIR spectrum of NMP treated barium titanate along with the untreated powder. The intensity of this broad peak in the range of 2600–3600 cm−1, which is assigned to the OH stretching vibration, was significantly reduced after the NMP treatment. The presence of some barium carbonate was also detected in as-received powders, which was also removed by the treatment. It is necessary to distinguish lattice hydroxyls from the naturally absorbed surface water because surface water does not cause lattice strain. The semi quantitative comparison of the band intensities show that the lattice OH content in this NMP treated barium titanate powder to be 0.35%.
FTIR spectrum, in the OH stretching region, of hydrothermal barium titanate powder calcined in air for 24 h at room temperature, 200 and 1100 °C are shown in Fig. 4. The broad spectrum attributable to OH groups decreases with increasing the calcining temperature. The spectrum of the BT powder aged in different pH waters has two sharp peaks, a larger peak at 3496 cm−1 corresponding to the surface OH and a smaller peak at 2931 cm−1 that corresponds to the peak position observed for the lattice OH, of 1426 and 1469 cm−1 to lattice and surface carbonate respectively and of 785 and 545 cm−1 to barium titanate. Semi quantitative comparison of the species by band intensity of OH at 2931 cm−1 was calculated to the ratio of the species by band intensity of barium titanate at 545 cm−1. The observed lattice OH content in both the different pH waters is tabulated in Table 1.
The TGA results obtained were analyzed for the weight loss in each sample. It is generally considered that the total weight loss as a combination of weight loss of hydroxyl ions, both surface and lattice, and carbonate ions. Based on these results, a study is being done on the TGA curves, shown in Fig. 5, for the exact temperature range and the amount of weight loss of the lattice OH content.
It was already mentioned that between the temperature 250–450 °C, the incorporated lattice hydroxyl ion diffuses to the surface [25]. Hence, at that particular temperature range the slope and the weight loss are being calculated and the results are tabulated in Table 2.
It is clearly evident that as the temperature increases the amount of water and hydroxyl groups deplete. Both the results from FTIR and TGA support the earlier statement.
Now it is very much understood that lattice OH plays an important role in phase transformation of barium titanate. The effect of the lattice OH content can be more clearly estimated by performing a dielectric study on the NMP treated barium titanate as well as the samples aged in different pH waters.
Dielectric constant values of the different samples were calculated from the measured capacitance data using the Eq. (1). The dielectric constant and loss values of the NMP treated barium titanate powder particles obtained by using castor oil as the binder phase is plotted in Fig. 6 and extrapolating this plot gives the dielectric constant of the NMP treated ceramic particles as 291 and the dielectric loss factor to be 0.04. Using the same technique, the dielectric constant value of the as-received powder particles is found to be 177 that were reported by our earlier work [37]. The dielectric constant of the commercial tetragonal powder particles, BT 219-6, in as-received condition is determined as 306 using the same procedure that was followed in our previous work [37, R. Kota, A.F. Ali, B.I. Lee, M.M. Sychov, Microelectron. Eng. (submitted)].
Similarly the dielectric constants of all the six samples that were aged in different pH waters were being determined using castor oil as the binder phase. Then the dielectric constant results were extrapolated and plotted against temperature and lattice OH content that are shown in Figs. 7 and 8 respectively. The dielectric constant values of samples aged in acidic water give higher values due to smaller amount of OH content present in them when compared to the samples aged in basic water. The loss factor for both acidic and basic waters was increased by a very small amount. It was observed that there was an excess weight loss in the powder aged in basic water. The reason for this behavior was the instability in the surface of the particle. There was a definite amount of carbonate as well as OH present on the surface when compared to the powder aged in acidic water.
The summary of the results is tabulated in Table 3.
4 Conclusions
Barium titanate treated with NMP contains a lower concentration of lattice hydroxyl group, resulting in a small lattice strain. The tetragonality in the hydrothermal barium titanate particles is restored by a one-step chemical treatment. The lattice hydroxyls in barium titanate were effectively extracted by the NMP treatment. As hypothesized, the lattice hydroxyl release is the reason of the tetragonality recovery. This method can be considered as a complementary treatment to promote the phase transition of cubic barium titanate and to synthesize tetragonal ferroelectric nanoparticles. XRD confirms the tetragonality with the peak split at 45° and c/a ratio is obtained as 1.0078. FTIR investigations of hydrothermal barium titanate particles revealed a spectrum with a broad OH band in the wave number range of 2600–3600 cm−1, indicating the presence of a significant concentration of surface OH groups in the film. A sharply-defined peak at 2931 cm−1, attributed to lattice OH species, was also found. The apparent amount of lattice OH content was reported to be 0.35% for barium titanate powder treated with NMP at 200 °C for 24 h. The simulation experiment adds value to the above statement as barium titanate calcined at 1100 °C in both acidic and basic waters show similar values which clearly indicates that elimination of OH initiates the tetragonality in a cubic barium titanate. Dielectric studies provide strong evidence to the hypothesis made as the dielectric constant of NMP treated barium titanate powder particle is 291, 64% higher than the value of as-received barium titanate, 177. Hence, extraction of hydroxyl ions, in particular the lattice OH, increases the dielectric constant of the powder.
References
S. Li, J.A. Eastman, Z. Li, C.M. Foster, R.E. Newnham, L.E. Cross, Phys. Lett. A 212, 341 (1996)
C.L. Wang, S.R.P. Smith, J. Phys. Condens. Matter. 7, 7163 (1995)
B. Jiang, L.A. Bursill, Phys. Rev. B 60, 9978 (1999)
W.L. Zhong, Y.G. Wang, P.L. Zhang, B.D. Qu, Phys. Rev. B 50, 698 (1994)
H. Huang, C.Q. Sun, P. Hing, J. Phys. Condens. Matter. 12, L127 (2000)
M.A. Pena, J.L.G. Fierro, Chem. Rev. 101, 1981 (2001)
A.S. Bhella, R. Guo, R. Roy, Mater. Res. Innov. 4, 3 (2000)
P.K. Dutta, R. Asiaie, S.K. Akbar, W. Zhu, Chem. Mater. 6, 1542 (1994)
P. Duran, D. Gutierrez, J. Tartaj, C. Moure, Ceram. Int. 28, 283 (2002)
P.R. Arya, P. Jha, A.K. Ganguli, J. Mater. Chem. 13, 415 (2003)
S. Bhattacharya, R. Tummalla, J. Mater. Sci. Mater. Electronics 11, 253 (2000)
D.H. Yoon, B.I. Lee, J. Ceram. Process. Res. 3, 41 (2002)
C.D. Chandler, C. Roger, M.H. Smith, J. Chem. Rev. 93, 1205 (1993)
D. Henning, M. Klee, W. Waser, Adv. Mater. 3, 334 (1991)
G. Arlt, D. Hennings, G. de With, J. Appl. Phys. 58, 1619 (1985)
I.J. Clark, T. Takeuchi, N. Ohtori D.C. Sinclair, J. Mater. Chem. 9, 83 (1999)
S. Schlag, H.F. Eicke, Solid State Commun. 91, 883 (1994)
K. Ishikawa, K. Yoshikawa, N. Okada, Phys. Rev. B 37, 5852 (1988)
Y. Kobayashi, A Nishikata, T. Tanase, M. Konno, J. Sol-Gel Sci. Technol. 29, 49 (2004)
T. Takeuchi, M. Tabuchi, K. Ado, K. Honjo, O. Nakamura, H. Kageyama, Y. Suyama, N. Ohtori, M. Nagasawa, J. Mater. Sci. 32, 4053 (1997)
L. Qi, B.I. Lee, P. Badheka, D.H. Yoon, W.D. Samuels, G.J. Exarhos, J. Eur. Ceram. Soc. 24, 3553 (2004)
L. Qi, B.I. Lee, P. Badheka, L.Q. Wang, P. Gilmour, W.D. Samuels, G.J. Exarhos, Mater. Lett. 59, 2794 (2005)
D. Hennings, S. Shreinmacher, J. Eur. Ceram. Soc. 9, 41 (1992)
S. Wada, T. Suzuki, T. Noma, J. Ceram. Soc. Jpn. 103, 1220 (1995)
T. Noma, S. Wada, M. Yano, T. Suzuki, J. Appl. Phys. 80, 5223 (1996)
D.F.K. Hennings, C. Metzmacher, B.S. Schreinemacher, J. Am. Ceram. Soc. 84, 179 (2001)
A.T. Chien, L. Zhao, M. Colic, J.S. Speck, F.F. Lange, J. Mater. Res. 14, 3330 (1999)
V.M. Fuenzalida, M.E. Pilleux, I. Eisele, Vacuum 55, 81 (1999)
S. Wada, T. Suzuki, T. Noma, J. Ceram. Soc. Jpn. 104, 383 (1996)
T. Yamamoto, H. Niori, H. Moriwake, Jpn. J. Appl. Phys. 39, 5683 (2000)
E. Ciftci, M.N. Rahaman, J. Mater. Sci. 36, 4875 (2001)
P. Badheka, L. Qi, B.I. Lee, J. Eur. Ceram. Soc. 26, 1393 (2006)
S.K. Patil, N. Shah, F.D. Blum, M.N. Rahaman, J. Mater. Res. 12, 3312 (2005)
B.I. Lee, J. Electroceramics 3, 51 (1999)
S. Lu, B.I. Lee, L. Mann, Mater. Lett. 43, 102 (2000)
S. Lu, B.I. Lee, Mater. Res. Bull. 35, 1303 (2000)
B.I. Lee, X. Wang, S.J. Kwon, H. Maie, R. Kota, J.H. Hwang, J.G. Park, M. Hu., Microelectron. Eng. 83, 463 (2006)
M.H. Frey, D.A. Payne, Phys. Rev. B 54, 3158 (1996)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kota, R., Lee, B.I. Effect of lattice hydroxyl on the phase transition and dielectric properties of barium titanate particles. J Mater Sci: Mater Electron 18, 1221–1227 (2007). https://doi.org/10.1007/s10854-007-9296-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-007-9296-3