Abstract
We prepared and investigated the composites of the spinel Li4Ti5O12 (LTO) and Marimocarbon (MC) by the hydrothermal method that could form uniform electric paths without aggregation of the LTO particles for high capacity and high rate lithium-ion batteries. MC consisted of many fine carbon nanofilaments (CNFs) intertwined with each other in a complicated fashion. There are vacant space volumes of hundred nanometers between the CNFs. LTO particles were deposited in the space volumes among the tangled CNFs of the MCs. LTO is one of the most attractive anode materials for lithium-ion batteries because of its structural stability and safety. The morphology, microstructure and elemental composition of the LTO/MC composites were characterized by scanning electron microscopy (SEM), and X-ray diffraction (XRD). A charge–discharge test revealed that the LTO/MC composite (MC 10 wt%, prepared using a fluidized bed flow-reactor) produced the specific capacity of 170 mA g−1 at 1C (1C = 175 mA g−1). The LTO/MC composite maintained the specific capacity of 47 mAh g−1 even in the high rate zone at 30C whereas only the pristine LTO the produced 15 mAh g−1 at this rate. The unique structure of the LTO/MC composites can contribute to improving the electrochemical performance of the LTO anode. The LTO/MC composites can provide an effective approach to improve the lithium-ion battery performances.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lithium-ion batteries are used in many electric devices, hybrid electric vehicles (HEVs) and electric vehicles (EVs) due to their high energy density, high power and long life [1,2,3,4]. The spinel Li4Ti5O12(LTO) is one of the most attractive anode materials due to its safety features. The LTO has a higher insertion potential of approximately 1.55 V versus Li+/Li which prevents the formation of metallic lithium. The LTO also exhibits the property referred to as “zero strain’’ which means an excellent cycle-life during charge/discharge processes [5,6,7,8,9,10].
However, the poor electronic conductivity and lithium-ion diffusion limit its electrochemical performance [11, 12]. To enhance the performance of LTO anodes, many efforts have been reported, such as ion doping [12,13,14], particle size reduction [15,16,17], use of carbon composites [18,19,20].
Carbon materials are widely used as conductive additives to enhance the conductivity. Xuefei Guo et al. prepared a carbon-coated LTO using an amphiphilic carbonaceous material as a carbon precursor, and showed an excellent rate capability and cycling life [21]. Eunae Kang et al. synthesized nano-sized LTO-carbon composites by block copolymer self-assembly and showed a highly improved electronic conductivity [22]. However, it is difficult to form a conductive path with a conventional carbon material.
Marimocarbon (MC) consists of many carbon nanofilaments (CNFs), and the CNFs are interwoven to form a spherical secondary shape. This carbon has an oxidized diamond core and radial CNFs [23, 24]). MC has a large space volume between the nanofilaments. The spaces can pass water molecules. This allows MC to easily disperse into a water solution [25] and some oxide materials can be easily deposited from ionic solutions. We have proved that MC acted as a conductive additive for anatase TiO2 [26] and MC acted as a catalyst support for fuel cells [25, 27,28,29,30].
In this study, we synthesized LTO/MC composites by deposition of LTO particles in the spaces between the CNFs of the MC using a hydrothermal process. Figure 1 shows a schematic diagram of the LTO/MC composite in which electrolytes carry broadly. The LTO particles were dispersed in the carbon networks of the MC. There are a few aggregations of the LTO particles in the CNFs. This structure enables LTO particles to have more contact with the CNFs and electrolytes. Applying LTO/MC composites improved the electrochemical performance of the LTO anodes.
Experimental
Preparation of LTO/MC composites
MC was synthesized by the decomposition of methane using the oxidized diamond-supported Ni catalyst [23, 24]. The reaction was carried out in a rotary or a fixed fluidized bed flow-reactor at 550 °C for 3 h (denoted MC-R or MC-F). Using MC-R prepared in a rotary fluidized bed type flow-reactor as the framework, the LTO/MC-R composites were synthesized by the deposition of Li and Ti to form LTO. Figure 2 shows the flow chart of the synthesis. Sieved MC-R smaller than 100 µm or 250 µm (MC-100R, or MC-250R, respectively) was added to 100 mL of a 0.4 M LiOH solution followed by ultrasonic wave irradiation for 1 h. The amount of the added MC-100R or MC-250R was determined to synthesize composites composed of LTO and MC in the weight ratio of 80:10 (denoted LTO/10MC-100R, or LTO/10MC-250R, respectively). The amount of LTO was based on the average amount of LTO synthesized by hydrothermal methods. Next, 5 mL of 30% hydrogen peroxide and 3.084 mL (10 mmol) of (C3H7O)4Ti (TTIP, 97%) were added to the MC suspension. After stirring for 10 min, the obtained suspension was transferred to a Teflon-lined autoclave and placed in an oven at 130 °C for 12 h. The precipitates were washed four times with deionized water, then freeze-dried in a vacuum. Finally, the products were calcined at 400 °C for 6 h in an Ar atmosphere. Samples using MC-F prepared in a fixed bed flow-reactor were synthesized by the same process. The amount of sieved MC-F of sizes less than 100 µm or 250 µm (denoted MC-100F, or MC-250F, respectively) was determined in order to synthesize composites composed of LTO with MC-100F or MC-250F in the weight ratio of 80:10 (denoted LTO/10MC-100F, or LTO/10MC-250F, respectively). Samples using MC-100R were synthesized by the same process. The amount of added MC-100R was determined in order to synthesize composites of LTO with MC-100R in the weight ratio of 70:20 or 60:30 (denoted LTO/20MC-100R or LTO/30MC-100R). For the blank experiment, pristine LTO without MC was prepared by the same process [31].
Characterizations
The composition and the crystalline phase of the samples were identified by X-ray diffraction (XRD, RIGAKU Ultima IV) using Cu Kα radiation in the region of 2θ = 10–80°. The surface area of the samples was evaluated by N2 gas adsorption measurements at 77 K (SHIMADZU TriStarII 3020). The specific surface areas were calculated using the Brunauer–Emmett–Teller (BET) equation. The morphologies and microstructures of the samples were characterized by field emission scanning electron microscopy (FE-SEM, HITACHI S-4100, 15 kV).
Electrochemical measurements
The working electrodes were fabricated as follows. First, the prepared samples (LTO/MC composites, and pristine LTO) were combined with polyvinylidene fluoride (PVDF) in the weight ratio of 90:10. They were dispersed in an N—methylpyrrolidone (NMP) solvent to form homogeneous slurries. The slurry was spread on a Cu foil using an applicator and dried at 100 °C for 1 h. After drying, the Cu foil was diecut into circles (15-mm diameter), then dried under vacuum at 120 °C for 12 h. The galvanostatic charge–discharge measurements were conducted using CR2032 coin cells in an Ar-filled glove box. The coin cells were assembled using LTO/MC composites or pristine LTO as the working electrode, Li metal as the counter electrode, and a microporous polypropylene film (Celgard 2400) as the separator. The electrolyte used was 1 M lithium hexafluorophosphate (LiPF6) dissolved in a mixture of ethylene carbonate (EC) and diethyl carbonate (DEC). The measurements were performed by a charge–discharge instrument (Hokuto Denko HJ1001SD8) at current densities between 1.0 and 2.5 V at 20 °C. Calculation of the capacities of mAh g−1 is only based on the weights of LTO. Electrochemical impedance spectroscopy (EIS) measurements were conducted using a three-electrode cell in an Ar-filled glove box. Li metal was used for both the counter and reference electrodes. The same separator and electrolyte of the coin cells were used as the galvanostatic charge–discharge measurements. The tests were measured by AUTOLAB (Metrohm) in the frequency range from 100 kHz to 0.1 Hz. The conductivities of the LTO/MC composites were evaluated by powder resistance measurements (EAGER CORPORATION).
Results and discussion
To enhance the electrochemical performances of the LTO/MC composites, we tried to reduce the particle size of the MC. Increasing the contact area for the MC can contribute to forming denser conductive paths. We succeeded in synthesizing smaller MC particles by using a rotary fluidized bed flow-reactor (Fig. 3). We synthesized more MC particles smaller than 100 µm in when using a rotary fluidized bed flow-reactor rather than a fixed bed flow-reactor. MC particles could be prevented from combining by their movement in the reactor.
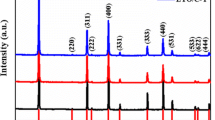
Figure 4 shows the XRD spectra of the LTO/MC-100R composites containing different amounts of MC and of pristine LTO. The XRD spectra of the pristine LTO showed peaks located at 18.5°, 35.6°, 43.3°, 47.4°, 57.2°, 62.8°, 66.2°,which could be indexed as the (1 1 1), (3 1 1), (4 0 0), (3 3 1), (3 3 3), (4 4 0), (5 3 1) planes of the spinel LTO (International Center for Diffraction Data (ICDD) No. 00–049-0207), respectively. According to a prior report [32], the reaction mechanism can be proposed as follows.
The hydrolysis reaction of the TTIP aqueous solution to form TiO2:
Lithium-ion inserts into the TiO2 to form mesophase lithium oxide:
Further reaction of the mesophase lithium titanium oxide and TiO2 can form the precursor of the Li4Ti5O12 phase:
In addition to these peaks, the XRD spectra of the LTO/10MC-100R composites showed a peak at 26°, which could be indexed as carbon. The XRD spectra of LTO/20MC-100R and LTO/30MC-100R showed the peaks of the spinel LTO as well as LTO/10MC-100R, along with a peak at 44°, which could be indexed as the (1 1 1) plane of diamond (ICDD No. 00-001-1249). The intensities of these peaks, which were not indexed as spinel LTO, became stronger with the increasing amount of added MC-100R. This tendency was caused by CNFs and the diamond core constructing MC-100R. Similarly, the XRD spectra of the LTO/10MC-250R, LTO/10MC-100F, and LTO/10MC-250F composites showed peaks corresponding to the spinel LTO that were derived from MC. They exhibited almost the same tendencies as the LTO/10MC-100R composites.
Figure 5 shows SEM images of the LTO/MC-100R composites at low and high magnifications. The low-magnification SEM images of the LTO/MC-100R composites (Fig. 2(a)–(c)) were similar to MC. The LTO particles, which are comprised of many actuate structures, were deposited in space volume among the CNFs. This could form complex conductive networks. The high-magnification SEM images of LTO/MC-100R (Fig. 2(a’)–(c’)) demonstrate that more CNFs appeared with an increasing amount of added MC-100R. These suggest that the LTO particles have more contact points with the CNFs. There was no major difference among the LTO/10MC-100R, LTO/10MC-250R, LTO/10MC-100F, and LTO/10MC-250F composites in their morphologies.
The BET surface areas of LTO, MC-R, LTO/10MC-100R, LTO/20MC-100R, and LTO/30MC-100R were 120, 129, 124, 139, and 132 m2 g−1, respectively. There was no major difference between the values for the MC and LTO/MC composites. This tendency was also observed in the other samples. The BET surface areas of LTO/10MC-250R, MC-F, LTO/10MC-100F, and LTO/10MC-250F were 125, 121, 119, and 125 m2 g−1, respectively. These results imply that the electrolyte can easily disperse in the LTO/MC composite particles even after the LTO particles are deposited in spaces among the CNFs of MC. This can improve contact between the electrolyte and active materials, thus increasing the reaction active sites of the lithium-ion.
Figure 6 shows the charge–discharge curves of the pristine LTO and LTO/MC composites at different rates from 1 to 30 C between 1.0 and 2.5 V (vs. Li/Li+). All the samples exhibited charge–discharge plateaus at approximately 1.55 V. Compared to the LTO/MC composites, the polarization of pristine the LTO increased more rapidly with the C-rate increasing. While the LTO/MC-R composites(Fig. 6(b), (c)) maintained nearly horizontal plateaus at 10C, the plateaus of the pristine LTO (Fig. 6(a)) sloped at the same rate. This result could indicate that the electronic conductive networks of the CNFs could effectively improve the performance of the LTO anodes. LTO/10MC-100R (Fig. 6(b)) exhibited almost the same length of its plateaus as LTO/10MC-250R (Fig. 6(c)). However, the polarization of LTO/10MC-100R was lower than that of LTO/10MC-250R in the high rate zone. This relationship between the composites using MC-R was observed in the composites prepared using MC-F. These results were attributable to increasing the contact area which formed denser electronic conductive paths. Comparing the MC-R with MC-F, the polarization of LTO/MC-100R (Fig. 6(b)) was lower than that of LTO/MC-100F (Fig. 6(d)) in the high rate zone. LTO/10MC-250R (Fig. 6(c)) and LTO/10MC-250F (Fig. 6(e)) also showed a similar relationship. MC-R contained a higher number of smaller particles than MC-F (Fig. 3), which could improve the charge–discharge performances.
Figure 7 shows comparisons of the rate performances of the pristine LTO, LTO/10MC-100R, LTO/10MC-250R, LTO/10MC-100F, and LTO/10MC-250F. The pristine LTO dramatically decreased in capacity at 10C, but the LTO/MC composites retained their capacities at the same rate. Also in the higher rate zones, the pristine LTO exhibited decreased capacities. LTO/10MC-100R and LTO/10MC-100F exhibited almost the same tendencies as LTO/10MC-250R and LTO/10MC-250F, respectively. Composites using MC-R decreased less than the composites using MC-F, especially in the high rate zone. The discharge capacities of the LTO/10MC-100R electrode were 170, 159, 145, 129, 85, and 47 mAh g−1 at 1, 2, 5, 10, 20, and 30C, respectively.
Figure 8 shows the EIS of LTO/10MC-100R and LTO/10MC-100F, while the inset is an equivalent circuit to fit the alternating current (AC) impedance spectra. Rs represents the solution resistance, Rct represents the charge transfer resistance, Zw represents the Warburg impedance, and Cd represents the double-layer capacitance. Table 1 summarizes the results of fitting the electrodes of LTO/10MC-100R, and LTO/10MC-100F. LTO/10MC-100R exhibited a lower Rct (31.8 Ω) than LTO/10MC-100F (48.6 Ω). The electric conductivity of LTO/10MC-100R is higher than that of LTO/10MC-100F. This result agreed with the charge–discharge results (Fig. 6(b), (d)).
We next investigated how the amounts of MC influenced the electrochemical performances. Figure 9 shows the powder resistances of LTO/MC-100R prepared by adding different amounts of MC-100R. The resistances decreased with the added MC-100R. This result agreed with the SEM images showing that the contact points between the LTO particles and CNFs increased with the increasing amounts of added MC. Figure 10 shows the charge–discharge curves of LTO/20MC-100R and LTO/30MC-100R. Figure 6(b) shows the charge–discharge curves of LTO/10MC-100R as previously described. The plateaus of the LTO/MC composites in the charge–discharge curves tended to be longer at every rate with the added MC (Figs. 6(b), 10(a), (b)). This can be attributed to improvement of the electronic conductivity in the LTO/MC composites by adding more MC (Fig. 9). Figure 11 compares the rate performances of the pristine LTO, LTO/10MC-100R, LTO/20MC-100R and LTO/30MC-100R composites by adding different amounts of MC-100R. With the increasing MC-100R, the discharge capacities of the LTO/MC-100R composites increased at every rate. Although the LTO/30MC-100R composites needed the highest amount of MC-100R, the LTO/30MC-100R composites showed the best electrochemical performance. The discharge capacities of LTO/30MC-100R were 183, 172, 158, 148, 134, and 113 mAh g−1 at 1, 2, 5, 10, 20, and 30C, respectively. By adding large amounts of MC-100R, the LTO/30MC-100R composite could offer excellent electron transport. The discharge capacity could increase by adding even more MC-100R. However, some discharge capacities surpassed the theoretical capacity of the spinel LTO (175 mAh g−1). Lithium-ion inserted into the graphene layers of the CNFs could have contributed to the lithium-ion storage.
Conclusions
We successfully synthesized LTO/MC composites by a hydrothermal synthesis process followed by a calcination treatment. Their uniform electric paths contributed to improving the specific capacity and rate capability compared to the pristine LTO. Sieving a smaller MC improved the charge–discharge performances. We observed that the LTO/MC composites showed increasing charge–discharge performances with the increasingly smaller MC. This is attributed to the increasing contact area. A smaller MC was synthesized by a rotary fluidized bed flow-reactor rather than a fixed type of flow-reactor. The LTO/MC composites show higher electrochemical performances when MC is synthesized by a rotary fluidized bed flow-reactor rather than a fixed type of flow-reactor. The LTO/MC composites showed higher discharge capacities with the increasing amount of added MC. This result is due to more conducting networks formed by the CNFs. In this study, the 30 wt% MC prepared using a rotary fluidized bed flow-reactor exhibited the best electrochemical performances.
We concluded that the LTO/MC composites are a promising anode material for lithium-ion batteries.
References
Tarascon J-M, Armand M (2001) Issues and challenges facing rechargeable lithium batteries. Nature 414:359–367
Yi TF, Yang SY, Xie Y (2015) Recent advances of Li4Ti5O12 as a promising next generation anode material for high power lithium-ion batteries. J Mater Chem A 3:5750–5777
Yang C, KungS. j. Lin and W. C. Chien, SH (2014) Li3V2(PO4)3/C composite materials synthesized using the hydrothermal method with double-carbon sources. J Power Sources 251:296–304
Ise K, Morimoto S, Harada Y, Takami N (2018) Large lithium storage in highly crystalline TiNb2O7 nanoparticles synthesized by a hydrothermal method as anodes for lithium-ion batteries. Solid State Ionics 320:7–15
Ferg E, Gummow RJ, Kock A, Thackeray MM (1994) Spinel Anodes for Lithium-Ion Batteries. J Electrochem Soc 141:L147–L150
Ohzuku T, Ueda A, Yamamoto N, Iwakoshi Y (1995) Factor affecting the capacity retention of lithium-ion cells. J Power Sources 54:99–102
Jansen AN, Kahaian AJ, Kepler KD, Nelson PA, Amine K, Dees DW, Vissers DR, Thackeray MM (1999) Development of a high-power lithium-ion battery. J Power Sources 81–82:902–905
Takami N, Inagaki H, Kishi T, Harada Y, Fujita Y, Hoshina K (2009) Electrochemical Kinetics and Safety of 2-Volt Class Li-Ion Battery System Using Lithium Titanium Oxide Anode. J Electrochem Soc 156:A128–A132
Goodenough JB, Kim Y (2010) Challenges for Rechargeable Li Batteries. Chem Mater 22:587–603
Hang T, Mukoyama D, Nara H, Takami N, Momma T, Osaka T (2013) Electrochemical impedance spectroscopy analysis for lithium-ion battery using Li4Ti5O12 anode. J Power Sources 222:442–447
Ge H, Chen L, Yuan W, Zhang Y, Fan Q, Osgood H, Matera D, Song XM, Wu G (2015) Unique mesoporous spinel Li4Ti5O12 nanosheets as anode materials for lithium-ion batteries. J Power Sources 297:436–441
Pu Z, Lan Q, Li Y, Liu S, Yu D, Lv XJ (2019) Preparation of W-doped hierarchical porous Li4Ti5O12/brookite nanocomposites for high rate lithium ion batteries at −20 °C. J Power Source 437(22689):1–10
Zhang Q, Verde MG, Seo JK, Li X, Meng YS (2015) Structural and electrochemical properties of Gd-doped Li4Ti5O12 as anode material with improved rate capability for lithium-ion batteries. J Power Sources 280:355–362
Liang Q, Cao N, Song Z, Gao X, Hou L, Guo T, Qin X (2017) Co-doped Li4Ti5O12 nanosheets with enhanced rate performance for lithium-ion batteries. Electrochim Acta 251:407–414
Zhao S, Ka O, Xian X, Sun L, Wang J (2016) Effect of primary crystallite size on the high-rate performance of Li4Ti5O12 microspheres. Electrochim Acta 206:17–25
Li X, Hu H, Huang S, Yu G, Gao L, Liu H, Yu Y (2013) Nano-sized Li4Ti5O12 anode material with excellent performance prepared by solid state reaction: The effect of precursor size and morphology. Electrochim Acta 112:356–363
Kashkooli AG, Lui G, Farhad S, Lee DU, Feng K, Yu A, Chen Z (2016) Nano-particle size effect on the performance of Li4Ti5O12 spinel. Electrochim Acta 196:33–40
Wang D, Shan Z, Liu X, Na R, Wang J, Liu H, j. Tian, (2018) High-rate Li4Ti5O12/porous activated graphene nanoplatelets composites using LiOH both as lithium source and activating agent. Electrochim Acta 262:9–17
H. p. Liu, G. w. Wen, S. f. Bi, C. y. Wang, J. m. Hao and P. Gao, (2016) High rate cycling performance of nanosized Li4Ti5O12/graphene composites for lithium ion batteries. Electrochim Acta 192:38–44
Cao N, Song Z, Liang Q, Gao X, Qin X (2017) Hierarchical Li4Ti5O12/C composite for lithium-ion batteries with enhanced rate performance. Electrochim Acta 235:200–209
Guo X, Wang C, Chen M, Wang J, Zheng J (2009) Carbon coating of Li4Ti5O12 using amphiphilic carbonaceous material for improvement of lithium-ion battery performance. J Power Source 214:107–112
Kang E, Jung YS, Kim G-H, Chun J, Wiesner U, Dillon AC, Kim JK, Lee J (2011) Highly Improved Rate Capability for a Lithium-Ion Battery Nano-Li4Ti5O12 Negative Electrode via Carbon-Coated Mesoporous Uniform Pores with a Simple Self-Assembly Method. Adv Funct Mater 21:4349–4357
Nakagawa K, Oda H, Yamashita A, Okamoto M, Sato Y, Gamo H, N-Gamo M, Ogawa K (2009) A novel spherical carbon. J Mater Sci 44:221–226. https://doi.org/10.1007/s10853-008-3081-4
Ando T, Nakagawa K, N-Gamo M, Oda H (2009) Cladophora-form carbon comprising carbon nanomaterials radially grown on a spherical core, process for producing the same and production apparatus. US Pat 7(608):331
Eguchi M, Baba K, Onuma T, Yoshida K, Iwasawa K, Kobayashi Y, Uno K, Komatsu K, KoboriN-GamoAndo MMT (2012) Influence of Ionomer/carbon ratio on the performance of a polymer electrolyte fuel cell. Polymers 4:1645–1656
Iwasawa K, Eguchi M, Miyoshi K, Ueda S, N-Gamo M, Ando T (2013) Preparation of TiO2/Marimo carbon composite. Transactions Mater Res Soc Jpn 38(4):573–577
Eguchi A, Okubo S, Yamamoto M, Kikuchi K, Uno Y, KobayashiN-GamoAndo MT (2010) Preparation of catalyst for a polymer electrolyte fuel cell using a novel spherical carbon support. J Power Sources 195:5862–5867
Eguchi M, Satou K, Iwasawa K, Nishitani-Gamo M, Ando T (2013) Preparation of the Pt-Co bimetallic catalyst on marimo carbon for PEFC. Transactions Mater Res Soc Jpn. 38(4):549–553
Baba K, N-Gamo M, Ando T, Eguchi M (2016) Durable Marimo-like carbon support for platinum nanoparticle catalyst in polymer electrolyte fuel cell. Electrochim Acta 213:447–451
Baba K, N-Gamo M, Ando T, Eguchi M (2017) Preparation of catalyst for polymer electrolyte fuel cell using the Marimo-like carbon. Transactions Mater Res Soc Jpn 42(2):51–56
Chen J, Yang L, Fang S, Tang Y (2010) Synthesis of sawtooth-like Li4Ti5O12 nanosheets as anode materials for Li-ion batteries. Electrochim Acta 55:6596–6600
Wu HY, Hon MH, Kuan CY, Leu IC (2015) Hydrothermal synthesis of Li4Ti5O12 nanosheets as anode materials for lithium ion batteries. RSC Adv 5:35224–35229
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Handling Editor: Kyle Brinkman.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hasegawa, K., Gunji, H., Kijima, R. et al. Electrochemical performance of marimocarbon/lithium titanate composites synthesized by hydrothermal method for lithium-ion batteries. J Mater Sci 56, 16602–16611 (2021). https://doi.org/10.1007/s10853-021-06319-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-021-06319-w