Abstract
Various proportions of sepiolite clay (1, 3, and 6 wt%) were blended with sulfonated polyether sulfone octyl sulfonamide (SPESOS) to improve the proton conductivity of SPESOS. Fourier-transform infrared spectroscopy, X-ray diffraction (XRD), and thermo-gravimetric analysis (TGA) were used to evaluate the structural functionalities, morphologies, and thermal stability of the composite membranes. FT-IR spectra indicated that no chemical reactions take place between the SPESOS and the clay. A lower degree of crystallinity in the SPESOS composite than that in pristine SPESOS was observed by XRD diffractograms. As observed in TGA, the elaborated membranes promoted the absorption of water due to the existence of sepiolite in the SPESOS. The presence of sepiolite nanoarchitecture material within SPESOS was found to have higher water retention, contact angle, and proton conductivity values than pristine SPESOS. These results reveal that the composite membranes display good hydrophilic character and higher ion exchange capacity than pristine SPESOS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Considerable research has been done in the field of ion-exchange membranes and their utilization in proton exchange membrane fuel cells (PEMFC). Aromatic polymers are exemplary for such applications since they bring together high thermal stability and easy handling. The aromatic ring is generally a favorable site for alteration, leading to the synthesis of polymers with better properties, for example, poly aryl ether ketone (PEEK), polybenzimidazole (PBI), and polyether sulfone (PES) [1,2,3,4]. PES is a low-cost polymer, which is regularly used in stage reversal manufacturing of polymer films for microfiltration, ultrafiltration, and gas separation, considering its superior mechanical strength and chemical stability [5]. However, PES is quite hydrophobic, and membrane fouling is one of the main disadvantages of the PES layer [6]. Chemical alteration of PES is utilized as a major approach to improve the hydrophilicity and anti-fouling property of the PES membrane. Sulfonation reaction is one of the chemical conversion approaches used to incorporate the negatively charged sulfonic groups to the PES membrane [7]. Specifically, sulfonated polyether sulfone (SPES) has exhibited numerous improvements to be used as a solid electrolyte for PEMFC [8]. Investigations have likewise been done in the use of PES in direct methanol fuel cells (DMFC). Synthesis and characterization of polymer composites of PES and either sulfonated polyether ketone or sulfonated poly sulfone have been investigated [9]. SPES contains desirable properties that fulfill all conditions required for its use in an ideal PEMFC. SPES has certain disadvantages due to the mechanical properties of PES. Different ratios of octylamine being incorporated into SPES backbone results in different types of SPESOS [10]. SPESOS is water-insoluble; hence, membranes are weak and exhibit a fluid variety of ionic conductivity and water uptake relative to the quantity of grafted octylamine. Numerous methods have been suggested to improve ionic conductivity and the mechanical properties of the membranes.
Polymer/clay composites are innovative materials with enhanced physical and mechanical properties when used as reinforcement in well-ordered polymers or conventional micro- and macro-composite polymers. The desirable characteristics of polymer composites include good elastic modulus, barrier property, flame retardant property, and durability [11]. Sepiolite is a natural fibrous mineral clay with a molecular formula of Si12O30Mg8(OH)4(H2O)4 8H2O. The structure of sepiolite structure comprises blocks of two tetrahedral silica sheets sandwiching an octahedral sheet of magnesium oxide hydroxide. The blocks form ribbons that are connected, producing an open channel similar to that of zeolites. This ribbon structure with interior channels permits a partial dispersion of organic and inorganic cations. The outermost sheet of silica is broken, leading to a number of silanol (Si–OH) groups being available at the outer layer of the sepiolite [12]. The presence of sepiolite in the polyamide matrix was found to improve the elastic modulus and heat deflection temperature in the composite [12]. The incorporation of sepiolite clay nanomaterial within sulfonated poly (ether ether ketone) (SPEEK) showed the highest proton conductivity value at high temperatures compared to pristine SPEEK membranes [13]. Thus, the presence of sepiolite clay leads to recovery of hydrophilicity and works as a connector to the ionic clusters in SPEEK, mainly due to homogeneous dispersion and formation of linkages with SPEEK [13]. In this perspective, the purpose of the present work was to synthesize a new kind of composite with SPESOS matrix incorporating sepiolite clay to enhance water retention and proton conductivity in the membrane. Sepiolite/SPESOS composite membrane is prepared with various ratios of sepiolite clay loading and characterized on the basis of their structure and morphology. Furthermore, the thermal stability, water retention, and proton conductivity of sepiolite/SPESOS composite will be analyzed to obtain an electrically conducting polymer composite that could be utilized in electrochemical sensors, thermal conductors, and related applications.
Experimental
Resources
Sulfonated polyether sulfone octyl sulfonamide SPESOS was synthesized in Eras Labo by the authors1. N, N’-dimethylacetamide (DMAc) was obtained from Acros, Sulfuric acid was obtained from Scharlau, and Sodium hydroxide was purchased from Laurylab.
Membrane preparation
Sulfonated polyether sulfone octyl sulfonamide (SPESOS) was synthesized according to the previously reported procedure [10]. The synthesized SPESOS with a DS of one proton per monomer unit (~ CEI = 2 meq/g) was used for the preparation of the sepiolite/SPESPS composite membrane [10]. A casting solution containing SPESOS (10 wt%) dispersed in DMAc (10 mL) was prepared by stirring for 15 min. A homogeneous transparent solution was obtained. The sepiolite clay, in different weight percentages (1, 3 and 6 wt%), was added to the 10 wt% SPESOS solution and the composite membranes were prepared by casting evaporation method. The synthesized membranes were dried at 80 °C. The thickness of all the membranes was about 100 µm.
Membrane characterizations
The different proton membranes (area = 5 × 5 cm2) were immersed separately in a sodium hydroxide solution (10–2 M) for 48 h [14]. The ion exchange capacity was determined by an acid–base assay of the sodium hydroxide solution used to neutralize the membrane. The ion exchange capacity was determined by the relationship:
where niNaOH is the initial number of moles of sodium hydroxide solution (10–2 M, 200 mL), nfNaOH is the number of moles of sodium hydroxide after the exchange, and Wdry is the dry mass of the membrane.
FT-IR data were collected using a Nicolet spectrophotometer (IR200 FT-IR) in transmission mode. The spectra of SPESOS and the different composite membranes were measured in the range 400–4000 cm–1. The automated Bruker D8 advance X-Ray spectrophotometer was used for recording X-Ray diffractograms at 2θ values between 5 and 60 degrees. The dry membrane samples were analyzed without any further treatment.
Thermogravimetric analysis (TGA) was performed using a Mettle TG analyzer. Samples weighing 2.5 mg were was vacuum dried at 100 °C for 24 h to remove residual solvent. The TGA curves were recorded by varying the temperature from room temperature to 900 °C, a sweep rate of 10 °C/min with a flow of argon gas at the rate of 40 mL/min.
Measurement of contact angle (CA) was performed with a Theta Optical Tensiometer (Attention) for the different membrane samples (area = 1.5 × 1.5 cm). A droplet of distilled water of five microliters was immobilized on the surface of the membrane with the help of a micro-syringe. A source of light was placed behind the sample before recording a photograph of the drop of water with a camera. The contact angle was calculated using computer software (Theta Attention) from the moment when no significant change in the surface was observed [15].
To determine the water uptake (WU) of the various membranes, the membranes were dried in an oven at 80 °C until constant masses were obtained. The membranes were immersed separately in distilled water for two days at room temperature. The water droplets adsorbed on the surface of the membranes were removed with the help of a hydrophilic paper. The difference in mass before and after the complete drying of the membrane was noted. From these values, the extent of water uptake was calculated, [16] as the relative weight increase per gram of the dry sample using the following Eq. (2):
where Wwet and Wdry are the weights of wet and dry membranes, respectively.
The cationic transport number was determined using Hittorf Cell. The cell consists of two symmetrical glass compartments separated by a membrane (4.15 cm2), as described earlier [17, 18]. The proton transport number was calculated as described in the earlier work [3, 10]. The cell was filled with 1 M aqueous H2SO4. The measurements were made for 188 min maintaining a current intensity of 100 mA. The amount of proton transfer is close to 10 mmol, considering the initial proton content in each compartment is 100 mmol.
The ionic conductivity of membranes was evaluated by electrochemical impedance spectroscopy, using a Biologic Science Instruments VSP potentiostat. The observations were made at different temperatures at a relative humidity of 0% and 100%. The disk-shaped membrane samples were placed between two platinum-plated titanium electrodes. The relative humidity was controlled using a sealed stainless-steel cell consisting of two cylindrical compartments (hot and cold) maintained at different temperatures. The relative humidity value was calculated from the ratio between the saturated vapor pressure of water at each temperature. High-frequency Nyquist patterns were recorded with frequency response analysis, sweeping the frequency between 1000 and 10 Hz with an oscillating voltage amplitude of 10 mV. Electrical resistance (R) was measured from the intercept of the Nyquist plot at high frequency with the real impedance axis [19, 20]. Ionic conductivity (σ, S/cm) was calculated using the following relation (3):
where e and R denote the thickness and the electrical resistance, respectively, and S is the surface area of the membrane placed between the two electrodes.
Results and discussion
Fourier transform infrared spectroscopy
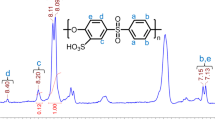
All the composite membranes synthesized were dense, transparent, and light yellow, which demonstrated the homogenization of organic and inorganic components. Figure 1 shows FT-IR of sulfonated polyether sulfone octyl sulfonamide (SPESOS), with a degree of sulfonation of one proton per monomer unit and the synthesized composites.
The spectra of the different composite membranes are quite similar, owing to the minimal content of clay in the composites. The presence of aromatic carbons can be detected from the FT-IR spectrum in the form of two intense bands at 1472 and 1578 cm–1. The methyl group of SPESOS can be confirmed from the band that appears at 1248 cm–1. The peak at 1150 cm–1 is due to the presence of the C–O–C group. The characteristic bands at 1090 and 1025 cm–1 were assigned to the symmetrical and asymmetrical stretching vibrations of the O=S=O group. Furthermore, the bands at 834 and 685 cm–1 are due to the vibrations of S–O and C–S groups, indicating the presence of the sulfonic group in composite membranes. The bands around 3400 and 2931 cm–1 denote the O–H vibration in the SPESOS and the various composite membranes. In addition, no considerable difference was noted between the FTIR spectra of the composite membranes and the reference membrane. This indicates that there is no chemical interaction between the SPESOS and the clay.
X-ray diffractograms
Figure 2 describes the diffractograms of clay and SPESOS composite membranes with different percentages of clay. The SPESOS membrane showed a significant difference in the major peak of SPESOS at 2θ at 13.4°.
The incorporation of clay resulted in poor macromolecular orientations of the polymer chains, shifting the major peak of SPESOS composite to a higher 2θ values. In SPESOS composites containing 1 wt% of clay, the shift is more significant compared with pure membrane. The intensity of the peak of sepiolite is very low, but in composites containing 3 and 6 wt% ratios of clay, it is more intense. But there is no shift in the position of the peak in the composite membrane. It can be concluded that the interaction with clay is more significant in the composite containing 1 wt% of clay. With the increase in the percentage of clay, the interaction is less in comparison. There is also a noticeable difference in the size of crystallite with different amounts of clay added. The crystallite size decreases with the sequential addition of clay in the composites, as illustrated in Table 1. There is a marked loss of crystallinity due to the introduction of phyllosilicate clay in the SPESOS matrix. The fibrous nature of sepiolite leads to the creation of oriented aggregates onto SPESOS planes that diminish the intensity of its major peak.
Thermo gravimetric analysis
Thermal stability studies of SPESOS and the composite membranes using TGA are reported in Fig. 3.
All membranes display three stages of weight loss. The first weight loss, around 100 °C, is associated with the loss of water from the matrix of SPESOS. The second significant loss in mass occurs at around 260 °C. This may be attributed to the deterioration of the sulfonic groups. The final loss in mass occurs at around 480 °C, which is due to the decomposition of the main matrix of the polymer. A comparison between the TGA of SPESOS and all SPESOS composite membranes shows no major differences in decomposition characteristics in the entire range of temperatures studied. The TGA curves of the composite membranes are shifted toward low temperatures as a function of the rate of sepiolite added to SPESOS. An in-depth analysis of TGA curves between room temperature and 250 °C showed the higher tendency of the composite membranes to trap water than the SPESOS membrane.
However, the presence of 6 wt%. clay has the greatest water loss at 150 °C of around 20%, while the 1 wt%. and 3 wt%. clay have a loss of 12% at the same temperature. On the other hand, the unmodified polymer has a water loss of 8% at 150 °C. This proves that the addition of sepiolite to SPESOS polymer promotes the absorption of water. This result was confirmed by measurements of the water uptake.
Contact angle
The drop shape analysis method has been applied to establish the surface properties of SPESOS that change during the addition of clay (Table 2).
Analysis of the contact angles of different membranes helps compare the super wettability of the SPESOS and the composites. The contact angle of the unmodified membrane (SPESOS) is 84.4°. It decreases to 81.7°, 81.2°, and 73.0° in the composites containing 1 wt%, 3 wt% and 6 wt% clay, respectively (Table 2). A decrease in the contact angle suggests an increase in hydrophilicity attributed to the incorporation of clay. High contact angles indicate that flat sheet membranes show excellent hydrophilic character. Contact angle measurements show the super wettability of composite membranes with respect to materials’ surfaces.
Commonly, the hydrophilicity of the membrane is related to hydrogen bond interactions between sulfonic functional groups and water molecules. The results indicate that the composite membrane will show good water uptake and ionic conductivity.
Water uptake
Table 2 illustrates the increase in uptake of water in SPESOS and the hybrid membranes as a function of the amount of sepiolite. It can be seen clearly that composite membranes tend to absorb more water than unmodified SPESOS (1.88 meq/g) membranes. These results can be explained by the hydrophilic characters of the sepiolite that play the main role in increasing the water uptake due to the presence of sulfonic groups in SPESOS polymer. Percentages of water uptake, presented in Fig. 5 for SPESOS, is 31%, whereas it is 57%, 66%, and 91% for the presence of 1 wt%, 3 wt%, and 6 wt % clay, respectively, in the composites. In general, the water uptake of composites membranes is 2–3 times more than that of SPESOS membrane owing to the amount of clay added. The water uptake rate is related to the distance between aggregates of SPESOS chains and to the ion exchange capacity that plays a significant role in electrochemical behavior. The addition of sepiolite may influence SPESOS microstructure by creating clusters as well as changing SPESOS dimension sizes. An increase in water uptake rate can be ascribed to an increase in the volume of aggregates and solvated protons circulation channels as a consequence of the addition of sepiolite in the SPESOS matrix.
Ionic exchange capacity
The results of ionic exchange capacity studies are displayed in Table 2. It can be noted that the minimum ion exchange capacity is 1.88 meq/g for SPESOS. The addition of sepiolite generates a slight augmentation of IEC values in the composites. This trend is because of the increase in the water uptake, which was confirmed by the TGA and contact angle results.
All obtained results are close to theoretical IEC values (2 meq/g), where the 6 wt% sepiolite/SPESOS membrane has an ion exchange capacity equal to 1.97 meq/g.
Proton transport number
Proton transfer number value was evaluated for all composite membranes and pristine SPESOS with a surface area equal to 4.15 cm2 by applying a current intensity at 100 mA and using an aqueous solution of sulfuric acid (Table 3) [10].
A higher transport number is closer to 1 for all composite membranes, which show a better selectivity to counter-ions (protons). Its values are dependent on the properties of the membrane, specifically its IEC, water uptake, selectivity, and thickness. The transport number for the composite membranes by Hittorf’s method is close to unity. This could be due to the higher ion conductivity in these membranes. These results also indicate that the composite membranes have a higher affinity toward cations (Table 3).
Proton conductivity
Figures 4 and 5 represent the variation in proton conductivity with temperature increase for the different composite membranes at a relative humidity of 0 and 100%. At 0% humidity (Fig. 4), the change in proton conductivity values with a temperature of SPESOS membrane is lower than that of composite membranes.
There is no significant difference in the conductivity among the composite membranes with different ratios of clay at the ambient temperature. This trend continues till a temperature of 70 °C. Beyond this temperature, there is a drastic increase in the conductivity and reaches 16 mS/cm at 120 °C for 1 wt% clay/SPESOS composite. The composite with an increase in clay ratio from 3 to 6 wt% shows lower conductivity than that of composite with clay ratio 1 wt.%. These results can be dependent on the system, pretreatment, synthesis, and equilibrium at temperatures above 70 °C. At 100% relative humidity (Fig. 5), the composite membranes show better results in terms of proton conductivity over the entire temperature range. In particular, the proton conductivity in the 1 wt% clay/SPESOS composite membrane reaches 450 mS/cm at 120 °C.
These results clearly reveal that the incorporation of sepiolite improves proton conductivity. The existence of sepiolite stimulates molecular water absorption and the preference for proton conduction pathways [21]. Their enhanced stability could be due to the ability of the membranes to retain water owing to the presence of sulfonated groups in SPESOS. The existence of 1 wt% sepiolite permits the sulfonic groups in the membrane to retain water and facilitate the proton transport, which reaches a value of 262 mS/cm at 100 °C. This value is comparable to other membranes such as Nafion or SPEEK, where the conductivity is about 130 and 148 mS/cm in the presence of clay [22, 23]. The further addition of clay (> 1 wt% of clay) decreases the conductivity. This could be because of the agglomeration of clay which blocks the transport and conduction of protons through the membrane. The Arrhenius equation gives the correlation between temperature and proton conductivity. The activation energy of conductivity can be calculated using the following Eq. (4):
where σ is the proton conductivity (S/cm), σ0 is the pre-exponential factor, R is the universal gas constant (8.314 J/mol. K), and T is the absolute temperature (K). Table 4 summarizes the values of the activation energies of SPESOS and its composite membranes.
The activation energies of the SPESOS and composite membranes mentioned in Table 4 were determined in experiments carried out at 100% RH. The composite membranes show lower values of activation energy than the ones prepared with unmodified SPESOS. Clearly, the incorporation of the filler influences the Ea, where the lowest value was obtained for the composite with 1% of added clay. This further collaborates with the results obtained for higher proton conductivity.
Conclusions
Sepiolite clay/SPESOS composites were successfully prepared and characterized. The properties of the composite membranes have been dramatically improved by the inclusion of clay onto SPESOS. FTIR spectra confirm that no chemical interaction between the SPESOS and the clay. X-ray diffractograms validate that the interaction with clay is more significant in the composite containing 1 wt% of clay. The water uptake of the composite membranes increased with increasing clay content owing to increase in hydrophilicity.
However, the proton conductivity of the 1 wt%. sepiolite/SPESOS composite membrane increased (437 mS/cm at 120 °C) when compared to the SPESOS membrane (47 mS/cm). These findings indicated that the sepiolite clay/SPESOS composite membranes, which possess excellent chemical and physical properties, show promise for their use as PEMs.
References
Chen G, Zhang H, Cheng J, Ma Y, Zhong H (2008) A novel membrane electrode assembly for improving the efficiency of the unitized regenerative fuel cell. Electrochem Commun 10:1373–1376. https://doi.org/10.1016/j.elecom.2008.07.002
Maier G, Meier-Haack J (2008) Sulfonated aromatic polymers for fuel cell membranes. Adv Polym Sci 216:1–62. https://doi.org/10.1007/12_2008_135
Mabrouk W, Ogier L, Vidal S, Sollogoub C, Matoussi F, Dachraoui M, Fauvarque JF (2012) Synthesis and characterization of polymer blends of sulfonated polyethersulfone and sulfonated polyethersulfone octylsulfonamide for PEMFC applications. Fuel Cells 2:179–187. https://doi.org/10.1002/fuce.201100051
Ahmed Z, Charradi K, Alsulami QA, Keshk SMAS, Chtourou R (2021) Physicochemical characterization of low sulfonated polyether ether ketone/Smectite clay composite for proton exchange membrane fuel cells. J Appl Polym Sci 138:49634–49642. https://doi.org/10.1002/app.49634
Rastegarpanah A, Mortaheb HR (2016) Surface treatment of polyethersulfone membranes for applying in desalination by direct contact membrane distillation. Desalination 377:99–107. https://doi.org/10.1016/j.desal.2015.09.008
Ahmad AL, Abdulkarim AA, Ooi BS, Ismail S (2013) Recent development in additives modifications of polyethersulfone membrane for flux enhancement. Chem Eng J 223:246–267. https://doi.org/10.1016/j.cej.2013.02.130
Zhang Y, Bruggen BVD, Pinoy L, Meesschaert B (2009) Separation of nutrient ions and organic compounds from salts in RO concentrates by standard and monovalent selective ion-exchange membranes used in electrodialysis. J Membr Sci 332:104–112. https://doi.org/10.1016/j.memsci.2009.01.030
Sanchez JY, Chabert F, Iojoiu C, Salomon J, El Kissi N, Piffard Y, Marechal M, Galiano H, Mercier R (2007) Extrusion: an environmentally friendly process for PEMFC membrane elaboration. Electrochim Acta 53:1584–1595. https://doi.org/10.1016/j.electacta.2007.04.022
Lau WJ, Ismail AF (2009) Effect of SPEEK content on the morphological and electrical properties of PES/SPEEK blend nanofiltration membranes. Desalination 249:996–1005. https://doi.org/10.1016/j.desal.2009.09.016
Mabrouk W, Ogier L, Matoussi F, Sollogoub C, Vidal S, Dachraoui M, Fauvarque JF (2011) Preparation of new proton exchange membranes using sulfonated poly(ethersulfone) modified by octylamine (SPESOS). Mater Chem Phys 128:456–463. https://doi.org/10.1016/j.matchemphys.2011.03.031
Paul DR, Robeson LM (2008) Polymer nanotechnology: nanocomposites. Polym J 49:3187–3204. https://doi.org/10.1016/j.polymer.2008.04.017
García-López D, Fernández JF, Merino JC, Santarén J, Pastor JM (2010) Effect of organic modification of sepiolite for PA6 polymer/organoclay nanocomposites. Compos Sci Technol 70:1429–1436. https://doi.org/10.1016/j.compscitech.2010.05.020
Charradi K, Ahmed Z, Thmaini N, Aranda P, Al-Ghamdi YO, Ocon P, Keshk SMAS, Chtourou R (2021) Incorporation of layered double hydroxide/sepiolite to improve the performance of sulfonated poly(ether ether ketone) composite membranes for proton exchange membrane fuel cells. J Appl Polym Sci 138:e50364. https://doi.org/10.1002/app.50364
Mabrouk W, Ogier L, Vidal S, Sollogoub C, Matoussi F, Fauvarque JF (2014) Ion exchange membranes based upon crosslinked polyethersulfone for electrochemical applications. J Membr Sci 452:263–270. https://doi.org/10.1016/j.memsci.2013.10.006
Ismail MF, Islam MA, Khorshidi B, Sadrzadeh M (2021) Prediction of surface charge properties on the basis of contact angle titration models. Mater Chem Phys 258:123933. https://doi.org/10.1016/j.matchemphys.2020.123933
Li YS, Zhao TS, Yang WW (2010) Measurements of water uptake and transport properties in anion-exchange membranes. Int J Hydrog Energy 35:5656–5665. https://doi.org/10.1016/j.ijhydene.2010.03.026
Agel E, Bouet J, Fauvarque JF (2001) Characterization and use of anionic membranes for alkaline fuel cells. J Power Sources 101:267–274. https://doi.org/10.1016/S0378-7753(01)00759-5
Barros KS, Marti-Calatayud MC, Scarazzato T, Bernardes AM, Espinosa DCR, Pérez-Herranz V (2021) Investigation of ion-exchange membranes by means of chronopotentiometry: a comprehensive review on this highly informative and multipurpose technique. Adv Colloid Interfac 293:102439. https://doi.org/10.1016/j.cis.2021.102439
Mabrouk W, Lafi R, Charradi K, Ogier L, Hafiane A, Fauvarque JF, Sollogoub C (2020) Synthesis and characterization of new proton exchange membrane deriving from sulfonated polyether sulfone using ionic crosslinking for electrodialysis applications. Polym Eng Sci 60:3149–3158. https://doi.org/10.1002/pen.25543
Mabrouk W, Lafi R, Fauvarque JF, Hafiane A, Sollogoub C (2021) New ion exchange membrane derived from sulfochlorated polyether sulfone for electrodialysis desalination of brackish water. Polym Advan Technol 32:304–314. https://doi.org/10.1002/pat.5086
Beauger C, Lainé G, Burr A, Taguet A, Otazaghine B, Rigacci A (2013) Nafion®-sepiolite composite membranes for improved proton exchange membrane fuel cell performance. J Membr Sci 430:167–179. https://doi.org/10.1016/j.memsci.2012.11.037
Charradi K, Ahmed Z, Escudero R, Aranda P, Ruiz-Hitzky E, Ocon P, Chtourou R (2019) Amelioration of PEMFC performance at high temperature by incorporation of nanofiller (sepiolite/layered double hydroxide) in Nafion membrane. Int J Hydrog Energy 44:10666–10676. https://doi.org/10.1016/j.ijhydene.2019.02.183
Charradi K, Ahmed Z, Aranda P, Chtourou R (2019) Silica/montmorillonite nanoarchitectures and layered double hydroxide-SPEEK based composite membranes for fuel cells applications. Appl Clay Sci 174:77–85. https://doi.org/10.1016/j.clay.2019.03.027
Acknowledgements
The authors would like to thank Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R185), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Yaroslava Yingling.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mabrouk, W., Charradi, K., Lafi, R. et al. Augmentation in proton conductivity of sulfonated polyether sulfone octyl sulfonamide using sepiolite clay. J Mater Sci 57, 15331–15339 (2022). https://doi.org/10.1007/s10853-022-07627-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-022-07627-5