Abstract
Layered double hydroxide (LDH) films have attracted extensive attention in Mg alloy anti-corrosion due to their unique physical barrier function and ion exchange performance. However, most of the LDH films on Mg alloy have problems of harsh preparation conditions, poor anti-corrosion stability, and poor adhesion. In this paper, a typical Li–Al-alanine (Ala) LDH film has been successfully prepared on AZ91D Mg alloy surface under milder conditions using multi-arc ion plating and in-situ dipping for the first time. Then an improved vacuum impregnation further modified the Li–Al-Ala LDH film in an ethanol solution of stearic acid (SA) to improve its protective stability. The potentiodynamic polarization curves and electrochemical impedance spectra (EIS) showed that the Li–Al-Ala LDH/SA film's corrosion resistance is more than three orders of magnitude higher than that of the blank Mg alloy in 3.5 wt% NaCl, showing a highly increased corrosion resistance. The long-term immersion experiments found that the surface structure and corrosion resistance of the Li–Al-Ala LDH/SA film did not change much after immersion in 3.5 wt% NaCl for 432 h. The bonding force between the film and the Mg alloy substrate can reach grade 0 based on the standard from International Standardization Organization (ISO). And the hydrophobicity of the film can remain stable with the water contact angle (WCA) being steady at about 140° after a certain distance of external abrasion, showing excellent mechanical wear resistance. The study provides a new milder approach to fabricating superhydrophobic LDH films with durable anti-corrosion resistance, good adhesion, and mechanical stability on Mg alloy surface.
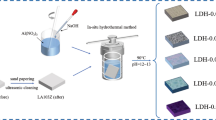
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mg alloy has been widely used due to its high specific strength, low density, and good castability [1]. However, the high chemical activity of Mg alloy leads to its poor corrosion resistance, which limits some of its applications [2]. At present, various coatings, such as chemical conversion coating [3], anodized coating [4], micro-arc oxidation coating [5], laser treatment films [6], and layered double hydroxide (LDH) films [7,8,9], have been prepared on Mg alloy surfaces to improve their corrosion resistance. LDH films have received extensive attention due to their excellent physical barrier effect and unique interlayer ion exchange function.
LDH is a general term for hydrotalcite and hydrotalcite-like compounds, which can be expressed by the general formula [M1-x2+ Mx3+ (OH)2]x+ (An−)x/n m H2O. In the formula, M2+ and M3+ represent the cation occupying the octahedral pores in the layered layer. An− represents the interlayer charge compensation anion, n is the charge of the intercalation anion, m is the number of water molecules, and X represents the molar ratio of M3+/(M2+ + M3+), with the range being generally between 0.20 and 0.33 [8, 9]. LDH films generally consist of a cationic layer composed of divalent M(II) or trivalent M(III) cations and an anionic layer comprised of organic or inorganic anions, where the anionic and cationic layers are charge-balanced. Thus, the overall LDH structure is electrically neutral [7]. However, recent studies showed that the cationic layer of LDH can not only be composed of divalent or trivalent metal cations. Other valence metal cations also can form the cationic layer of LDH, such as Li+, Ti4+, Zr4+, and Sn4+ [10,11,12,13]. It also pointed out that LDH's specific ion exchange capacity can chemically modify the anions between the layers of LDH, leading to the anion layer containing more than one kind of anions [14, 15]. The common interlayer anion exchange order of LDH is PO43− > CO32− > SO42− > OH− > F− > Cl− > Br− > NO3− > I−, generally speaking, high-valent anions are easier to exchange for low-valent anions [16, 17]. The unique interlayer anion exchangeability of LDH enables it to capture corrosive anions (such as Cl−), thereby effectively preventing corrosive anions from attacking the base metal. In addition, the good physical barrier effect of LDH can further block corrosive media from contacting metal substrates, thereby effectively improving the corrosion protection performance of the base metal [18].
Currently, a large number of studies have used co-precipitation [19, 20], hydrothermal treatment [21, 22], and electrochemical deposition [23, 24] to prepare LDH films on Mg alloy surfaces. Gu and his co-workers [19] synthesized an aluminum silicate-modified Ni–Al-LDH film on the surface of Mg alloy by co-precipitation. Still, some studies pointed out that LDH film obtained by this method has poor adhesion to the base Mg alloy [8, 9]. Li et al. [21] synthesized the Mg–Al-LDH film by hydrothermal method, and the obtained film had a good bonding force with the substrate, achieving a more substantial protective effect. However, the preparation of LDH films by hydrothermal methods requires high temperature and a high-pressure environment [21, 22]. Also, the reaction time is too long, resulting in excessive energy consumption in the preparation process. Wu et al. [23] prepared a Zn–Al-LDH film on the surface of Mg alloy by electrochemical deposition in a relatively short time. The obtained LDH film has good corrosion protection and adhesion to the substrate. However, the electrodeposition process involves both chemical and electrochemical processes, resulting in the properties of the obtained LDH films being affected by the deposition current/potential, the concentration of the deposition solution, and the temperature [23, 24]. The complex deposition process means that the preparation of LDH films by electrodeposition consumes not only electrical energy but also has many influencing factors in the preparation process, which is difficult to control. Therefore, finding a more environmentally friendly and convenient method to prepare LDH films with good adhesion and corrosion resistance on the surface of Mg alloys is a research hotspot in the field of Mg alloy anti-corrosion.
The vast majority of LDH films prepared are formed based on the isomorphous replacement of M2+ in the cationic layer by M3+ and then covalent bonding with hydroxyl groups. Therefore, the preparation conditions of most LDH films with excellent corrosion resistance are relatively harsh, and the energy consumption is high [21, 22]. Li–Al LDH, which can be expressed as [LiAl2(OH)6] (An−)1/n · m H2O, is formed by the insertion of metal ions into lattice holes [25]. Because of its unique film-forming mechanism, Li–Al LDH film can be prepared by immersing Al alloy in LiNO3 solution under normal pressure, medium temperature, and alkaline environment in a short time, and the obtained film always shows good corrosion resistance and binding force [26]. Yan et al. [27] found that when Li+ and most of the metal ions (except Al3+) constitute the host layer of LDH, Li+ causes the smallest structural unit (MO6 octahedron) of LDH to be severely distorted, thus making it impossible for Mg and Li to form LDH films under the milder conditions described above. Therefore, if introducing Al into the surface of Mg alloy, and then the LDH film on Mg alloy can be prepared by using the Al layer as the intermediate layer, the LDH films' preparation conditions on Mg alloy can be much milder. Also, the Mg alloy covered with the obtained film would have good corrosion resistance.
Corrosion durability is an important index to evaluate the corrosion resistance of the films to the substrate. The LDH film contains many hydrophilic hydroxyl groups, so it is difficult for the hydrophilic LDH film to maintain its high protective durability in aqueous solutions containing aggressive anions (e. g., Cl−) [28]. Some studies [29, 30] have used fluorine-containing compounds or long-chain saturated fatty acids to modify LDH films with low surface energy to prepare superhydrophobic LDH films. It is attempted to prevent the contact between the film and water and the penetration of corrosive media through the superhydrophobicity of the film, thereby further improving the protective durability of the LDH film [28,29,30]. However, most current methods for preparing superhydrophobic films are to directly immerse the samples in the precursor [28,29,30,31]. At this time, the low surface energy compounds can only accumulate on the surface of the film layer. They cannot penetrate the film layer or disperse uniformly on the micro-nano structure of the film layer, which leads to a loss in superhydrophobicity once the LDH film is slightly worn [31, 32]. Our previous study [33] found that the modified vacuum impregnation method can thoroughly wet the surface of the film layer with the precursor solution and fully introduce the precursor solution into the alumina nanopores by using the pressure difference between the inside and outside under the vacuum conditions. The thus obtained layered composite film layer has a uniform structure from the inside to the outside, avoiding performance changes caused by structural changes caused by external forces.
In order to obtain an LDH film on the Mg alloy surface with durable anti-corrosion resistance and good mechanical stability under milder conditions, this paper first rapidly prepared Li–Al-Ala LDH film on the Mg alloy surface by multi-arc ion plating and in-situ dipping. Then an improved vacuum impregnation was used for superhydrophobic modification of the obtained Li–Al-Ala LDH film in an ethanol solution of SA. Finally, the paper systematically studied the modified Li–Al-Ala LDH/SA films' structure, composition, and corrosion protection performance by scanning electron microscope (SEM), X-ray diffraction (XRD), Fourier Transform Infrared Spectroscopy (FTIR), X-ray photoelectron spectroscopy (XPS), water contact angle (WCA), electrochemical test, abrasion test, and long-term immersion experiment.
Experimental method
Materials
The AZ91D Mg alloy (compositions: Al of 8.5–9.5wt%, Zn of 0.45–0.9 wt%, Mn of 0.17–0.4 wt%, Si < 0.08wt%, Fe < 0.004 wt%, Ni < 0.001 wt%, balanced Mg) was cut into blocks with a size of 30 mm × 30 mm × 0.5 mm for later use as a substrate. All the chemical reagents applicable to this experiment were purchased from Beijing Inno Chem Science & Technology Co, including LiOH·H2O (AR, 99%), NaCl (AR, 99%), NaOH (AR, 99%), L-Alanine (Ala, C3H7NO2, 98%) and stearic acid (SA, C18H36O2, 99%) and are used without further purification. The solutions used in the experiments are all corresponding aqueous solutions, except that the SA solution is made of ethanol as a solvent. Deionized water was prepared by a water purifier (UPW-N15UV/N30UV, Shanghai INESA scientific).
Experimental details
Synthesis of Li–Al LDH and Li–Al-Ala LDH films
Firstly, the AZ91D Mg alloy was ground with 200, 400, 800, 1200, and 2000 grit SiC paper and ultrasonically cleaned in ethyl alcohol for 10 min. Then the Mg alloy sample was immersed in 2 M NaOH solution for 1 min to remove surface oxides by ultrasonic cleaning and then ultrasonically cleaned in ethyl alcohol for 10 min. Finally, the samples were dried with cold air for later aluminum plating treatment. Aluminum plating on the Mg alloy surface is realized by vacuum multi-arc ion plating. The target used for the coating was 99.999% aluminum, the coating current was 70 A, the coating temperature was 200 °C, and the coating time was 1 h. After the end, take out the Mg alloy sample and put it in a desiccator for use.
In the typical synthesis of Li–Al-Ala LDH film, 0.1 mol/L LiNO3 and 0.025 mol/L L-Ala were dissolved in deionized water to form a homogeneous solution, and 1 M LiOH was used to adjust the pH of the solution to 10.0. Then, the aluminized Mg alloy sample was immersed in the mixed solution and reacted at 60 °C for 30 min to obtain a Li–Al-Ala LDH film. After the reaction, the samples were taken out, rinsed with deionized water, and dried in cold air for use. Li–Al LDH film is synthesized using the same method as above, except that Ala is not added during preparation.
Synthesis of Li–Al-Ala LDH/SA film
The improved vacuum impregnation method [33] was used to acquire the Li–Al-Ala LDH/SA film. Briefly, the obtained Li–Al-Ala LDH film was vacuumed in the dried environment for 30 min and then transferred into the immersing solution composed of 0.01 mol/L SA ethanol solution to vacuum immersion for another 30 min. Finally, the samples were taken out and air-dried for subsequent performance testing. The schematic illustration for preparing the Li–Al-Ala LDH/SA film is shown in Fig. 1
Characterization and measurements
Microstructures and surface morphologies of the films were investigated by SEM (S-4800, Hitachi, Japan) equipped with an energy dispersive X-ray spectrometer (EDS) at an acceleration voltage of 10 kV. The structure of the obtained films was characterized by powder XRD (Rigaku Ultima IV, Japan) with Cu Κα radiation in the 2θ range of 5–80°. FTIR was used to describe the distinct functional groups of the films. The FTIR measurements were performed using the ATR method with air as the background on a Nicolet iS50 produced by Thermo Scientific, with a scan range of 400–4000 cm−1. The XPS analysis was performed on a VG ESCALAB MARK II spectrometer with the Mg Ka radiation (1253.6 eV), operating at the constant pass energy mode of 50 eV. The surface charging effect was corrected by fixing the C1s peak at the binding energy of 284.6 eV. The WCAs were measured at room temperature using a CA instrument (JC2000DF, China). Each WCA was the average of at least five measurements obtained at different surface positions using 5.0 μL droplets of water.
The samples' corrosion resistance performance was evaluated using an electrochemical workstation (CS2350H, Wuhan Corrtest Instruments, China) in 3.5 wt% NaCl solution. A standard three-electrode system consisting of the specimens (working electrode), Pt sheet (2 cm × 2 cm, counter electrode), and a saturated calomel electrode (reference electrode) were adopted. All samples were tested at room temperature (25 ± 2 °C), and the exposed area was 1 cm2. EIS was obtained at the open circuit potential (Eocp) in a frequency range from 100 to 10 mHz with an amplitude perturbation of 10 mV. A potentiodynamic polarization curve was performed using a sweep rate of 5 mV/s in the interval of (1.0 V + Eocp) ~ (−0.5 V + Eocp). Before each electrochemical test, the working electrodes were first immersed in a 3.5 wt.% NaCl solution for 30 min to obtain a stable Eocp. All electrochemical data are average values obtained after at least three tests.
Results and discussion
Characterization of Li–Al-Ala LDH and Li–Al-Ala LDH/SA films
Figure 2 shows the SEM micrographs of Li–Al-Ala LDH and the modified Li–Al-Ala LDH/SA films prepared on the Mg alloy. It can be seen in Fig. 3a1 and b1 that both films show the lamellar structure, which is the characteristic structure of LDH film, already reported in previous studies [9]. This morphology indicates that the metal hydroxide has been successfully bonded to the internal anion [21, 22]. The SEM images of blank Mg alloy and aluminized Mg alloy can also be seen in Fig. S1(a-b). It shows that after the multi-arc ion aluminizing treatment of the smooth Mg alloy (Fig. S1a), a relatively rough layered structure appears on the surface of the Mg alloy (Fig. S1b). The main component of the uneven film is aluminum (Fig. S1c), and the thickness is about 15 μm (Fig. S1d). After the rough aluminized film was immersed in LiNO3 for 30 min, as described in the experimental part, a unique LDH film's lamellar structure was obtained (Fig. 3a1–b1). The rapid formation of LDH film indicates that the intermediate treatment of pre-aluminum plating can effectively speed up the construction of LDH film on the surface of Mg alloy. After the obtained LDH film is modified with SA, the film still maintains a complete lamellar structure (Fig. 3b1). The lamellar structure indicates that modification by SA with low surface energy has no effect on the film's surface morphology and may only reduce the film's surface energy. At the same time, since the LDH film is a micro-nano structure film composed of nanosheets, the LDH film modified by SA has a micro-nano structure and low surface energy substances. Combining these two factors is the key to achieving superhydrophobic properties [31]. The insets of Fig. 3a1 and b1 show that the LDH films' WCA increased from 87.3 to 153.7° after SA modification. The increased WCA also indicates that the combination of micro-nano structure (LDH) and low surface energy substances (SA) can make the film layer from hydrophilic (Fig. 3a1) to superhydrophobic (Fig. 3b1). Besides, the thickness of the SA-modified LDH film increased by around 10 nm, as seen in the cross-sectional images Fig. 3(a2–b2), which is mainly due to the coverage of SA on the surface of LDH film. It is generally accepted that the organic compound modified film is beneficial in reducing the minor defects on the surface of the original film, thereby increasing the corrosion protection performance of the overall film [34, 35].
Meanwhile, it can be seen from the cross-sectional view that (Fig. 3a2–b2), after SA modification, the side of the film is denser and more uniform, which means that the vacuum impregnation makes SA fully enters between the laminates and fills the laminate gap. The SA modification makes the laminate gap smaller, thus, leading to an enhanced physical barrier of the film to the corrosive medium. Furthermore, the entire laminate structure of the SA-modified LDH film is the same from the inside out, composed of a micro-nano laminate structure and low surface energy substance SA. This uniform structure from the inside out can make the film layer not lose its hydrophobic properties due to changes in its design and composition when external forces wear it [31]. Before (Fig. 3a3) and after (Fig. 3b3) SA modification, the elemental composition of the film layer remained unchanged, and both contained C, N, and O, mainly derived from Ala and SA. But the relative contents of the three elements changed, caused by the additional introduction of SA.
Figure 3a–c shows the XRD patterns of blank Mg alloy, aluminized Mg alloy, Li–Al-Ala LDH, and Li–Al-Ala LDH/SA films. Compared with the blank Mg alloy, there are many characteristic peaks (2θ = 38.41° and 44.67°) (JCPDS: 04–0787) [36] of Al on the surface of Mg alloy after multi-arc ion plating. It mainly grows along the (111) and (220) crystal planes (Fig. 3a). At the same time, due to high-temperature ion plating, there are a small amount of Al2O3 peaks located at 2θ = 72.69° (JCPDS: 46–1131) [37]. In addition to the characteristic peaks of Mg and Al in the XRD pattern (Fig. 3b), steamed bread-like peaks appeared between 2θ = 10° and 2θ = 20°(Fig. 3c), which are the characteristics peaks of LDH from (003) and (006) planes after the aluminized Mg alloy being immersed in LiNO3(JCPDS: 42–0729) [38]. The appearance of the LDH characteristic diffraction peak confirms that Li–Al LDH films can be successfully prepared on Mg alloy surfaces by pre-aluminizing under the above experimental conditions. However, after being modified by SA, the diffraction peak of the (003) plane shifted to a higher 2θ from 11.7 to 12.6°, and the diffraction peaks became less sharp than the Li–Al-Ala LDH film. This change of 2θ value suggests a decrease in basal spacing and a decrement in crystallinity of the (003) plane of Li–Al-Ala LDH/SA compared to that of Li–Al-Ala LDH films [39]. However, the small changes in basal spacing and crystallinity did not cause the surface morphology changes of the LDH film (Fig. 2a1, b1).
FTIR(Fig. 3d) and XPS(Fig. 3e-l) techniques were utilized further to determine the chemical composition of the LDH films. In Fig. 3d, both curves' infrared absorption peaks at 3741 cm−1, and 2337 cm−1 are attributed to the free hydroxyl vibration peak and CO2 adsorption on the film surface. The other two sets of absorption peaks with similar positions are located in 1535 cm−1, 1550 cm−1, and 1389 cm−1, 1365 cm−1 belong to the bending vibration of water molecules, the stretching vibration of C = O, and the stretching vibration of NO3− [29, 40], the symmetrical bending of CH3 in the LDH interlayer spacing, respectively [31]. The bands at the lower wavenumber (400–700 cm−1) arise from M–O and Me-OH bond vibrations (M = Li or Al) [10, 41]. Comparing the FTIR spectra before (red curve) and after (blue curve) SA modification (Fig. 3d), it shows that three more characteristic absorption peaks at the wavelength of 2978 cm−1, 2908 cm−1, 1057 cm−1 appeared, and an absorption peak located at 3425 cm−1 disappeared after SA modification. The two newly emerged peaks (2978 cm−1 and 2908 cm−1) originate from the asymmetric and symmetric stretching of CH2 in SA [42], and the other peak at 1057 cm−1 is attributed to the −COOH group in SA [42]. The disappeared band at 3425 cm−1 is attributed to the antisymmetric stretching of NH2 from Ala [43, 44]. The possible reason for the disappearance of this peak is that, after SA modification, the surface of LDH film is covered by SA, resulting in the weakening of Ala's signal. These peaks confirm that the Li–Al-Ala LDH film has been successfully synthesized on the aluminized Mg alloy surface under experimental conditions. Also, SA has modified the LDH film presumably through physical adsorption or chemical reactions.
Figure 3e–l shows the XPS spectra of LDH films before and after SA modification. It can be seen from the full spectrum of XPS (Fig. 3e) that the types of peaks before and after SA modification do not change, and all of them contain peaks from Li1s, Al 2p, Al2s, C1s, N1s, O1s, and OKLL, indicating that the element type has not changed after SA modification. The XPS spectrum shows that the binding energy of Al2p(Fig. 3f), Li1s(Fig. 3g), and O1s (Fig. 3h) located at 74.5 eV and 74.4 eV, 55.2 eV and 55.3 eV, 531.8 eV and 531.7 eV, are corresponding to Al–OH, Li–OH and OH/ OH−, respectively [28, 39]. N1s spectra of the two LDH films can be deconvoluted into two groups of peaks appearing at 398.88 eV, 398.08 eV (Fig. 3i) and 406.48 eV, 407.28 eV (Fig. 3j). The former group of peaks is derived from C-N bonds in Ala [43, 44], and the latter group of peaks belongs to NO3−in the LDH films. Binding energy at 283.98 eV (Fig. 3k), 287.48 (287.78) eV (Fig. 3l) and 288.85 (288.38) eV (Fig. 3l) are contributed to the presence of C–C, CO32− and O–C = O respectively [29, 40]. SA only contains three elements C, H, and O, so the introduction of SA may mainly affect the relative content of different bonding types of these three elements [42].
The change in the relative content of different bonding types can be measured by the difference in the peak area ratio. Table 1 list the fitting results of C1s spectra in Li–Al-Ala LDH and Li–Al-Ala LDH/SA films. The relative content of C–C bonds increased significantly, indicating that SA, which contains many C-H bonds, was successfully loaded on the LDH film. However, the relative content of carboxylate (O–C = O) shows an apparent decrease, which may also result from the SA. After introducing SA into the LDH film, the O–C = O on the surface of the LDH film is jointly provided by SA and Ala. Because the proportion of O–C = O in SA is much smaller than that in Ala, the SA introduction will increase the absolute content of O–C = O but a significant decrease in the relative content. The conclusion can also be used to explain the change in O–H. For carbonate (CO32−), which is mainly affected by CO2 in the air, SA modification or not has little effect on its absolute and relative content. Therefore, SA has been successfully loaded on the LDH film based on the above analysis. The introduction of SA mainly affects the relative content of different valence states of elements. Still, it does not affect the type of bonding, indicating SA has modified the LDH film mainly through physical adsorption.
Corrosion Resistance of Li–Al-Ala LDH and Li–Al-Ala LDH/SA films
Tafel polarization curves are employed to estimate the corrosion resistance of the LDH films, aluminized Mg alloy, and bare Mg alloy. The results are presented in Fig. 4a, and the detailed polarization parameters are listed in Table 2. Ecorr represents the corrosion potential, icorr represents the corrosion current density, βa the anodic Tafel slope, and βc the cathodic Tafel slope. Generally speaking, a lower icorr and a more positive Ecorr are related to a lower corrosion rate and better resistance [19]. It was shown in Table 2 that the corrosion potential of aluminized Mg alloy has a negative shift from −1.516 to −1.633 V. The negative change may be caused by the aluminized layer with negative self-corrosion potential compared with that of Mg alloy [36, 45].
Meanwhile, the corrosion current density of aluminized Mg alloy is slightly lower than that of blank Mg alloy, but the decrease is not apparent. It shows that simple aluminizing treatment cannot effectively improve the corrosion resistance of Mg alloy. After the Li–Al-Ala LDH film was prepared on the surface of Mg alloy, the corrosion potential is still more negative than that of Mg alloy but more positive than aluminized Mg alloy. The change in corrosion potential indicates that the presence of the aluminized intermediate layer increases the corrosion tendency of the Mg alloy substrate. Still, the formation of the LDH films can appropriately reduce this tendency.
Furthermore, the corrosion current density of the Li–Al-Ala LDH film decreased by about two orders of magnitude compared with blank Mg alloy, proving that the LDH film can significantly reduce the corrosion rate of Mg alloy and improve its corrosion resistance. This enhancement of corrosion resistance may be due to the physical barrier effect and the capacity to absorb the LDH film [26]. Ala can act as an inhibitor and prevent the metal from corrosion [44, 45]. After the LDH film was further modified with SA, the corrosion potential of the obtained film directly moved to −1.497 V, which was more positive even than that of the Mg alloy (−1.516 V). The corrosion current density is further reduced to 1.318 × 10–7 A/cm2, almost three orders of magnitude lower than the blank Mg alloy (1.940 × 10–4 A/cm2), suggesting its practical protection effect on the underlying Mg alloy substrates. This excellent corrosion resistance can be attributed to two aspects: the SA modification decreases the surface energy of the LDH film and makes it superhydrophobic (Fig. 2b1), effectively preventing water molecules and Cl− from eroding the substrate. The other comes from the enhanced physical barrier (Fig. 2b2), ion exchange, and an inhibitor Ala of the LDH film [43, 44].
EIS was employed to investigate further the corrosion behavior of bare Mg alloy, aluminized Mg alloy, Li–Al-Ala LDH, and the superhydrophobic Li–Al-Ala LDH/SA films (Fig. 4b–f). As shown in Fig. 4b–d, the capacitance loops over the entire frequency range of the two LDH films coated samples are much larger than that of the bare Mg alloy, indicating a better corrosion resistance of the LDH film-coated samples. The aluminized Mg alloys have a more excellent total corrosion resistance than blank Mg alloys (Fig. 4c). Also, there is an inductive reactance in the bare Mg alloy's Nyquist plot (Fig. 4d), indicating pitting corrosion on the Mg alloy surface [46].
Three equivalent circuits (Fig. 4g–i) were used to stimulate the EIS results to quantitatively compare the corrosion resistance of different films. The corresponding fitting results are shown in Table 3. Generally, the capacitive loop in the high and middle-frequency region mainly characterizes the outer film's electrochemical process. The electrochemical behavior in the low-frequency region is primarily attributed to the interface between the film and the substrate. Meanwhile, the Nyquist plots of the EIS in Fig. 4b–d are not perfect semicircles, which means the tested system is not an ideal capacitor. So, the constant phase element CPE is used to describe the system's capacitance, and n (0.5 ≤ n ≤ 1) is introduced to calibrate the actual capacitance. Factor n represents the deviation coefficient of the capacitance obtained from the system from the ideal capacitance C. Factor n also represents the frequency power of CPE; n = 1 means that CPE is a perfect capacitor (C) [33]. So, as depicted in Fig. 4b–d, the equivalent circuit in Fig. 4g is used to fit the EIS data with inductive loops. Another one in Fig. 4h is used to stimulate the EIS data of aluminized Mg alloy and Li–Al-Ala LDH films. For the superhydrophobic sample Li–Al-Ala LDH/SA film, due to the existence of "air film" on the surface [47], the equivalent circuit in Fig. 4i is used to simulate the corrosion process. In Fig. 4h–i, Rs represents the solution resistance, Rair and CPEair are the resistance and capacitance of the "air films", Rm and CPEm are the LDH films' resistance and capacitance, Rct is the charge transfer resistance, CPEdl is the double layer capacitance, RL is the inductance resistance, and L means the electrical inductance. The Rtotal value (Table 3) calculated by Eq. (1) represents the total resistance of different samples and can be used to evaluate the films' anti-corrosion resistance.
It can be seen from Table 3 that after the LDH film is coated on the surface of the Mg alloy, its Rtotal is significantly higher than that of the blank and aluminized Mg alloy. With the further hydrophobic modification of the LDH film, the Rtotal reaches the maximum value three orders of magnitude higher than the blank Mg alloy. The significant increase in Rtotal indicates that after the Mg alloy surface is covered with LDH film, its corrosion resistance is significantly enhanced, reaching the maximum after SA modification. This variation law is consistent with the law of corrosion resistance obtained in the polarization curves (Fig. 4a, Table 2).
The superhydrophobicity is generally the result of the combined micro-nano structure and low surface energy compounds. The "air film" in the superhydrophobic design can effectively prevent the attack of water molecules and Cl−. However, when the superhydrophobic film is immersed in the solution, the strong hydraulic pressure will cause the "air film" to rupture, thus losing its protective effect. So the "air film" resistance (Rair) exhibited in Li–Al-Ala LDH/SA film is smaller than that of the inner layer of the film (Rm). In addition to the Rair, the Rm after SA modification is about two orders of magnitude higher than the unmodified Li–Al-Ala LDH film. The increased Rm indicates that SA modification forms an "air film" on the surface to hinder corrosive media entry and enables the entire LDH film to have (super) hydrophobic properties from the inside out. The (super) hydrophobic film can prevent corrosive media from entering the film layer. This conclusion can also be seen in the later increase in the electrochemical reaction resistance (Rct). The film modified by SA not only has a larger Rm on the surface layer, but the Mg alloy covered by the Li–Al-Ala LDH/SA film also has a larger Rct than that of the Li–Al-Ala LDH film. This phenomenon also shows that the low surface energy substance SA has entered the inside of the film layer, which leads to the inside film layer also having a (super)hydrophobic effect. The (super)hydrophobicity inside the film layer prevents water molecules and corrosive media from being in good contact with the substrate, thus showing a larger electrochemical reaction resistance (Rct).
The total capacitance \(\left( {CPE_{air} + CPE_{m} + CPE_{dl} } \right)\) of LDH after SA modification also decreases. Combining with the changes in the surface structure and film thickness of the film in SEM (Fig. 2a1–b2), it can be seen that the decrease in capacitance is mainly due to the changes in the total area of the LDH film. Since the modification of SA fills the interlayer gap of the film, the gap is reduced, leading to a decreased total area of the film (Fig. 2a2). Also, SA modification increases 10 nm in film thickness (Fig. 2b2), so the capacitance of the SA-modification LDH film is diminished.
Corrosion durability of Li–Al-Ala LDH/SA film
Corrosion durability is an important index to evaluate the protection stability of the LDH film. The long-term durability of the Li–Al-Ala LDH/SA films was investigated using EIS. The EIS spectra of the LDH films before and after immersing in 3.5 wt% NaCl aqueous solution at 25 ± 2 °C are shown in Fig. 5. It can be seen that the capacitive arc radius (Fig. 5a) and the modulus (Fig. 5b) of the Li–Al-Ala LDH/SA film decreased by about an order of magnitude after immersing in 3.5 wt% NaCl for 432 h. However, it is still much higher than Li–Al-Ala LDH film without immersion. The phenomenon proves that though Li–Al-Ala LDH/SA film's corrosion resistance decreased after a long-time immersion, it is still larger than the Li–Al-Ala LDH film. Figure 5c shows the Li–Al-Ala LDH/SA film's phase angle diagram before immersion. In the phase angle diagram, a small peak in the high-frequency region and a prominent broad peak in the medium–low frequency indicates that a circuit diagram with three time constants can simulate the film's corrosion process (Fig. 4i). Only two peaks appeared in the middle-low frequency region (Fig. 5c) for the Li–Al-Ala LDH/SA film after long-time immersion and the Li–Al-Ala LDH film without immersion. So the corrosion process of the two films can be simulated by the circuit diagram with two time constants (Fig. 4h). The disappearing peak in the high-frequency region of the Li–Al-Ala LDH-SA films may represent the superhydrophobic film's "air film resistance" (Rair). When the film is immersed in a corrosive medium for a long time, the Rair will disappear due to water pressure. The relevant electrochemical data fitted by Z-view software are listed in Table 4.
It is shown in Table 4 that the most apparent change of the Li–Al-Ala LDH/SA film after long-time immersion is the disappearance of the air film resistance (Rair). The film resistance (Rm) and electrochemical reaction resistance (Rct) also dropped by an order of magnitude. The change in the resistances shows that the corrosion resistance of the Li–Al-Ala LDH/SA film will decrease after long-term immersion. The main reason is that the superhydrophobicity of the film partially disappeared due to long-term immersion. In particular, the disappearance of the "air film" will cause corrosive ions (Cl−) to gradually penetrate into the lamellar structure of the film layer during long-term immersion, exchange with anions, and then fix the Cl− between the lamellae to achieve an anti-corrosion effect. However, with the prolongation of immersing time, the corrosion inhibitor Ala ions and other anions (e.g., NO3−) in the LDH film gradually decreased, weakening the anion exchange capacity of the LDH film, thus leading to the weakening of the corrosion resistance.
At the same time, Table 4 shows that the total corrosion resistance (Rtotal) of the Li–Al Ala LDH/SA film after immersion for 432 h is still higher than that of Li–Al Ala LDH film without immersing. It is shown in Fig. 6a that the surface of the film layer has a lamellar structure, with small clusters appearing locally, which makes the whole film layer look denser. In Fig. 6b, a small number of corrosion products accumulate on the surface of the film layer, so the overall film resistance (Rm) is larger than that of Li–Al Ala LDH film. The macroscopic images of Li–Al Ala LDH/SA film after immersion for 432 h can also be seen in Fig. S2a–b. It can be seen that the surface of the film layer becomes slightly black after immersion, and a small number of corrosion points appear on the surface, which indicates that local pitting corrosion occurred on the surface of the sample. However, a relatively complete film can still protect the substrate from corrosion. It is generally accepted that when the above two films are not immersed, the amount of Ala in the LDH films is the same, and the only difference is whether the film contains SA. After the Li–Al Ala LDH/SA film immersing for a long time, embedded Ala will decrease due to ion exchange with Cl− (Fig. 6c), and the corrosion resistance will decrease. However, the Rct of the Li–Al Ala LDH/SA film after immersion for a long time is still greater than that of the Li–Al Ala LDH film without immersing. Thus it can be speculated that the only reason is SA modification. The vacuum impregnation allows SA to fully enter the film layer's interior, enhancing the compactness and hydrophobicity of the inner layer of the film, thereby effectively preventing the exchanged Cl− from contacting the substrate. Then the interface resistance (Rct) between the substrate and the film layer is enhanced so that the corrosion resistance of the substrate is improved.
Mechanical stability of Li–Al-Ala LDH/SA film
Based on the previous analysis, it can be seen that the superhydrophobicity of LDH is caused by the combined effect of the micro-nano structure of the LDH film and the low surface energy compound SA. However, these characteristics are highly susceptible to mechanical abrasion, which could alter the surface morphology and chemistry. The mechanical durability of the Li–Al-Ala LDH/SA film was carried out by pushing a weight with a 100 g weight back and forth across the surface in a straight-line direction on 600 grit sandpaper (Fig. 7a). Figure 7b shows the wettability changes of the film with an abrasion distance from 0 to 200 cm. It can be seen that when the abrasion distance increases from 0 to 60 cm, the hydrophobicity of the film decreases rapidly, and the WCA decreases from 153.7 to 141.9°. The main reason is that the modification by vacuum impregnation will cause part of the SA to exist on the surface of the LDH film in the form of physical adsorption. The hardness of the low surface energy SA film is relatively small, and the bonding force with the LDH film is relatively low (physical adsorption). When an external force wears it, the SA on the surface is quickly worn off, and the overall hydrophobicity of the film decreases. However, as the abrasion distance continued to extend (60 → 200 cm), the WCA of the film decreased very little (141.9° → 140.3°, decline rate:1.12%), and the hydrophobicity remained unchanged, which indicated that the film had good mechanical stability. Figure 7c–d shows the LDH film's surface morphology and energy spectrum after 200 cm abrasion. Compared with the Li–Al-Ala LDH/SA film without abrasion (Fig. 2b1, b3), it can be seen that after 200 cm of abrasion, the surface structure of the film layer remains unchanged, and it is still a regular lamellar structure. The content of each element also remains intact, and only the C element content is slightly reduced due to the abrasion of the surface SA.
When an external force wears the Li–Al-Ala LDH/SA film, its WCA first decreases and then remains stable, opposite to the trend with the WCA first decreasing slowly and then sharply in recent research [31, 32, 48]. The reason is that based on the formation mechanism of the superhydrophobic surface, the abrasion will lead to a sharp decrease in the content of SA adsorbed on the film surface, resulting in an apparent reduction in the WCA of the film (153.7° → 141.9°). However, vacuum impregnation can make SA enter into the rough LDH film as much as possible to ensure that the structure and composition of the entire composite film are consistent from the inside to the outside. After that, the inner layer SA is adsorbed between the layers of LDH. The structure and chemical composition of the overall LDH film will not be changed even if the external continuous wear and tear, thus maintaining the mechanical stability of the LDH film layer. Therefore, it is reasonable to speculate that it may be more effective to enhance the mechanical wear resistance of the film by maintaining the stability of the structure and composition of the LDH film than to improve its wear resistance by simply enhancing the hardness of the film.
Since the Li–Al-Ala LDH film has good mechanical wear resistance, as long as the bonding force between the film layer and the substrate is suitable, it can ensure that the film layer always has an excellent protective effect on the substrate. Figure 8 shows the macroscopic (a) and optical microscope (b) images of the Li–Al-Ala LDH/SA film on the Mg alloy surface after the cross-cutting test. It can be seen from Fig. 8a that the edge of the incision is nearly smooth, and there is no apparent delamination or peeling phenomenon after the 3 M tape is removed (Fig. 8b). The film's adhesion level can reach level 0 based on the standard from ISO, indicating that the Li–Al-Ala LDH/SA film has good adhesion to the Mg alloy substrate. Table 5 lists recent work for preparing LDH films on Mg alloy surface and the related properties of the resulting films.
Photograph of the Li–Al-Ala LDH/SA film after the cross-cut tape test (a) and the enlarged image of the region shown in Fig. 8a (b)
Conclusions
The Li–Al-Ala LDH film has been successfully prepared on the surface of Mg alloys by combining multi-arc ion plating and in-situ dipping under mild conditions. The obtained films were dense and uniform with a typical lamellar structure. Electrochemical tests show that the Li–Al-Ala LDH film can increase the Mg alloy's corrosion resistance by two orders of magnitude. Vacuum immersion of the Li–Al-Ala LDH film in SA ethanol solution can make the film from hydrophilic (87.3°) to superhydrophobic (WCA = 153.7°), and the corrosion resistance of the Li–Al-Ala LDH/SA film can be further improved by an order of magnitude. Furthermore, there was no apparent corrosion pitting on the Li–Al-Ala LDH/SA film's surface, and it still had a good corrosion protection effect on Mg alloy after it was immersed in 3.5 wt% NaCl for 432 h. Based on the ISO standard, the bonding force between the film and the Mg alloy substrate can reach grade 0. And the hydrophobicity of the film remains stable (WCA≈140°) with the extension of the wear distance after mechanical abrasion of 60 cm, showing an excellent mechanical abrasion resistance. The durable mechanical abrasion resistance of the film results from the structure and composition of the Li–Al-Ala LDH/SA film obtained by the improved vacuum impregnation is homogeneous from the inside out.
References
Wang XJ, Xu DK, Wu RZ et al (2018) What is going on in magnesium alloys? J Mater Sci Technol 34(02):245–247
Esmaily M, Svensson JE, Fajardo S et al (2017) Fundamentals and advances in magnesium alloy corrosion. Prog Mater Sci 89:92–193
Deepa B, Gopalakrishnan P, Ravi K (2019) Morphological studies on the development of chemical conversion coating on the surface of Mg-4Zn alloy and its corrosion and biomineralization behavior in simulated body fluid. J Alloy Compd 812:152146. https://doi.org/10.1016/j.jallcom.2019.152146
Zhang G, Wu L, Tang A et al (2017) A Novel Approach to Fabricate Protective Layered Double Hydroxide Films on the Surface of Anodized Mg-Al Alloy. Adv Mater Interfaces 4(12):1700163. https://doi.org/10.1002/admi.201700163
Ly X, Yang S, Nguyen T (2020) Effect of equal channel angular pressing as the pretreatment on microstructure and corrosion behavior of micro-arc oxidation (MAO) composite coating on biodegradable Mg-Zn-Ca alloy. Surf Coat Technol 395:125923. https://doi.org/10.1016/j.surfcoat.2020.125923
Taltavull C, Torres B, López AJ et al (2013) Novel laser surface treatments on AZ91 magnesium alloy. Surf Coat Technol 222:118–127
Jing C, Dong B, Raza A et al (2020) Corrosion inhibition of layered double hydroxides for metal-based system. Nano Mater Sci 3:47–67
Guo L, Wu W, Zhou Y et al (2018) Layered double hydroxide coatings on magnesium alloys: a review. J Mater Sci Technol 34:1455–1466
Hu J, Gan M, Ma L et al (2015) preparation and enhanced properties of polyaniline / grafted intercalated ZnAl-LDH nanocomposites. Appl Surf Sci 328:325–334
Zhang Y, Liu J, Li Y et al (2015) A facile approach to superhydrophobic Li–Al-layered double hydroxide film on Al-Li alloy substrate. J Coat Technol Res 12(3):595–601
Arias S, Sousa LV, Barbosa CBM et al (2018) Preparation of NiAlZr-terephthalate LDH with high Al and Zr content and their mixed oxides for cyclohexane dehydrogenation. Appl Clay Sci 166:137–145
Shen Y, Yin K, An C et al (2018) Design of a difunctional Zn-Ti LDH supported PdAu catalyst for selective hydrogenation of phenylacetylene. Appl Surf Sci 456:1–6
Zhu Y, Zhe A, Jing H (2016) Single-atom and small-cluster Pt induced by Sn (IV) sites confined in an LDH lattice for catalytic reforming. J Catal 341:44–54
Tichit D, Layrac G, Gérardin C (2019) Synthesis of layered double hydroxides through continuous flow processes: a review. Chem Eng J 369:302–332
Liu Q, Ma J, Wang K et al (2017) BiOCl and TiO2 deposited on exfoliated ZnCr-LDH to enhance visible-light photocatalytic decolorization of Rhodamine B. Ceram Int 43(7):5751–5758
Hunter BM, Hieringer W, Winkler JR et al (2018) effect of interlayer anions on [NiFe]-LDH nanosheet water oxidation activity. Energy Environ Sci 9(5):1734–1743
Fedel M, Zanella C, Ferrari L et al (2021) effect of the synthesis parameters of in situ grown Mg-Al LDH on the filiform corrosion susceptibility of painted AA5005. Electrochim Acta 381(10):138288. https://doi.org/10.1016/j.electacta.2021.138288
Chen J, Song Y, Shan D et al (2015) In Situ Growth Process of Mg-Al Hydrotalcite Conversion Film on AZ31 Mg Alloy. J Mater Sci Technol 7(4):384–390
Gu Z, Huang Y, Wang Y et al (2019) An aluminum silicate modified Ni-Al LDH film to improve the corrosion resistance of AZ31 Mg alloy. Mater Lett 252:304–307
Wu W, Sun X, Zhu CL et al (2020) Biocorrosion resistance and biocompatibility of Mg-Al layered double hydroxide/poly-L-glutamic acid hybrid coating on magnesium alloy AZ31. Prog Org Coat 147:105746. https://doi.org/10.1016/j.porgcoat.2020.105746
Li LX, Xie ZH, Fernandez C et al (2019) Development of a thiophene derivative modified LDH coating for Mg alloy corrosion protection. Electrochim Acta 330:135186. https://doi.org/10.1016/j.electacta.2019.135186
Cheng S, Zhang D, Li M et al (2021) Osteogenesis, angiogenesis and immune response of Mg-Al layered double hydroxide coating on pure Mg. Bioactive Mater 6(1):91–105
Wu F, Liang J, Peng Z et al (2014) Electrochemical deposition and characterization of Zn-Al layered double hydroxides (LDH) films on magnesium alloy. Appl Surf Sci 313:834–840
Yao PY, Zhong Li, Ran XX et al (2021) Controllable synthesis of NiCo-LDH/Co(OH)2 @PPY composite via electrodeposition at high deposition voltages for high-performance supercapacitors. J Alloys Compounds 875:160042. https://doi.org/10.1016/j.jallcom.2021.160042
Besserguenev AV, Fogg AM, Francis RJ (1997) Synthesis and Structure of the Gibbsite Intercalation Compounds [LiAl2(OH)6]X {X = Cl, Br, NO3 and [LiAl2(OH)6]Cl·H2O Using Synchrotron X-ray and Neutron Powder Diffraction. Chem Mater 9(1):241–247
Ji L, Li K, X L et al (2019) Enhanced corrosion protection property of Li–Al layered double hydroxides (LDH) film modified by 2-guanidinosuccinic acid with excellent self-repairing and self-antibacterial properties. Appl Surf Sci 480:384–394
Yan H, Lu J, Wei M et al (2008) Theoretical study of the hexahydrated metal cations for the understanding of their template effects in the construction of layered double hydroxides. J Mol Struct (Thoechem) 866(1):34–45
Zhou M, Pang X, Wei L et al (2015) In-situ grown superhydrophobic Zn-Al layered double hydroxides films on magnesium alloy to improve corrosion properties. Appl Surf Sci 337:172–177
Li J, Yuan T, Zhou C et al (2020) Facile Li–Al layered double hydroxide films on Al alloy for enhanced hydrophobicity, anti-biofouling and anti-corrosion performance. J Mater Sci Technol 79(3):230–242
Kuang J, Ba Z, Li Z et al (2019) Fabrication of a superhydrophobic Mg-Mn layered double hydroxides coating on pure magnesium and its corrosion resistance. Surf Coat Technol 361:75–82
Wu L, Wu J, Zhang Z et al (2019) Corrosion resistance of fatty acid and fluoroalkylsilane-modified hydrophobic Mg-Al LDH films on anodized magnesium alloy. Appl Surf Sci 487:569–580
Jena G, Thinaharan C, George RP et al (2020) Robust nickel-reduced graphene oxide-myristic acid superhydrophobic coating on carbon steel using electrochemical co-deposition and its corrosion resistance. Surf Coat Technol 397:125942. https://doi.org/10.1016/j.surfcoat.2020.125942
Du X, Cai D, Ou Q et al (2021) Fabrication and characterization of the hierarchical AAO film and AAO-MnO2 composite as the anode foil of aluminum electrolytic capacitor. Surf Coat Technol 419:127286. https://doi.org/10.1016/j.surfcoat.2021.127286
Chen Y, Liu Y, Xie Y et al (2021) Preparation and anti-corrosion performance of superhydrophobic silane/graphene oxide composite coating on copper. Surf Coat Technol 423(9):127622. https://doi.org/10.1016/j.surfcoat.2021.127622
Chen Y, Liu Y, Xie Y et al (2022) Preparation of hydrophobic silane/graphene oxide composite coating implanted with benzotriazole to improve the anti-corrosion performance of copper. J Alloy Compd 893:162305. https://doi.org/10.1016/j.jallcom.2021.162305
Zhang D, Wei B, Wu Z et al (2016) A comparative study on the corrosion behavior of Al, Ti, Zr and Hf metallic coatings deposited on AZ91D magnesium alloys. Surf Coat Technol 303:94–102
Pan K, Wang F, Wei S et al (2020) Low-temperature solution synthesis and characterization of enhanced titanium dioxide photocatalyst on tailored mesoporous γ-Al2O3 support. Compos Commun 19:82–89
Ma J, Chang M, He H et al (2022) Corrosion resistance of Li–Al LDHs film modified by methionine for 6063 Al alloy in 3.5 wt% NaCl solution. Coatings 12(4):507. https://doi.org/10.3390/coatings12040507
Geetanjali M, Barsha D, Diptipriya S et al (2017) Orientation of organic anions in Zn-Al layered double hydroxides with enhanced antibacterial property. Original article 34:516–527
Lin K, Luo X, Pan X et al (2018) Enhanced corrosion resistance of LiAl-layered double hydroxide (LDH) coating modified with a Schiff base salt on aluminum alloy by one step in-situ synthesis at low temperature. Appl Surf Sci 463:1085–1096
Hou L, Li Y, Sun J et al (2019) Enhancement corrosion resistance of Mg-Al layered double hydroxides films by anion-exchange mechanism on magnesium alloys. Appl Surf Sci 487:101–108
Sobhana L, Zhang X, Kesavan L et al (2017) Layered double hydroxide interfaced stearic acid—Cellulose fibers: a new class of superhydrophobic hybrid materials. Colloids Surf A 522:416–424
Hamed E, El-Rehim S, El-Shahat MF et al (2012) Corrosion inhibition of nickel in H2SO4 solution by alanine. Mater Sci Eng, B 177(5):441–448
Ashraf MA, Liu Z, Peng W et al (2019) Amino acid and TiO2 nanoparticles mixture inserted into sol-gel coatings: an efficient corrosion protection system for AZ91 magnesium alloy. Prog Org Coat 136:105296–105296
Wei B, Cheng Y, Liu Y et al (2021) corrosion and wear resistance of AZ31 Mg alloy treated by duplex process of magnetron sputtering and plasma electrolytic oxidation. Trans Nonferrous Metals Soc China 31(8):2287–2306
Umer M, Ameeq F, Kaab T et al (2022) Corrosion behavior of AZ31 magnesium alloy with calcium addition. Corros Sci 199:110205. https://doi.org/10.1016/j.corsci.2022.110205
Cao Y, Zheng D, Luo J et al (2019) Enhanced corrosion protection by Al surface immobilization of in-situ grown layered double hydroxide films co-intercalated with inhibitors and low surface energy species. Corros Sci 164:108340. https://doi.org/10.1016/j.corsci.2019.108340
Du X, Liu Y, Chen D et al (2022) Co-electrodeposition of silane and graphene oxide on copper to enhance the corrosion protection performance. Surf Coat Technol 436:128279. https://doi.org/10.1016/j.surfcoat.2022.128279
Syu J, Uan J, Lin M et al (2013) Optically transparent Li–Al-CO3 layered double hydroxide thin films on an AZ31 Mg alloy formed by electrochemical deposition and their corrosion resistance in a dilute chloride environment. Corros Sci 68:238–248
Lin M, Chang F, Uan J (2010) Synthesis of Li–Al-carbonate layered double hydroxide in a metal salt-free system. J Mater Chem 20(31):6524–6530
Tang Y, Wu F, Fang L et al (2019) A comparative study and optimization of corrosion resistance of ZnAl layered double hydroxides films intercalated with different anions on AZ31 Mg alloys. Surf Coat Technol 358:594–603
Lin JK, Uan JY (2009) Formation of Mg, Al-hydrotalcite conversion coating on Mg alloy in aqueous HCO3-/CO32- and corresponding protection against corrosion by the coating. Corros Sci 51(5):1181–1188
Anjum MJ, Zhao J, Asl V et al (2019) In-situ intercalation of 8-hydroxyquinoline in Mg-Al LDH coating to improve the corrosion resistance of AZ31. Corros Sci 157:1–10
Funding
This study was funded by the Key-Area Research and Development Program of Guangdong Province (2020B010186001), Guangdong Basic and Applied Basic Research Foundation (2020B1515120093, 2020A1515110825), Science and Technology Project of Guangdong Province (2020B121202002) and the National Natural Science Foundation of China (52101079).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Naiqin Zhao.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, C., Huang, Y., Li, J. et al. Preparation of superhydrophobic Li–Al-Ala LDH/SA film with enhanced corrosion resistance and mechanical stability on AZ91D Mg alloy. J Mater Sci 57, 14780–14798 (2022). https://doi.org/10.1007/s10853-022-07582-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-022-07582-1