Abstract
Enkephalins have been shown to retard cell proliferation in various human cancer cell lines, including breast, soft tissue, gastrointestinal, brain, pancreatic, and liver cell cultures. In this study, we have synthesized polymeric PLGA [poly (lactide-co-glycolic acid)]–poloxamer nanoparticles loaded with enkephalins in order to achieve a consistent release of these neuropeptides. In order to provide targeting abilities toward cancerous cells, these nanoparticles were functionalized with RGD (Arg-Gly-Asp-amide, a cancer cell marker). The synthesized nanoparticles (NPs) were further thoroughly characterized for biological activity including cytotoxicity, anticancer activity as well as cancer cell uptake abilities. Interestingly, it was observed that the fluorescent marker containing cancer cell-targeting peptide (RGD) conjugated nano-formulation(s) exhibited good uptake and internalization into cancer cells. The results indicate that the developed enkephalin-RGD-PLGA nanoparticles can serve as suitable targeted drug delivery vehicle/system for existing conventional drugs as well as in increasing the efficacy of drugs.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Enkephalins are pentapeptide neuropeptides belonging to endogenous opioids family which also include dynorphins, endorphins, endomorphins and nociception [1]. These opioid peptides play role in neurotransmission as well as pain modulation through opioid receptors (ORs) [2, 3]. Enkephalins exist in two forms containing methionine and leucine residues, at terminal position, respectively. While amino acid sequence of methionine-enkephalin (Met-enk) is Tyr-Gly-Gly-Phe-Met, the leucine-enkephalin (Leu-enk) consists of Tyr-Gly-Gly-Phe-Leu (Fig. 1). The functioning of enkephalins is mainly modulated via δ- and μ-opioid receptors [4, 5].
Met-enk is a tonically active inhibitory neuropeptide and functions in both normal and abnormal cells through receptors. It binds to the opioid receptors present on surfaces of immune cells such as NK cells (natural killer cells), macrophages, T cells and also on tumor cells. Thereby, it up-regulates the activity of immune cells and may inhibit tumor growth [6]. Also, Met-enk has been shown to retard cell proliferation in various human cancer cell lines, including breast, soft tissue, gastrointestinal, brain, pancreatic and liver cell cultures [7,8,9]. Basically, Met-enk along with opioid receptors forms a regulatory biological axis that modulates cell proliferation by retarding G1/S cell cycle progression under homeostatic conditions and in neoplasia and thus arrests tumor progression [10]. In a recent study, the anticancer effect of Met-enk using A375 cells was explored which aimed at investigating the inhibition effect of Met-enk on human melanoma which revealed that this neuropeptide can inhibit growth and induce apoptosis of A375 cells [11]. These studies suggested that Met-enk should be investigated as a primary therapy for different cancers as well as an adjuvant for existing chemotherapeutic techniques.
Apart from anticancer and immunosuppressant functions, enkephalins are also known to be neurotransmitters in pain perception and modulation [12]. Due to non-addictive opioid analgesic activity, these neuropeptides might have extraordinary potential in acute and chronic pain management [13]. In particular, Leu-enk has been extensively investigated for its antinociception [14] and analgesic activities [15].
Recently, the therapeutic potential of these neuropeptides has been extensively studied and utilized for the treatment of variety of diseases, including cancer [3]. However, their broad applications are constrained by their poor oral bioavailability because of rapid degradation in the gut or in the blood and excretion from the organism leading to low therapeutic effect [16, 17]. Moreover, their chemical features such as high molecular mass, charge, hydrophilic groups also hamper their cellular uptake [18]. There have been exhaustive attempts along the lines of development of successful therapeutic system based on these neuropeptides. Various strategies include lipidization [19], glycosylation, substitution of L- by D-amino acids in peptide to enhance stability against enzymatic proteolysis [20] and covalent linkage to BBB penetrating proteins to get transportable neuropeptides [18,19,20]. In an approach, Leu/Met-enkephalins were reversibly lipophilized to 9-fluorenylmethoxycarbonyl (Fmoc)-derived lipophilic prodrug analogs. These analogs were claimed to undergo slow and spontaneous hydrolysis to give native enkephalins under physiological conditions [18]. But most of the attempts made so far in this direction showed rather limited success. Chemical modification of peptides to enhance stability and lipophilicity often resulted in biologically inactive derivatives, which was also the case in neuropeptides covalently linked to BBB transport vectors. Further, the efficiency of releasing the neuropeptides from the inactive-conjugate by enzymatic cleavage in its active native form in central nervous system is quite low [21,22,23,24,25].
With the advent of nanotechnology, the use of nanoparticles as carrier moieties is emerging as successful approach in this context. Recently, a nanoparticle system based on thermoresponsive aggregation of polymer, poly [(di (ethylene glycol) monomethyl ether methacrylate)-ran-(oligo (ethylene glycol) monomethyl ether methacrylate], was reported as a carrier system for Met-enkephalin which was also conjugated with RGD (a cancer targeting peptide) [26]. However, it suffered from a serious limitation that the loading of neuropeptide required its chemical bioconjugation to polymer which aggregated at specific temperature to give mesoglobule or nanoparticle. Thus, the release of biologically active form of enkephalin after delivery is ambiguous. Also, the polymer used is not of biodegradable nature.
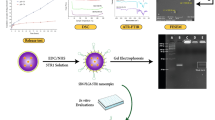
Further, in this direction, a number of reports are available for the development of nanoparticle delivery systems for Dalargin (a Leu-enkephalin analog) using various polymers [26,27,28]. Although derivatization strategy has been widely investigated in order to ensure enhanced stability and lipophilicity to overcome the blood–brain barrier crossing abilities of these enkephalins, they suffer from serious shortcomings like rendering the therapeutic significance of peptide by chemically modifying it to inactive form. Further, the incorporation of non-biodegradable moieties like Fmoc or non-degradable polymers raises another concern which may lead to toxicity. Even after dissociation of derivatives to give active form of peptide, the remaining part should be non-toxic and easily disposable by body. Thus, instead of modifying the basic structure of these peptides, developing a biodegradable and targeting delivery depot in the form of nanoparticles can be a better approach in order to completely exploit the therapeutic benefit of these peptides. Keeping this in mind, in this study, we have synthesized polymeric PLGA [poly (lactide-co-glycolic acid)]–poloxamer nanoparticles loaded with enkephalins in order to achieve a consistent release of these neuropeptides. Further, we also explored the effect of chitosan coating on nanoparticles which is considered of mucoadhesive nature.
In order to provide the nanoparticles with targeting abilities toward cancerous cells, RGD (Arg-Gly-Asp-amide, a cancer cell marker) was decorated on surface of nano-formulation(s). To study the encapsulation efficiency and drug release profile using fluorescence, FITC was conjugated to enkephalins. The synthesized nanoparticles were further thoroughly characterized for biological activity including cytotoxicity, anticancer activity as well as cancer cell uptake abilities.
Experimental section
Materials
[Met5] Enkephalin acetate salt hydrate (≥ 95.0%, HPLC) (Met-enk), Leucine Enkephalin acetate salt hydrate (≥ 95.0%, HPLC) (Leu-enk), Fluorescein 5-isothiocyanate (≥ 97.5%, HPLC) (FITC), poly (D, L-lactide-co-glycolide) acid (PLGA) (lactic/glycolic acid 75:25 ratio, M wt. 66,000–107,000), poloxamer (Kolliphor® P 188), Sephadex-G25 gel, 1-Ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) solution and N-hydroxysuccinimide (NHS) were purchased from Sigma-Aldrich, India. Poly-(vinyl alcohol) (PVA) (M. wt. 15,000) was purchased from Fluka, India. Sodium carbonate (Na2CO3), dimethyl sulfoxide (DMSO), agarose gel, glycerol, acetone, ethidium bromide and phosphotungstic acid were procured from CDH, India. Arg-gly-asp-amide (RGD) was prepared using solution phase peptide chemistry. De-ionized water of grade I, obtained from Merck-Millipore purification unit having resistivity of 18.2 mΩ, was used in all experiments.
Methods
Synthesis of Met-enk-FITC and Leu-enk-FITC bioconjugate
Methionine enkephalin (Met-enk) and leucine enkephalin (Leu-enk) were tagged with a fluorescent dye (FITC) using reported bioconjugation technique [29]. Briefly, 1 mg/mL stock solution of FITC was prepared freshly by dissolving 500 µg of FITC in 500 µL of DMSO. To protect the solutions from photobleaching, amber-colored microcentrifuge tubes were used. Further, 0.1 M Na2CO3 buffer solution was prepared by dissolving 0.052 g in 5 mL of de-ionized water and adjusting its pH to 9.2. 1 mg/mL solution of Met-enk (or Leu-enk) was prepared in buffer followed by addition of 100 µL of freshly prepared FITC solution. The reaction mixture was mixed well by shaking and incubating at 4 °C for 8 h. After 8 h, the reaction was quenched by the addition of 2.6 mg (50 mM) of ammonium chloride, and the reaction mixture was again incubated for 2 h after complete mixing at 4 °C. The bioconjugates so prepared were purified to remove excess unbound FITC using gel filtration chromatography. Briefly, a thin column was loaded with sephadex-G25 gel which was kept immersed in 1xPBS buffer 24 h. The prepared Met-enk-FITC bioconjugate (or Leu-enk-FITC bioconjugate) was loaded and eluted with 1xPBS buffer. The fractions containing conjugate were collected. The whole apparatus was maintained at 25 °C.
Characterization of Met-enk-FITC and Leu-enk-FITC bioconjugates using electrophoresis
The purified bioconjugates were characterized using gel electrophoresis. A horizontal agarose gel system was employed in the experiment. The agarose gel (1.8%) was prepared by dissolving agarose in 1 × TAE (Tris acetate-EDTA) buffer (pH 8) at boiling temperature. After slight cooling, 0.5 µg/mL of ethidium bromide was added and the molten agarose was poured into a gel tray fixed within the gel caster. Next, a teeth comb was placed on a side slot and the gel was left to cool and solidify for 1 h after ensuring proper leveling of gel to achieve an even thickness with identical wells. The solidified gel was transferred to mini-sub cell electrophoresis tub along with TAE buffer for submersion of gel beneath 5 mm of liquid. 10 µL each of Met-enk-FITC bioconjugate, Leu-enk-FITC bioconjugate and FITC were separately mixed with 10 µL of glycerol and were loaded into the wells carefully. The gel was run for 1 h using 18 V (steady current) per 1 cm of gel (180 V). The gel was removed from electrophoresis cell and was visualized in UV chamber, and images were taken under UV illumination as well as under normal light.
Synthesis of Met-enk-FITC loaded PLGA–poloxamer NPs and Leu-enk-FITC loaded PLGA–poloxamer NPs
Met-enk-FITC loaded and Leu-enk-FITC loaded polymeric NPs were prepared by using modified single emulsion method. 500 µL of purified Met-enk-FITC conjugate (or Leu-enk-FITC conjugate) was sonicated with 2 mL of 5 mg/mL solution of PLGA in acetone for 30 s at 50 W using probe ultrasonicator (Q-sonica, India). The formed emulsion was extruded by passing through a mini-extruder (Avanti mini-extruder) fitted with 0.2-micron membrane filter for 21 cycles to achieve monodispersed nanoemulsion with homogenous particle size. Further, this emulsion was dispersed into 2 mL aqueous solution containing 0.05% PVA and 0.05% poloxamer (Kolliphor P 188). The resultant solution was again extruded to 21 cycles and the stirred for 4 h at 700 rpm at room temperature. The NPs were purified using centrifugation and re-dispersion into 1 × PBS buffer.
Synthesis of Met-enk-PLGA–poloxamer-chitosan NPs and Leu-enk-PLGA–poloxamer-chitosan NPs
For preparing chitosan-coated Met-enk-PLGA–poloxamer NPs, method similar to previously mentioned in Sect. 2.2.3 was used. Briefly, 100 µL of chitosan solution (1 mg/mL in 0.1% acetic acid) was added dropwise to 1 mL of Met-enk-PLGA–poloxamer nanoparticles prepared in previous section. The reaction mixture was stirred at 200 rpm for 2 h at room temperature. The chitosan-coated nanoparticles so formed were purified by centrifugation to remove any unbound chitosan.
Functionalization of nanoparticles with cancer targeting peptide (RGD)
The nanoparticles were functionalized with arg-gly-asp-amide (RGD) using carbodiimide cross-linking chemistry. Briefly, to 1 mL of Met-enk-PLGA–poloxamer-chitosan nanoparticles, 100 µL aqueous 1-Ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) solution (100 µM), 200 µL of N-hydroxysuccinimide (NHS) solution (100 µM) and 100 µL RGD solution (100 µM) were added. The reaction mixture was incubated at room temperature for 24 h under mild stirring. The nanoparticles were washed with de-ionized water using centrifugation and were finally dispersed in 1 × PBS buffer.
Size determination of enkephalin-loaded nanoparticles using DLS
All the prepared nanoparticles were analyzed for size distribution using DLS (Malvern Nano ZS). The samples were prepared by diluting the prepared nanoparticles to 50% with de-ionized water.
Morphology analysis using TEM
The shape, size as well as surface morphology of the synthesized nanoparticles were analyzed using TEM. For the preparation of sample grids, a drop of sample was loaded on the carbon-coated copper grid and the excess of liquid was removed using an absorbing paper. Further, the grid was inverted onto a drop of 1% (w/v) phosphotungstic acid in order to stain the sample nanoparticles negatively. The grid was then allowed to air dry for 5–6 h. For imaging, the electron microscope (Hitachi Model H-7500) was operated at 120 kV.
Fluorescence spectroscopy
Fluorescence spectra were recorded on a fluorescence spectrophotometer (Agilent Cary Eclipse) at room temperature with excitation and emission wavelengths of λex = 495 nm and λem = 515 nm, respectively. The excitation and emission slit widths were10 nm and 5 nm, respectively, while the lamp voltage (PMT) was 650 V.
Encapsulation efficiency
In order to determine the encapsulation efficiency (EE) for enkephalin-loaded nanoparticles, fluorescence spectroscopy was employed. Standard curves were plotted between concentration of Met-enk-FITC conjugate or Leu-enk-FITC conjugate and fluorescence intensity at 515 nm. The equations obtained were utilized to calculate the concentrations of conjugates in the samples. In order to determine the encapsulation efficiencies, the amount of conjugates remaining un-entrapped in the supernatant was estimated and EE% was calculated using formula for encapsulation efficiency.
Drug release profiles for enkephalin-loaded nanoparticles
The in vitro release of Met-enk-FITC and Leu-enk-FITC bioconjugates from synthesized polymeric nanoparticles was studied using PBS buffer maintained at pH 7.4 and temperature 37 °C using incubator shaker. Briefly, 1500 μL of nanoparticles suspension mixed with 500 μL of 1 × PBS was loaded into dialysis tubing (Sigma-Aldrich, cut-off 1200 kD) which was further sealed on both sides. The tubing was suspended into 25 mL of 1 × PBS buffer solution maintained at 37 °C and constant shaking of 250 rpm. Aliquots of 0.5 mL were retrieved from the diffusion media at time intervals of 30 min, and the media was replenished with fresh buffer each time to maintain the sink conditions. The aliquots were analyzed using fluorescence microscopy, and concentration of Met-enk-FITC or Leu-enk-FITC was estimated using above obtained standard curves and equations. Finally, the release profiles were plotted between time and drug release percentage for 5 h.
Cell viability assay and IC50 determination
In the present study, MTT assay was performed to evaluate the cytotoxic potency and efficacy of enkephalin neuropeptides-based formulations along with carrier system consisting of PLGA, poloxamer and chitosan. Briefly, HeLa cells (4 × 104 cells/well) were seeded in a 96-well plate (in triplicate) with 100 µL of media 18–24 h prior to experiment. Then, the media was replaced with the different concentrations of neuropeptide-based formulation(s) (Met-FITC-PLGA-RGD, Leu-FITC-PLGA-RGD, Met-FITC-PLGA-ch, Leu-FITC-PLGA-ch, Met-FITC-PLGA-ch-RGD, Leu-FITC-PLGA-ch-RGD) diluted in media and incubated for 24 h. After incubation, treatment groups containing media were removed and 100 µL media containing 10% v/v MTT solution (5 mg/mL) was added to cells and incubated for 4 h [30,31,32]. After completion of MTT incubation period, media was removed completely and formazan crystals thus formed were dissolved in 100 µL of DMSO. The solubilized formazan gave purple color, which was measured spectrophotometrically at 570 nm using micro-plate reader. The results obtained were expressed as percent viability = [A570 (treated cells)-background/A570 (untreated cells)-background]/100. Typically, viability experiments were done in triplicates on three different days. Similarly, assays were done to evaluate the inhibitory concentrations of various treatment groups using the narrowed range of concentration in cytotoxicity assay. Hence, IC50 values of different treatment groups were calculated and compared based on representative data obtained on a single day. All the results were expressed as mean ± standard deviation (s.d.) of three independent values. Results were estimated by one-way ANOVA using software SPSS followed by post hoc analysis for the in vitro experiments. The p value ≤ 0.05 was considered statistically significant.
In vitro cellular uptake studies
To investigate the localization of synthesized nanoparticles into cancer cells, the HeLa cells were seeded into a six-well plate with cover slips and incubated with Met-FITC-PLGA-RGD, Leu-FITC-PLGA-RGD, Met-FITC-PLGA-ch, Leu-FITC-PLGA-ch, Met-FITC-PLGA-ch-RGD and Leu-FITC-PLGA-ch-RGD nanoparticle (Met-FITC-50 ng/ml and Leu-FITC-50 ng/ml) containing medium for 4 h at 37 °C. The cells with no treatment were kept as control. After the incubation, the cells were gently rinsed with PBS to fully remove non-specifically adsorbed drug. Next, the cells were fixed with 4% paraformaldehyde for 30 min at room temperature. The cells were again washed with PBS and were subjected to laser scanning confocal microscopy. All images were captured under the same instrumental settings and analyzed with image analysis software.
Results and discussion
Preparation of RGD grafted polymeric nanoparticles containing enkephalins for targeted antitumor activity
Synthesis of Met-enk-FITC and Leu-enk-FITC bioconjugates
Firstly, met and Leu-enk neuropeptides were tagged with fluorescent dye FITC (Met-enk-FITC and Leu-enk-FITC) (Fig. 3a). FITC was used for bioconjugation owing to its high quantum yield and good conjugate stability [33]. During the conjugation, a covalent bond was formed between isothiocyanate group of FITC and primary amine of both met-enk and leu-enk peptides. Conventionally, encapsulation efficiency used to be determined using sophisticated instruments like high-performance liquid chromatography (HPLC). The determination of encapsulation efficiency using HPLC has several disadvantages such as high cost of instrument, time consuming, require trained person, and complex sample preparation. Therefore, in order to overcome the above limitation, FITC acting as fluorophore has been conjugated to both Met-enk and Leu-enk neuropeptides in order to facilitate easy determination of encapsulation efficiency and monitor drug release from nano-formulation(s) using highly sensitive fluorescence spectroscopy. In addition, FITC will also enable the tracking of nano-formulation(s) in biological studies such as cancer cell uptake of nanoparticles with the aid of fluorescence microscopy.
Characterization of Met-enk-FITC and Leu-enk-FITC using gel electrophoresis
Gel electrophoresis is used to monitor the electrophoretic mobility of charged species in a gel matrix, typically agarose or polyacrylamide, when an electric field is applied across it. For macromolecules such as DNA, RNA and bioconjugates, the overall size, shape, and charge density influence the direction and distance moved in the gel [34,35,36]. On the smaller scale, these techniques are routinely used to separate and purify bioconjugates and are also quite often used as rapid and powerful tool for confirming biomolecular attachment to the nanomaterials through discrete changes in mobility.
The enkephalin bioconjugates, prepared and purified as discussed in above section, were analyzed using agarose gel electrophoresis to confirm the conjugation with FITC. Figure 2 shows the images of agarose gel loaded with both bioconjugates and FITC under normal light and under UV illumination.
It was observed that the conjugates as well as FITC moved from negative to positive electrode under the influence of electric field. Further, it could be noticed that both fluorescent conjugates (Met-enk-FITC and Leu-enk-FITC) moved at a slower pace than FITC owing to their lower mobility due to larger sizes confirming the successful binding with FITC (Fig. 2). Additionally, it is to be noted that no additional bands were observed in gel.
Synthesis of Met-enk-FITC loaded PLGA–poloxamer NPs and Leu-enk-FITC loaded PLGA–poloxamer nanoparticles
Polymeric nanoparticles are essential delivery carrier for peptides, protein and oligonucleotides in pharmaceutical sciences owing to several advantages such as controlled release of drug, enhanced bioavailability, efficacy and stability and reduced toxicity for in vivo analysis. Among the different characteristics of polymeric nanoparticles, size is one of the most significant properties that is useful for their easy facilitation and interaction with biological system and, therefore, is necessary to opt for method that will produce homogeneous particles and free of toxins. Keeping this in mind, Met-enk-FITC and Leu-enk-FITC-loaded polymeric nanoparticles were prepared by using optimized and modified single emulsion technique. Single emulsion technique has gained widespread attention owing to the effectiveness of method in controlling size, homogeneity and for its simplicity as compared to conventional in situ polymerization of monomer which possess disadvantage owing to the presence of untreated monomer and free radicals. Single emulsion technique basically involved two steps; in the first step, FITC labeled neuropeptides and PLGA polymer, dissolved in organic solvent, were made into emulsion with the aid of probe sonicator and extruder was employed to gain homogeneity in nanoparticles. In the second step, poloxamer 188 and PVA dissolved in water were mixed with emulsion formed in first step to obtain stable nanoemulsion. Poloxamer 188 is the FDA-approved non-ionic linear block copolymer which enhances the structural stability of nanoparticles [37]. Also, during the synthesis, PVA mainly acted as stabilizer to get desired homogeneous nano-formulation(s) (Fig. 3b) [38]. The synthesized nanoparticles were characterized using DLS to determine hydrodynamic size.
Surface modification of Met-enk-PLGA–poloxamer NPs and Leu-enk-PLGA–poloxamer NPs with chitosan
Nanoparticle systems containing chitosan have attracted increasing attention in pharmaceutical and biomedical fields as these systems may be exploited also for prolonging drug retention at the adsorption site, thus allowing reduction of the frequency of dosing and minimizing the side effects. As the drugs are preferentially administered orally, by inhalation or transdermally, it is very important to find carriers endowed with good mucoadhesivity. Especially, for intranasal administration, mucoadhesive, biocompatible and biodegradable polymers have usually been chosen as the adjuvant materials among which chitosan and its derivatives play a significant role.
The Met-enk-PLGA–poloxamer NPs and Leu-enk-PLGA–poloxamer NPs prepared in above section were coated with chitosan via electrostatic interaction between negatively charged PLGA surface and positively charged chitosan (Fig. 3C). The chitosan-coated nanoparticles so prepared were further purified using centrifugation to remove any unbound chitosan and were characterized using DLS.
Functionalization of nanoparticles with cancer targeting peptide (RGD)
Functionalizing the anticancer drug-loaded nanoparticles with tumor-targeting peptide like arginyl-glycyl-asparticacid (RGD) can render it selectively recognizable and bind to αvβ3 integrin overexpressed by the tumor endothelium leading to improved accumulation of nanoparticles in the tumor tissue, thus enhancing anticancer activity along with reduced side effects. In order to functionalize the outer surface of synthesized nanoparticles with cancer cell targeting tripeptide RGD, carbodiimide chemistry (using EDC-NHS) was utilized. This bioconjugation technique utilizes a crosslinker, 1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride (EDC.HCl) along with a hydrophilic active group, N-hydroxysulfosuccinimide (sulfo-NHS) for the generation of a stable amide bond between carboxylic acid-functionalized moiety and a primary amine-containing moiety [36]. In this experiment, RGD was conjugated to the surface of Met-enk-PLGA–poloxamer-chitosan NPs and Leu-enk-PLGA–poloxamer-chitosan NPs conjugating the free amine groups of chitosan present on the nanoparticles with –COOH moiety of RGD-amide peptide (Fig. 3d). The RGD functionalized nanoparticles were further purified using centrifugation to remove any unbound peptide and other chemical reactants.
Characterization of synthesized nanoparticles
The different types of nanoparticles synthesized as per earlier sections were further characterized using various techniques.
Size determination using DLS
From Table 1, it can be observed that the sizes of the uncoated enkephalin-loaded PLGA poloxamer nanoparticles were below 100 nm in both cases. The chitosan modification of nanoparticles led to the slight increase in average sizes of nanoparticles as observed by DLS. There was significant increase in observed diameter of nanoparticles after conjugation with RGD. The possible reason for this may be the slight aggregation of nanoparticles. As RGD-functionalization employs use of cross-linking agents, it can also lead to increased interaction between nanoparticles along with binding to RGD which can be the reason for increased viscosity resulting in observed increased size and polydispersity in DLS analysis.
Morphology analysis using TEM
In order to analyze the morphology and texture of the synthesized nanoparticles, TEM was employed. It can be observed from TEM images (Fig. 4) that the particles were spherical in all cases.
TEM images of a Met-enk-FITC loaded PLGA–poloxamer-RGDNPs, b Met-enk-FITC loaded PLGA–poloxamer–chitosan NPs, c Met-enk-FITC loaded PLGA–poloxamer-chitosan-RGD NPs, d Leu-enk-FITC loaded PLGA–poloxamer-RGDNPs, e Leu-enk-FITC loaded PLGA–poloxamer-chitosan NPs and f Leu-enk-FITC loaded PLGA–poloxamer-chitosan-RGD NPs stained with 1% w/v phosphotungstic acid
Further, the RGD-modified chitosan-coated nanoparticles showed tightly packed particles which also explain the increased size in DLS analysis. On closely observing the RGD-modified nanoparticles, a thin layer can be observed on the nanoparticle surface which may be attributed to cross-linking due to binding with RGD.
Encapsulation efficiency
In order to determine the encapsulation efficiency (EE), fluorescence spectroscopy was employed. Standard curves were plotted between concentration of Met-enk-FITC conjugate or Leu-enk-FITC conjugate and fluorescence intensity at 515 nm (as shown in Fig. 5). The equations obtained led to linear curve and were utilized to calculate the concentrations of conjugates in the samples.
In order to determine the encapsulation efficiencies, the Met-enk-FITC loaded PLGA nanoparticles and Leu-enk-FITC loaded PLGA nanoparticles as prepared in the first step were centrifuged and the amount of conjugates which remained un-entrapped in the supernatant was estimated. The amount of conjugates in the nanoparticles was calculated by subtracting the free conjugates in supernatant from the total mass of conjugates in the initial solution. Further, EE % was calculated for both kinds of nanoparticles assuming full conversion of added polymer into nanoparticles. The encapsulation efficiency was determined by the following relations, respectively:
Encapsulation efficiency (EE%),
The encapsulation efficiency for Met-enk-FITC-loaded polymeric nanoparticles was found out to be 54.4 ± 0.34%. On the other hand, the encapsulation efficiency of Leu-enk-FITC-loaded polymeric nanoparticles was 24.0 ± 0.22% (Table 2).
In vitro release study of Met-enk-FITC and Leu-enk-FITC conjugates from nanoparticles
The in vitro release of Met-enk-FITC and Leu-enk-FITC conjugates from nanoparticles in PBS at 37 °C was investigated by using dialysis diffusion method. The release behavior of Met-enk-FITC conjugate from polymeric nanoparticles exhibited a rapid burst release and about 60% of the conjugate was released in first 120 min and it reached to 100% gradually in 280 min (Fig. 6a). The release of Leu-enk-FITC conjugate also exhibited similar pattern as 45% of the conjugate was released in first 100 min followed by gradual release upto 100% in next 300 min (Fig. 6b).
Cell viability assay
The different nano-formulation(s) based on Met-enkephalin and Leu-enkephalin were evaluated for cytotoxicity using MTT assay. The cell viability profiles show that all the nanoparticle formulations were non-toxic upto 5 ng/ml of concentration and more than ~ 70% cells were viable with all treatment groups (Fig. 7). It was observed that the synthesized nanoparticles showed killing effect against HeLa cancer cell line even at very low concentrations of drug and viability came down to ~ 50% on increasing the concentration to 25 ng/ml or more. Further, it can be noted that nanoparticles without RGD (Met-FITC-PLGA-ch and Leu-FITC-PLGA-ch) showed least cytotoxicity toward HeLa cell line. It leads to the inference that introduction of RGD led to the increase in cell-killing efficiency of nanoparticles. Statistical analysis results also indicated that cancer cell-targeting peptide (RGD) conjugated nano-formulation(s) significantly killing HeLa cancer cell lineage as compared to the nanoparticles without RGD (p ≤ 0.05). Further, these studies led us to an interesting observation that the absence of chitosan coating further improved the cell-killing efficiency of nanoparticles. The reason for this may be because chitosan coating delays the drug release from nanoparticles decreasing its anticancer activity.
In vitro cytotoxicity analysis of a Met-FITC-PLGA-ch, Met-FITC-PLGA-ch-RGD and Met-FITC-PLGA-RGD; b Leu-FITC-PLGA-ch, Leu-FITC-PLGA-ch-RGD and Leu-FITC-PLGA-RGD nanoparticles against HeLa cells on incubation for 24 h. (n = 3) Values represent mean ± s.d. (n = 3), analyzed using one-way ANOVA followed by post hoc test. p ≤ 0.05 is considered significant
Determination of IC50
In order to evaluate the anticancer properties of the prepared nanoparticles, the IC50 values of synthesized formulations against HeLa cell line were determined by analyzing inhibitory rate at extended range of concentrations of nano-formulation(s) (Fig. 8). The percentage of inhibitory rate was plotted versus concentration of treatment groups. The values at 50% inhibition rate were used to calculate IC50 values of different formulations.
The IC50 values of different formulations (Table 3) further confirmed that the presence of cancer cell-targeting peptide RGD improves the anticancer activity of formulations by increasing the cell binding and cellular uptake of nanoparticles.
In vitro cellular uptake studies
To verify the co-localization and cellular internalization of RGD conjugated nanoparticles and non-RGD conjugated nanoparticles, enkephalin-FITC loaded nanoparticles were incubated with HeLa cells for 4 h at 37 °C. After removal of the culture medium, the cells were fixed with paraformaldehyde and observed by laser scanning confocal microscopy (LSCM) to monitor the cellular uptake of the nanoparticles. Figures 9 and 10 show the FITC fluorescence images of control (cells with no treatment), chitosan conjugated, chitosan-RGD conjugated and only RGD conjugated PLGA nanoparticles loaded with Met-enk and Leu-enk. Apparently, both kinds of nanoparticles were present in the form of diffused bright spots in the cell cytoplasm, and the fluorescence signal (FITC) was randomly distributed in the cells.
It can be observed that the nanoparticles without RGD, i.e., Met-FITC-PLGA-ch nanoparticles and Leu-FITC-PLGA-ch nanoparticles could not internalize into the cells and the fluorescence signal in both cases was similar to control cells (Figs. 9b, 10b). Interestingly, on the other hand, the uptake intensity of RGD conjugated nanoparticles was higher than that of the non-RGD conjugated nanoparticles after incubation for the same time. Further, it can also be observed that RGD conjugated nanoparticles without chitosan were able to internalize more than the chitosan-containing nanoparticles.
Additionally, this cellular uptake trend of nanoparticles can also be explained on the basis of size of the nanoparticles. The smaller nanoparticles were able to internalize into cells more efficiently than larger-sized nanoparticles. Although chitosan has been studied as carrier molecule in many studies, but our studies prove that enkephalin containing PLGA-RGD nanoparticles can be much better approach for the development of drug delivery system.
Therefore, these results illustrated that RGD conjugated nanoparticles could more easily enter the cell which may lead to better anticancer efficacy, but the presence of chitosan hinders the cellular uptake.
Conclusions
As to in vitro evaluation, cytotoxicity studies show that the Met-enkephalin and Leu-enkephalin-loaded nanoparticles are only moderately cytotoxic at higher concentrations. Interestingly, it was observed that these fluorescent markers containing cancer cell-targeting peptide (RGD) conjugated nano-formulation(s) exhibited good uptake and internalization into cancer cells. These results indicate that the developed enkephalin-RGD-PLGA nanoparticles can serve as suitable targeted drug delivery vehicle/system for existing conventional drugs as well as increase the efficacy of drugs. In this regard, Cell-targeting peptides/ultra-short peptides are one of the most important choices available among the existing biomolecules for specific recognition of the desired target. cell-targeting peptides (RGD) are generally specific sequences that can bind to a wide range of targeted cancer cells with high affinity and specificity. It is worth mentioning here, the developed RGD conjugated nano-formulation may reduce the cost of discovery of new anti-cancer drugs and consequently reduce the economy burden, especially for developing countries like India. Moreover, such bio nano-formulation-based delivery systems can prove to be helpful in addressing the problem of multi-drug resistance (MDR). As the whole system is biocompatible in nature, since it constitutes PLGA (which is a biodegradable polymer), enkephalins (which are naturally occurring small peptides which do not evoke any immune response, but, in fact, have immunosuppressant and antiproliferative properties) and RGD (which is a small cancer marker binding peptide), this nanoformulation has the potential to be a suitable model for anticancer therapy alone or in conjunction with other drugs.
References
Mongi-Bragato B, Avalos MP, Guzmán AS et al (2018) Enkephalin as a pivotal player in neuroadaptations related to psychostimulant addiction. Front Psych. https://doi.org/10.3389/fpsyt.2018.00222
Xu H, Shi X, Li X et al (2020) Neurotransmitter and neuropeptide regulation of mast cell function: a systematic review. J Neuroinflammation 17:356–371. https://doi.org/10.1186/s12974-020-02029-3
Yeo XY, Cunliffe G, Ho RC et al (2022) Potentials of neuropeptides as therapeutic agents for neurological diseases. Biomedicines 10:343–370. https://doi.org/10.3390/biomedicines10020343
Neugebauer V, Mazzitelli M, Cragg B et al (2020) Amygdala, neuropeptides, and chronic pain-related affective behaviors. Neuropharmacology 170:108052–108115. https://doi.org/10.1016/j.neuropharm.2020.108052
Palmer CB, Meyrath M, Canals M et al (2022) Atypical opioid receptors: unconventional biology and therapeutic opportunities. Pharmacol Ther 233:108014–108029. https://doi.org/10.1016/j.pharmthera.2021.108014
Wang R, Zhang Y, Shan F (2019) Interaction of opioid growth factor (OGF) and opioid antagonist and their significance in cancer therapy. Int Immunopharmacol 75:105785. https://doi.org/10.1016/j.intimp.2019.105785
Wang X, Jiao X, Meng Y et al (2018) Methionine enkephalin (MENK) inhibits human gastric cancer through regulating tumor associated macrophages (TAMs) and PI3K/AKT/mTOR signaling pathway inside cancer cells. Int Immunopharmacol 65:312–322. https://doi.org/10.1016/j.intimp.2018.10.023
Huang H, Liu B, Qu N et al (2021) Research progress of opioid growth factor in immune-related diseases and cancer diseases. Int Immunopharmacol 99:107713–107723. https://doi.org/10.1016/j.intimp.2021.107713
Wang X, Tian J, Jiao X et al (2018) The novel mechanism of anticancer effect on gastric cancer through inducing G0/G1 cell cycle arrest and caspase-dependent apoptosis in vitro and in vivo by methionine enkephalin. Cancer Manag Res 10:4773–4787. https://doi.org/10.2147/CMAR.S178343
Wang D-M, Wang G-C, Yang J et al (2016) Inhibition of the growth of human melanoma cells by methionine enkephalin. Mol Med Rep 14:5521–5527. https://doi.org/10.3892/mmr.2016.5941
Bai X, Cao X, Qu N et al (2021) Methionine enkephalin activates autophagy and stimulates tumour cell immunogenicity in human cutaneous squamous cell carcinoma. Int Immunopharmacol 96:107733–107745. https://doi.org/10.1016/j.intimp.2021.107733
Lueptow LM, Fakira AK, Bobeck EN (2018) The contribution of the descending pain modulatory pathway in opioid tolerance. Front Neurosci. https://doi.org/10.3389/fnins.2018.00886
Kumar M, Pandey RS, Patra KC et al (2013) Evaluation of neuropeptide loaded trimethyl chitosan nanoparticles for nose to brain delivery. Int J Biol Macromol 61:189–195. https://doi.org/10.1016/j.ijbiomac.2013.06.041
Komatsu T, Katsuyama S, Mizoguchi H et al (2014) Spinal ERK2 activation through δ2-opioid receptors contributes to nociceptive behavior induced by intrathecal injection of leucine-enkephalin. Peptides (NY) 54:131–139. https://doi.org/10.1016/j.peptides.2014.01.014
Budka J, Kowalski S, Chylinska M et al (2021) Opioid growth factor and its derivatives as potential non-toxic multifunctional anticancer and analgesic compounds. Curr Med Chem 28:673–686. https://doi.org/10.2174/0929867327666200304122406
Shechter Y, Tsubery H, Mironchik M et al (2005) Reversible PEGylation of peptide YY 3–36 prolongs its inhibition of food intake in mice. FEBS Lett 579:2439–2444. https://doi.org/10.1016/j.febslet.2005.03.044
Shechter Y, Mironchik M, Saul A et al (2007) New technologies to prolong life-time of peptide and protein drugs in vivo. Int J Pept Res Ther 13:105–117. https://doi.org/10.1007/s10989-006-9052-1
Shechter Y, Heldman E, Sasson K et al (2010) Delivery of neuropeptides from the periphery to the brain: studies with enkephalin. ACS Chem Neurosci 1:399–406. https://doi.org/10.1021/cn100001j
Uchegbu IF, Carlos M, McKay C et al (2014) Chitosan amphiphiles provide new drug delivery opportunities. Polym Int 63:1145–1153. https://doi.org/10.1002/pi.4721
Malatesta M, Galimberti V, Cisterna B et al (2014) Chitosan nanoparticles are efficient carriers for delivering biodegradable drugs to neuronal cells. Histochem Cell Biol 141:551–558. https://doi.org/10.1007/s00418-013-1175-9
Kim GC, Cheon DH, Lee Y (2021) Challenge to overcome current limitations of cell-penetrating peptides. Biochim Biophys Acta (BBA) Proteins Proteom 1869:140604–140624. https://doi.org/10.1016/j.bbapap.2021.140604
Jafari B, Pourseif MM, Barar J et al (2019) Peptide-mediated drug delivery across the blood-brain barrier for targeting brain tumors. Expert Opin Drug Deliv 16:583–605. https://doi.org/10.1080/17425247.2019.1614911
Newman MR, Benoit DSW (2018) In vivo translation of peptide-targeted drug delivery systems discovered by phage display. Bioconjug Chem 29:2161–2169. https://doi.org/10.1021/acs.bioconjchem.8b00285
Hoppenz P, Els-Heindl S, Beck-Sickinger AG (2020) Peptide-drug conjugates and their targets in advanced cancer therapies. Front Chem. https://doi.org/10.3389/fchem.2020.00571
Islam Y, Leach AG, Smith J et al (2020) Peptide based drug delivery systems to the brain. Nano Express 1:12002–12026. https://doi.org/10.1088/2632-959X/ab9008
Szweda R, Trzebicka B, Dworak A et al (2016) Smart Polymeric Nanocarriers of Met-enkephalin. Biomacromolecules 17:2691–2700. https://doi.org/10.1021/acs.biomac.6b00725
Ansari R, Sadati SM, Mozafari N et al (2020) Carbohydrate polymer-based nanoparticle application in drug delivery for CNS-related disorders. Eur Polym J 128:109607–109621. https://doi.org/10.1016/j.eurpolymj.2020.109607
Godfrey L, Iannitelli A, Garrett NL et al (2018) Nanoparticulate peptide delivery exclusively to the brain produces tolerance free analgesia. J Control Release 270:135–144. https://doi.org/10.1016/j.jconrel.2017.11.041
Zhang L, Li G, Gao M et al (2016) RGD-peptide conjugated inulin-ibuprofen nanoparticles for targeted delivery of Epirubicin. Colloids Surf B 144:81–89. https://doi.org/10.1016/j.colsurfb.2016.03.077
Fotakis G, Timbrell JA (2006) In vitro cytotoxicity assays: comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol Lett 160:171–177. https://doi.org/10.1016/j.toxlet.2005.07.001
Liang H, Jin C, Tang Y et al (2014) Cytotoxicity of silica nanoparticles on HaCaT cells. J Appl Toxicol 34:367–372. https://doi.org/10.1002/jat.2953
Sapsford KE, Tyner KM, Dair BJ et al (2011) Analyzing nanomaterial bioconjugates: a review of current and emerging purification and characterization techniques. Anal Chem 83:4453–4488. https://doi.org/10.1021/ac200853a
Chaganti LK, Venkatakrishnan N, Bose K (2018) An efficient method for FITC labelling of proteins using tandem affinity purification. Biosci Rep. https://doi.org/10.1042/BSR20181764
Surugau N, Urban PL (2009) Electrophoretic methods for separation of nanoparticles. J Sep Sci 32:1889–1906. https://doi.org/10.1002/jssc.200900071
Pyell U (2010) Characterization of nanoparticles by capillary electromigration separation techniques. Electrophoresis 31:814–831. https://doi.org/10.1002/elps.200900555
Danhier F, Ansorena E, Silva JM et al (2012) PLGA-based nanoparticles: an overview of biomedical applications. J Control Release 161:505–522. https://doi.org/10.1016/j.jconrel.2012.01.043
Gu J-H, Ge J-B, Li M et al (2013) Poloxamer 188 protects neurons against ischemia/reperfusion injury through preserving integrity of cell membranes and blood brain barrier. PLoS ONE 8:e61641–61652. https://doi.org/10.1371/journal.pone.0061641
Javed R, Zia M, Naz S et al (2020) Role of capping agents in the application of nanoparticles in biomedicine and environmental remediation: recent trends and future prospects. J Nanobiotechnol 18:172–187. https://doi.org/10.1186/s12951-020-00704-4
Acknowledgements
Sarabjit Kaur thanks Council of Scientific and Industrial Research (CSIR), India, for her senior research fellowship. NishimaWangoo acknowledges funding from Council of Scientific and Industrial Research (CSIR), New Delhi, India, via grant no. 02(0302)/17/EMR-II.
Author information
Authors and Affiliations
Contributions
S.K. designed and carried out the synthesis, functionalization and characterization studies. S.K.P. carried out the biological activity studies including cell viability and in-vitro cellular uptake studies. D.S. assisted with the experimental work and manuscript editing. R.K.S. designed the experiments and assisted in data analysis and drafting of the manuscript. N.W. designed, supervised and funded the project overall, along with the idea conception with S.K. and supervision in experiments and drafting of the manuscript. All the authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interests
There are no conflicts to declare.
Additional information
Handling Editor: Annela M. Seddon.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaur, S., Pandey, S.K., Sharma, D. et al. Enkephalin loaded and RGD decorated PLGA–poloxamer nanoparticles for effective targeting in cancer cells. J Mater Sci 57, 17416–17432 (2022). https://doi.org/10.1007/s10853-022-07691-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-022-07691-x