Abstract
The large-scale consumption and discharge of antibiotic tetracycline (TC) urge us to search for a highly efficient and eco-friendly technology to remove it. In this work, face-contact ZnSnO3@g-C3N4 core–shell heterojunction was successfully constructed via one-step calcination route. The experimental data indicate that the photocatalytic TC removal performance of ZnSnO3@g-C3N4 (1:3) reaches 90.8% within 120 min under the same condition compared with bulk g-C3N4 (32% degradation) and ZnSnO3 (9% degradation). The improved photocatalytic activity is ascribed to the formation of core–shell structure between ZnSnO3 and g-C3N4 which not only enlarges visible light response but also effectively separates electron–hole pairs. Meanwhile, this face-contact ZSO-CN photocatalyst displays much more contact interfaces than the point-contact ZSO-CN photocatalyst, and the contact interfaces could play the part of efficient channels for charge transfer. Finally, the photocatalytic reaction mechanism on ZnSnO3@g-C3N4 was also stated at length through active species capture and electron spin resonance (ESR) tests. And the possible intermediates products were discussed through the liquid chromatography–mass spectrometry (LC–MS) analysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the abuse of antibiotics, the random disposal of drug waste and the wanton discharge of drug wastewater, the purification of wastewater containing antibiotics has become a problem of human concern [1]. Semiconductors-based photocatalysis is considered as a promising and effective technology to remove organic contaminants in the aqueous solution because of its simple equipment and easy control without secondary pollution [2,3,4,5,6,7,8,9,10,11]. Up to the present, various metal oxide semiconductors have been developed as photocatalysts, such as TiO2, ZnO, SnO2, Bi2O3, BiVO4 and ZnSnO3 [12,13,14,15,16]. Among them, ZnSnO3 has attracted great interest in the photocatalytic field due to its high electron mobility, fast electrical conductivity and strong negative reduction potential of excited electrons [17, 18]. However, like most wide-band-gap semiconductors, ZnSnO3 cannot be excited to generate photogenerated carriers in the visible region, which leads to practical limitation [19].
Considerable research efforts have been devoted to expanding the photocatalytic activity of ZnSnO3 in the range of visible light, which include doping elements to broaden the visible light absorbance [20] and carbon-based material sensitization to enhance the visible-light-response range [21]. Besides, coupling the ZnSnO3 with the visible-light-driven semiconductors to form heterojunctions is also an advisable strategy [22]. Recently, graphitic carbon nitride (g-C3N4) has been attracted much attention because of its relatively narrow band gap, low cost, high chemical stability and innocuity [23,24,25,26,27]. In recent years, many reports of employing g-C3N4 to improve the photocatalytic performance of wide-band-gap semiconductors have been published. For example, Tan et al. modified the TiO2 with g-C3N4, which expands the light absorption of the photocatalysts and exhibits the favorable photocatalytic H2 evolution efficiency under visible light [28]. Tang’s group synthesized g-C3N4/ZnO and displayed the excellent visible light photodegradable efficiency by degrading 94% of Rhodamine B (RhB) in two hours, which is mainly caused by the heterostructure between g-C3N4 and ZnO; the photoexcited electrons in CB of g-C3N4 can be transferred into the CB of ZnO, thus effectively separating electrons and holes [29]. Zhang et al. fabricated visible-light-response Bi2O2CO3/g-C3N4 heterojunction photocatalysts which showed greatly improved activity for removing the three model pollutants of CH3CHO, RhB and Cr (VI) [30]. Concerning the research on g-C3N4/ZnSnO3 composite, Chen’s group has utilized zero-dimensional (0D) ZnSnO3 nanoparticles to coat on the surface of two-dimensional (2D) g-C3N4 nanosheets forming the g-C3N4/ZnSnO3 heterojunction to effectively enhance its photocatalytic activity under visible light irradiation [31]. However, with respect to the contact area at the interface of the heterojunction, the reactive sites of this point contact formed by 0D/2D heterojunction are often less than that of the face-contact heterojunction [32]. Thus, in order to further enhance the photocatalytic activity, the construction of g-C3N4/ZnSnO3 heterojunction with face-contact is more reasonable.
As is known, to design and develop the core–shell structure could provide more channels for charge transport and more surface reactive sites. For example, Hou et al. prepared BiVO4@TiO2 core–shell hybrid mesoporous nanofibers for photocatalytic hydrogen production, exhibiting the superior photocatalytic H2 production efficiency benefiting from the high specific surface area with increased reaction sites [33]. Meanwhile, Liu’s group prepared the Cd2Ge2O6/CdS core–shell structure photocatalysts, which significantly improve the charge migration capability due to the large-area contact of core–shell structure [34]. In addition, the construction of core–shell structure can also improve the stability of the core material by covering with a stable shell material. An et al. prepared the core–shell Ag2CO3@g-C3N4 photocatalyst, aiming at the prevention of the photo-corrosion of Ag2CO3 through the outer g-C3N4 shell in the composite [35]. Similarly, Jia et al. reported hybrid core–shell microgels with Cu2O nanocubes as the core and thermosensitive PNIPAM as the shell, which presents excellent colloidal stability [36]. Consequently, systematic design and controllable prepared g-C3N4/ZnSnO3 with core–shell nanostructure is expected to achieve the high active visible-light-driven heterojunction photocatalyst.
In this work, face-contact ZnSnO3@g-C3N4 core–shell heterojunction was successfully constructed via the calcination route. This composite photocatalyst is used for degrading TC under visible light irradiation (λ > 420 nm). It not only expands the absorption of visible light but also effectively separates the electrons and holes; thus, the photocatalytic degradation performance of TC is greatly improved. Moreover, the possible degradation mechanism over ZnSnO3@g-C3N4 was investigated through active species capture and electron spin resonance (ESR) experiments.
Experimental section
Synthesis of g-C3N4
Typically, 2 g melamine was in a covered crucible heated to 550 °C maintained for 3 h with a ramp speed of 5 °C/min. After cooling to room temperature, the resulting solids were ground to obtain the g-C3N4 powder.
Synthesis of ZnSn(OH)6 cubes
ZnSn(OH)6 cubes were prepared using a typical synthetic method according to the previous report [16]. First, 0.158 g of SnCl4·5H2O was dissolved in 20 mL of deionized water and stirred at room temperature. The pH of the mixed solution was adjusted to 12.0 by adding NaOH solution, and 0.1295 g of ZnSO4·7H2O was added under stirring at 60 °C until the appearance of a white precipitate. It was then aged at 60 °C for 12 h, cooled to room temperature and washed six times with distilled water and absolute ethanol to remove residual ions. Finally, the white precipitate was dried at 60 °C to obtain ZnSn(OH)6 cubes powder.
Synthesis of ZnSnO3@g-C3N4
The synthetic process of ZnSnO3@g-C3N4 is shown in Scheme 1. Briefly, 200 mg of ZnSn(OH)6 cubes was immersed in 20 mL deionized water, and then a certain mass of cyanamide solution (50% aqueous solution) was added. Next, the mixed solution was sonicated at 60 °C for 2 h and stirred for 12 h. It was then centrifuged, dried and calcined at 580 °C for 2 h. The obtained ZnSnO3@g-C3N4 samples were expressed as ZSO@CN (X), where X refers to the mass ratio of ZnSn(OH)6 and cyanamide. The pure ZnSnO3 sample was fabricated under the same conditions in the absence of cyanamide and labeled as ZSO.
The characterizations and photocatalytic experiments can be listed in the Supporting Information.
Results and discussion
The morphologies of ZSO and ZSO@CN (1:3) were observed by scanning electron microscope (SEM) and transmission electron microscope (TEM). From the SEM image of ZSO (Fig. 1a), it can be seen that the ZSO possesses a cube-like morphology with smooth surfaces and the average size of around 200 nm. After introducing the CN, the morphology of ZSO@CN composite is hardly changed in comparison with pure ZSO (Fig. 1b). And the specific morphologies of the synthesized samples were further employed by the TEM measurement. As can be seen in Fig. 1c, d, the lattice spacing on the crystal we measured is 0.262 nm, which corresponds to the (110) plane of ZnSnO3 nanocubes [37]. Notably, for the ZSO@CN composite, the layer of CN is coated on the outer surface of nanocube-like ZSO material, which is approximately 4.0 nm (Fig. 1e, f) to form a distinct core–shell structure.
To estimate the distribution of the elements in the ZSO@CN composite, the HAADF-SEM and elements mapping were carried out. As shown in Fig. 2a–f, the HAADF-SEM and C, N, Zn, Sn and O elements are detected and all the elements are continuous and concentrating distribution, unambiguously identifying that CN has been successfully coated on the outer surface of ZSO cubic nanostructure, although the C and N elements in the elements mapping are not obvious due to the low content of CN on the outer surface.
In Fig. 3, X-ray diffraction (XRD) patterns of CN, ZSO and ZSO@CN (1:3) were recorded. It is observed that there are two characteristic diffraction peaks at (002) and (100) planes in the XRD pattern of CN, which were ascribed to in-plane structural packing motifs and the interlayer accumulation of aromatic system [38]. For the porous ZSO cubes, the characteristic diffraction peaks are assigned to the (012), (110) and (116) planes, respectively [39]. The XRD pattern of as-prepared ZSO@CN (1:3) sample almost shows the typical peaks of ZSO. However, the characteristic peaks of CN shell could not be observed in the XRD pattern of ZSO@CN (1:3) sample, which is due to the shell thickness that belongs to a short-range ordered structure and the limited amount and low intensity of CN [40].
As illustrated in Fig. 4, the Fourier transform infrared (FT-IR) spectra of ZSO, pure CN and ZSO@CN (1:3) samples are adopted. From the spectra of CN, several peaks between 1230 and 1650 cm−1 are observed, which ascribe to the aromatic C–N stretching and C=N stretching vibration modes [41]. The other two peaks about 808 and 3200–3400 cm−1 are observed separately in the spectra: The 808 cm−1 can be attributed to the breathing mode of triazine unit, whereas the 3200–3400 cm−1 to the stretching vibration of N–H [42]. For pristine ZSO, the peaks located at 475 and 600 cm−1 can be attributed to the vibration modes of Zn–O and Sn–O [43]. Similar to the XRD results, the main characteristic peaks of ZSO@CN (1:3) are basically consistent with ZSO in the FT-IR spectra. In the ZSO@CN (1:3) core–shell composite photocatalyst, it is difficult to observe the main characteristic peaks of CN, which is due to the low content of CN in the ZSO@CN (1:3).
X-ray photoelectron spectroscopy (XPS) technique was performed for confirming the chemical valences and compositions of CN, ZSO and ZSO@CN (1:3), and the corresponding spectra are displayed in Fig. 5. Figure 5a shows two main elements (C and N) in the survey spectrum of CN and three main elements (Zn, Sn and O) in the survey spectrum of ZSO, respectively. However, there are no obvious diffraction peaks representing C and N elements in the survey spectrum of ZSO@CN, which is due to the little content of CN in ZSO@CN, but reflected the peak intensities of the C 1 s and N 1 s high-resolution XPS spectra. There are two obvious diffraction peaks located at 288.2 eV and 284.6 eV in the high-resolution XPS spectrum of C 1 s for CN (Fig. 5b), which belongs to sp2 C–C bonds and sp2-bonded carbon (N–C=N), respectively [44]. The N 1 s spectrum of CN (Fig. 5c) can be decomposed into three peaks located at 398.5, 399.2 and 400.7 eV, which are corresponded to C–N–C, N–(C)3 and N–H, respectively [29]. The Zn 2p spectrum of ZSO (Fig. 5d) should be deconvoluted by two peaks at 1021.6 and 1044.7 eV which are associated with Zn 2p3/2 and Zn 2p1/2. In the Sn 3d spectrum of ZSO (Fig. 5e), the peaks appear at 486.3 eV for Sn 3d3/2 and 494.8 eV for Sn 3d5/2, respectively. The O 1 s spectra of ZSO (Fig. 5f) centered at 530.1 and 531.4 eV are attributed to the metal oxide bonds and –OH groups, respectively [45]. From the high-resolution XPS spectra of ZSO@CN (1:3), the positive shifts of five elements are seen, which are due to the heterojunction formed successfully.
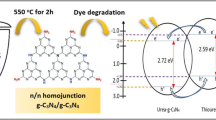
In order to study the optical properties of as-prepared photocatalysts, the UV–Vis diffuse reflectance spectra of CN, ZSO and ZSO@CN (1:3) samples are measured and displayed in Fig. 6a. It can be seen that pristine CN shows an obvious absorption edge at around 478 nm, and in the visible light range of 400–800 nm pure ZSO shows a weak absorption. When coupling ZSO core with CN shell, the absorption capacity of ZSO@CN (1:3) composite photocatalyst has been enhanced compared with pure phase ZSO, which indicated that the addition of CN could improve the utilization rate of ZSO to light, so it was beneficial to the improvement in photocatalytic performance. According to the Tauc formula, the band gap values of ZSO and CN can be calculated to be 3.25 and 2.73 eV (Fig. 6b), respectively.
The visible-light-driven photocatalytic performance of the prepared photocatalysts was tested by the degradation of TC solution. Before the photocatalytic experiment, in the dark for 30 min, all the samples reached the adsorption equilibrium. The photocatalytic test results (Fig. 7a) reveal that compared to individual CN (32% degradation) and ZSO (9% degradation) within the same irradiation time, the ZSO@CN core–shell composite photocatalysts owned the superior photocatalytic activity. Notably, the photocatalytic activity of the ZSO@CN core–shell structure depends on the quality of CN introduction. With the increase in CN mass content (from 1:0.5 to 1:3), the photocatalytic activity of ZSO@CN core–shell structure was enhanced, and ZSO@CN (1:3) exhibited the optimum TC degradation rate of 90.8% after 120-min irradiation. Further increasing the content of CN mass can lead to a decrease in the ZSO@CN core–shell structure. This is attributed to the excessive CN (1:5 and 1:10 in the core–shell structure when the amount of CN is higher than its optimum deposition) which could lead to agglomeration state of the CN surface under such conditions without forming an effective core–shell interface, which hinders the transfer of photogenerated electrons [46]. As shown in Fig. 7b, all prepared photocatalysts follow the pseudo-first-order rule. Based on the calculated k values, the ZSO@CN (1:3) owns the highest TC degradation k value (0.019 min−1), which is around 6.33 and 31.67 times higher than that of CN (0.003 min−1) and ZSO (0.0006 min−1), respectively. Besides, for exploring the mineralization degree of TC during the photocatalysis, total organic carbon (TOC) removal rate over the ZSO@CN under visible light illumination was tested. As exhibited in Fig.S1, the removal rate of TOC was only 0.5% without photocatalyst, while ZSO@CN (1:3) photocatalyst reduced TOC by 46.2%. The results display that the ZSO@CN composite had good mineralization efficiency for the degradation of TC. In order to further reveal the advantage of the as-prepared photocatalyst with surface contact, we carried out a comparative experiment of ZSO nanoparticles coated on the surface of CN nanosheets to form point-contact ZSO-CN based on previous report [31]. The results of TC degradation and the pseudo-first-order reaction kinetics and k values of the as-prepared photocatalysts with different contact modes for TC degradation were further investigated. As displayed in Fig. 7c, it can be seen that the degradation of TC solution of the ZSO-CN with face contact is always higher than that with point contact. From Fig. 7d, the face-contact ZSO-CN shows the higher TC degradation k value (0.019 min−1), which is about 2.71 times higher than that of point-contact ZSO-CN (0.007 min−1). It is obvious that the face-contact ZSO-CN photocatalyst displays much more contact interfaces than the point-contact ZSO-CN photocatalyst, and the contact interfaces could play the part of efficient channels for charge transfer [47], leading to the higher photocatalytic degradation performance of face-contact ZSO-CN composite photocatalyst.
a TC degradation over as-prepared photocatalysts under visible light irradiation and b the corresponding pseudo-first-order reaction kinetics and rate constants. c TC degradation over ZSO-CN composite photocatalysts with different contact modes under visible light irradiation and d the corresponding pseudo-first-order reaction kinetics and rate constants
The BET analysis was carried out, and the result is shown in Fig.S2. It can be seen that the specific surface area of ZSO@CN (40.3 m2 g−1) is higher than that of ZSO (35.1 m2 g−1). This result reveals that the increasing specific surface area in ZSO@CN can provide more activity sites and be beneficial to improve the photocatalytic activity. Photocurrent experiments were carried out to reveal the separation and migration properties of photoinduced electron–hole pairs. The results are shown in Fig. 8a. Because of the wide band gap of ZSO, it is hard to use visible light, and the photocurrent intensity of pristine ZSO is very low. Notably, the photocurrent intensity for pure CN is higher than that of ZSO, which is consistent with UV–Vis diffuse reflectance spectra. Evidently, the ZSO@CN (1:3) photocatalyst displayed the highest photocurrent intensity, implying that the core–shell heterostructure between CN and ZSO is beneficial to promote the separation of electrons and holes. Similarly, compared to other samples (Fig. 8b), the impedance arc radius of the ZSO@CN (1:3) photocatalyst was the smallest, which also verified that charge transfer is promoted in the core–shell heterostructure.
Additionally, nanosecond (ns) time-resolved fluorescence decay spectra were recorded to further investigate charge transfer and separation (Fig. 9a). The corresponding parameters are summarized in Table 1; the fluorescence peak decay of ZSO@CN (1:3) (τ1 = 1.08 ns, τ2 = 4.82 ns) was shorter than that of the CN with half-lives of τ1 = 2.05 ns and τ2 = 6.50 ns. Meanwhile, the corresponding average fluorescence lifetimes (τav) of CN and ZSO@CN (1:3) are obtained to be 5.21 and 3.22 ns, respectively. The shorter lifetime indicates that the recombination rate of photogenerated charge carriers decreases, which makes the separation efficiency of photoinduced charge higher, according to the previous reports [48]. Hence, the τav of ZSO@CN (1:3) is shorter than that of CN, which further proves that the interfacial charge transfer can promote charge separation. In order to assess the stability of ZSO@CN composite photocatalyst, the recycling experiment was performed and the result is exhibited in Fig. 9b. After four recycles, the photocatalytic degradation rate of TC remained above 83%, revealing that the ZSO@CN (1:3) composite possesses the outstanding photocatalytic stability.
In order to confirm the main active species of ZSO@CN, different scavengers, such as nitrogen (N2), isopropyl (IPA) and disodium ethylenediamine tetra-acetic acid (EDTA), were added into the reaction solution for capturing superoxide radicals (·O2−), hydroxyl radicals (·OH) and holes (h+), respectively. As shown in Fig. 10a, the addition of IPA into the reaction system did not significantly inhibit the degradation rate of TC. However, after adding N2 and EDTA into the reaction system, the photocatalytic degradation rate of ZSO@CN distinctly decreased from 91 to 49% and 33%, respectively. As shown in Fig. 10b, taking ZSO@CN composite under visible light irradiation, electron spin resonance (ESR) test was carried out to further clarify the existence of ·O2− active substance in the photocatalysis. In the absence of light, there is no obvious peak of ·O2−. Under visible light irradiation, the peak value of the ·O2− crack of the ZSO@CN composite was significantly enhanced. In summary, as we all know, ·O2− and h+ are produced during the photocatalytic process and play the significant roles in the degradation of TC over ZSO@CN core–shell composite photocatalyst.
To further explore the main intermediate products during the photocatalysis over ZSO@CN (1:3) photocatalyst, the liquid chromatography–mass spectrometry (LC–MS) technique was performed and the corresponding spectrum is exhibited in Fig.S3. It is well known that the substance with a molecular weight of 445 is TC [49]. Moreover, m/z values of 410, 388, 337, 241, 201, 154 and 141 were also formed in the degradation process, which were labeled as TC2–TC8, respectively, and the possible degradation pathways are revealed in Fig. 11, through further consideration of intermediates. Based on previous reports [50, 51], three typical functional groups (phenolic group, double bond and amine group) were easily attacked by O2− and h+ [52, 53]. TC2 was formed through breaking bond reaction from TC. TC3 and TC4 were formed via dehydration reaction, losing N-methyl and ring opening reaction. The products TC5, TC6, TC7 and TC8 with smaller molecular weight were formed by ring opening reaction and the breaking bond reaction. The above reaction is actually produced by the synergistic effect of O2− and h +. Finally, most of the intermediate products would be degraded into CO2 and H2O.
According to the above analysis, the possible photocatalytic mechanism over ZSO@CN core–shell heterostructure photocatalyst was initially described and is discussed in Fig. 12. By comparing the energy levels of ZSO with CN, it is exactly right to find that the energy levels of ZSO and CN are well-matched overlapping band structures for forming the type II heterojunction [46]. When exposed to visible light, due to the narrower band gap of CN (2.73 eV), the photoinduced electrons and holes were produced, while for ZSO (Eg = 3.25 eV), it was difficult to produce photogenerated charges during the visible light due to its broader band gap. The suitable energy band positions of CN and ZSO were inferred and are drawn in the right of Fig. 12. Specifically, the photoexcited electrons could transfer from CB of CN (− 1.16 eV vs. NHE) to the CB of ZSO (− 0.15 eV vs. NHE) and left the photoinduced holes on the VB of CN; hence, the separation rate of photogenerated electron–hole pairs was promoted effectively. The CB potential of ZSO was more negative than O2/·O2− (− 0.046 eV vs. NHE); photogenerated electrons on the CB of ZSO can capture O2 to form ·O2− directly [54]. Owing to the VB of ·OH/OH− (2.38 eV vs. NHE) was higher CN (1.53 eV vs. NHE), therefore the h+ directly oxidizes TC in VB of CN through photocatalytic reaction. At the same time, the process can be described as follows:
Conclusions
In summary, ZSO@CN core–shell heterojunction was successfully constructed via a simple calcination route to improve the photocatalytic performance for the degradation of TC under visible light irradiation. The results indicated that the photocatalytic activity of as-prepared face-contact core–shell ZSO@CN is higher than that of pure ZSO, CN and point-contact ZSO-CN. The reason for higher photocatalytic degradation performance of face-contact ZSO-CN composite photocatalyst is mainly ascribed to the core–shell structure between CN and ZSO, which could own much more contact interfaces than that of point-contact ZSO-CN photocatalyst that play the part of efficient channels for charge transfer, leading to the higher photocatalytic degradation performance of face-contact ZSO-CN composite photocatalyst. The capture experiments indicated that both ·O2− and h+ played significant roles in the photocatalysis. This study laid a foundation to prepare for more ZSO-containing composite photocatalysts and promote the purification of wastewater containing organic pollutants.
References
Gothwal R, Shashidhar T (2015) Antibiotic pollution in the environment: A review. Clean-Soil, Air, Water 43:479–489
Guo F, Shi W, Li M, Shi Y, Wen H (2019) 2D/2D Z-scheme heterojunction of CuInS2/g-C3N4 for enhanced visible-light-driven photocatalytic activity towards the degradation of tetracycline. Sep Purif Technol 210:608–615
Guo F, Li M, Ren H, Huang X, Hou W, Wang C, Shi W, Lu C (2019) Fabrication of p-n CuBi2O4/MoS2 heterojunction with nanosheets-on-microrods structure for enhanced photocatalytic activity towards tetracycline degradation. Appl Surf Sci 491:88–94
Lu CY, Guo F, Yan QZ, Zhang ZJ, Li D, Wang LP, Zhou YH (2019) Hydrothermal synthesis of type II ZnIn2S4/BiPO4 heterojunction photocatalyst with dandelion-like microflower structure for enhanced photocatalytic degradation of tetracycline under simulated solar light. J Alloy Compd 811:151976–151986
Zhu Q, Sun Y, Na F, Wei J, Xu S, Li Y, Guo F (2019) Fabrication of CdS/titanium-oxo-cluster nanocomposites based on a Ti32 framework with enhanced photocatalytic activity for tetracycline hydrochloride degradation under visible light. Appl Catal B: Environ 254:541–550
Shi W, Li M, Ren H, Guo F, Huang X, Shi Y, Tang Y (2019) Construction of a 0D/1D composite based on Au nanoparticles/CuBi2O4 microrods for efficient visible-light-driven photocatalytic activity. Beilstein J Nanotechnol 10:1360–1367
Shi W, Li M, Huang X, Ren H, Guo F, Yan C (2020) Three-dimensional Z-Scheme Ag3PO4/Co3(PO4)2@Ag heterojunction for improved visible-light photocatalytic degradation activity of tetracycline. J Alloy Compd 818:152883–152893
Yang S, Liu C, Wang J, Lin X, Hong Y, Guo F, Shi J (2020) Enhanced photocatalytic activity of g-C3N4 quantum dots/Bi3.64Mo0.36O6.55 nanospheres composites. J Solid State Chem 287:121347–121355
Shi W, Liu C, Li M, Lin X, Guo F, Shi J (2020) Fabrication of ternary Ag3PO4/Co3(PO4)2/g-C3N4 heterostructure with following Type II and Z-Scheme dual pathways for enhanced visible-light photocatalytic activity. J Hazard Mater 389:121907–121918
Shi W, Li M, Huang X, Ren H, Guo F, Tang Y, Lu C (2020) Construction of CuBi2O4/Bi2MoO6 p-n heterojunction with nanosheets-on-microrods structure for improved photocatalytic activity towards broad-spectrum antibiotics degradation. Chem Eng J 394:125009–125019
Jia MY, Yang ZH, Xu HY, Song PP, Xiong WP, Cao J, Zhang YR, Xiang YP, Hu JH, Zhou CY, Yang Y, Wang WJ (2020) Integrating N and F co-doped TiO2 nanotubes with ZIF-8 as photoelectrode for enhanced photo-electrocatalytic degradation of sulfamethazine. Chem Eng J 388:124388–124401
Wang C, Xu B-Q, Wang X, Zhao J (2005) Preparation and photocatalytic activity of ZnO/TiO2/SnO2 mixture. J Solid State Chem 178:3500–3506
Chen L, Zhang Q, Huang R, Yin SF, Au CT (2012) Porous peanut-like Bi2O3-BiVO4 composites with heterojunctions: one-step synthesis and their photocatalytic properties. Dalton Trans 41:9513–9518
Borhade AV, Baste YR (2017) Study of photocatalytic asset of the ZnSnO3 synthesized by green chemistry. Arab J Chem 10:S404–S411
Guo F, Sun H, Cheng L, Shi W (2020) Oxygen-defective ZnO porous nanosheets modified by carbon dots to improve their visible-light photocatalytic activity and gain mechanistic insight. New J Chem 44:11215–11223
Guo F, Huang X, Chen Z, Ren H, Li M, Chen L (2020) MoS2 nanosheets anchored on porous ZnSnO3 cubes as an efficient visible-light-driven composite photocatalyst for the degradation of tetracycline and mechanism insight. J Hazard Mater 390:122158–122170
Bing Y, Zeng Y, Liu C, Qiao L, Sui Y, Zou B, Zheng W, Zou G (2014) Assembly of hierarchical ZnSnO3 hollow microspheres from ultra-thin nanorods and the enhanced ethanol-sensing performances. Sensors Actuat B: Chem 190:370–377
Guo F, Huang X, Chen Z, Sun H, Shi W (2020) Investigation of visible-light-driven photocatalytic tetracycline degradation via carbon dots modified porous ZnSnO3 cubes: Mechanism and degradation pathway. Sep Purif Technol 253:117518–117528
Huang J, Xu X, Gu C, Wang W, Liu J (2012) Size-controlled synthesis of porous ZnSnO3 cubes and their gas-sensing and photocatalysis properties. Sensors Actuat B: Chem 171–172:572–579
Guo R, Tian R, Shi D, Li H, Liu H (2019) S-Doped ZnSnO3 Nanoparticles with narrow band gaps for photocatalytic wastewater treatment. ACS Appl Nano Mater 2:7755–7765
Shi W, Shu K, Huang X, Ren H, Li M, Chen F, Guo F (2020) Enhancement of visible-light photocatalytic degradation performance over nitrogen-deficient g-C3N4/KNbO3 heterojunction photocatalyst. J Chem Technol Biotechnol 95:1476–1486
Liu Y, Yang ZH, Song PP, Xu R, Wang H (2018) Facile synthesis of Bi2MoO6/ZnSnO3 heterojunction with enhanced visible light photocatalytic degradation of methylene blue. Appl Surf Sci 430:561–570
Lin L, Yu Z, Wang X (2019) Crystalline carbon nitride semiconductors for photocatalytic water splitting. Angew Chem Int Ed 58:6164–6175
Shi W, Ren H, Huang X, Li M, Tang Y, Guo F (2020) Low cost red mud modified graphitic carbon nitride for the removal of organic pollutants in wastewater by the synergistic effect of adsorption and photocatalysis. Sep Purif Technol 237:116477–116485
Sun H, Guo F, Pan J, Huang W, Wang K, Shi W (2021) One-pot thermal polymerization route to prepare N-deficient modified g-C3N4 for the degradation of tetracycline by the synergistic effect of photocatalysis and persulfate-based advanced oxidation process. Chem Eng J 406:126844–126856
Murugesan P, Moses JA, Anandharamakrishnan C (2019) Photocatalytic disinfection efficiency of 2D structure graphitic carbon nitride-based nanocomposites: a review. J Mater Sci 54:12206–12235. https://doi.org/10.1007/s10853-019-03695-2
Mousavi M, Hamzehloo M, Ghasemi JB (2020) Deposited CuBi2O4 and Bi3ClO4 nanoparticles on g-C3N4 nanosheet: a promising visible light-induced photocatalyst toward the removal of tetracycline hydrochloride and rhodamine B. J Mater Sci 55:7775–7791. https://doi.org/10.1007/s10853-020-04573-y
Tan YG, Shu Z, Zhou J, Li TT, Wang WB, Zhao ZL (2018a) One-step synthesis of nanostructured g-C3N4/TiO2 composite for highly enhanced visible-light photocatalytic H2 evolution. Appl Catal B Environ 230:260–268
Lv H, Ji G, Yang Z, Liu Y, Zhang X, Liu W, Zhang H (2015) Enhancement photocatalytic activity of the graphite-like C3N4 coated hollow pencil-like ZnO. J Colloid Interface Sci 450:381–387
Zhang Q, Xu B, Yuan S, Zhang M, Ohno T (2017) Improving g-C3N4 photocatalytic performance by hybridizing with Bi2O2CO3 nanosheets. Catal Today 284:27–36
Huang X, Guo F, Li M, Ren H, Shi Y, Chen L (2020) Hydrothermal synthesis of ZnSnO3 nanoparticles decorated on g-C3N4 nanosheets for accelerated photocatalytic degradation of tetracycline under the visible-light irradiation. Sep Purif Technol 230:115854
Jin Y, Jiang D, Li D, Chen M (2017) Construction of ultrafine TiO2 nanoparticle and SnNb2O6 nanosheet 0D/2D heterojunctions with abundant interfaces and significantly improved photocatalytic activity. Catal Sci Technol 7:2308–2317
Hou H, Wang L, Gao F, Yang X, Yang W (2019) BiVO4@TiO2 core–shell hybrid mesoporous nanofibers towards efficient visible-light-driven photocatalytic hydrogen production. J Mater Chem C 7:7858–7864
Liu J, Zhang G (2014) Ion-exchange synthesis of one-dimensional Cd2Ge2O6/CdS core–shell composites and their enhanced visible-light photocatalytic activity. Appl Surf Sci 319:291–297
An WJ, Sun KL, Hu JS, Cui WQ, Liu L (2020) The Z-scheme Ag2CO3@g-C3N4 core-shell structure for increased photoinduced charge separation and stable photocatalytic degradation. Appl Surf Sci 504:114345–114355
Jia H, Roa R, Angioletti-Uberti S, Henzler K, Ott A, Lin X, Möser J, Kochovski Z, Schnegg A, Dzubiella J, Ballauff M, Lu Y (2016) Thermosensitive Cu2O–PNIPAM core–shell nanoreactors with tunable photocatalytic activity. J Mater Chem A 4:9677–9684
Lo M-K, Lee S-Y, Chang K-S (2015) Study of ZnSnO3-nanowire piezophotocatalyst using two-step hydrothermal synthesis. J Phys Chem C 119:5218–5224
Tang L, Feng C, Deng Y, Zeng G, Wang J, Liu Y, Feng H, Wang J (2018) Enhanced photocatalytic activity of ternary Ag/g-C3N4/NaTaO3 photocatalysts under wide spectrum light radiation: The high potential band protection mechanism. Appl Catal B: Environ 230:102–114
Mu Haq Z, Zhang XC, Rahman N, Khan S, Khatoon R, Hassan SS, Ye Z, Zhu L (2019) A two-step synthesis of microsphere-decorated fibers based on NiO/ZnSnO3 composites towards superior ethanol sensitivity performance. J Alloy Compd 777:73–83
Wang Y, Yang W, Chen X, Wang J, Zhu Y (2018) Photocatalytic activity enhancement of core-shell structure g-C3N4@TiO2 via controlled ultrathin g-C3N4 layer. Appl Catal B: Environ 220:337–347
Wang J, Huang J, Xie H, Qu A (2014) Synthesis of g-C3N4/TiO2 with enhanced photocatalytic activity for H2 evolution by a simple method. Int J Hydrogen Energ 39:6354–6363
Tan Y, Shu Z, Zhou J, Li T, Wang W, Zhao Z (2018b) One-step synthesis of nanostructured g-C3N4/TiO2 composite for highly enhanced visible-light photocatalytic H2 evolution. Appl Catal B: Environ 230:260–268
Park H, Park TJ, Huh YS, Choi BG, Ko S, Lee SY, Hong WH (2010) Immobilization of genetically engineered fusion proteins on gold-decorated carbon nanotube hybrid films for the fabrication of biosensor platforms. J Colloid Interface Sci 350:453–458
Yang YH, Li XL, Lu C, Huang WH (2019) G-C3N4 Nanosheets Coupled with TiO2 Nanosheets as 2D/2D heterojunction photocatalysts toward high photocatalytic activity for hydrogen production. Catal Lett 149:2930–2939
Yuvaraj S, Amaresh S, Lee YS, Selvan RK (2014) Effect of carbon coating on the electrochemical properties of Co2SnO4 for negative electrodes in Li-ion batteries. RSC Adv 4:6407–6416
Shi W, Shu K, Sun H, Ren H, Li M, Chen F, Guo F (2020) Dual enhancement of capturing photogenerated electrons by loading CoP nanoparticles on N-deficient graphitic carbon nitride for efficient photocatalytic degradation of tetracycline under visible light. Sep Purif Technol 246:116930-116939
Yuan YJ, Shen ZK, Wu ST, Su YB, Pei L, Ji ZG, Ding MY, Bai WF, Chen YF, Yu ZT, Zou ZG (2019) Liquid exfoliation of g-C3N4 nanosheets to construct 2D–2D MoS2/g-C3N4 photocatalyst for enhanced photocatalytic H2 production activity. Appl Catal B: Environ 246:120–128
Wang L, Jin P, Duan S, She H, Huang J, Wang Q (2019) In-situ incorporation of Copper(II) porphyrin functionalized zirconium MOF and TiO2 for efficient photocatalytic CO2 reduction. Sci Bull 64:926–933
Barhoumi N, Olvera-Vargas H, Oturan N, Huguenot D, Gadri A, Ammar S, Brillas E, Oturan MA (2017) Kinetics of oxidative degradation/mineralization pathways of the antibiotic tetracycline by the novel heterogeneous electro-Fenton process with solid catalyst chalcopyrite. Appl Catal B: Environ 209:637–647
Deng F, Zhao L, Luo X, Luo S, Dionysiou DD (2018) Highly efficient visible-light photocatalytic performance of Ag/AgIn5S8 for degradation of tetracycline hydrochloride and treatment of real pharmaceutical industry wastewater. Chem Eng J 333:423–433
Zhao S, Chen J, Liu Y, Jiang Y, Jiang C, Yin Z, Xiao Y, Cao S (2019) Silver nanoparticles confined in shell-in-shell hollow TiO2 manifesting efficiently photocatalytic activity and stability. Chem Eng J 367:249–259
Xie ZJ, Feng YP, Wang FL, Chen DN, Zhang QX, Zeng YQ, Lv WY, Liu GG (2018) Construction of carbon dots modified MoO3/g-C3N4 Z-scheme photocatalyst with enhanced visible-light photocatalytic activity for the degradation of tetracycline. Appl Catal B: Environ 229:96–104
Wang DD, Li J, Xu ZF, Zhu YR, Chen GX, Cui Z (2019) Synthesis of g-C3N4/NiO p-n heterojunction materials with ball-flower morphology and enhanced photocatalytic performance for the removal of tetracycline and Cr6+. J Mater Sci 54:11417–11434
Sheng Y, Wei Z, Miao H, Yao W, Li H, Zhu Y (2019) Enhanced organic pollutant photodegradation via adsorption/photocatalysis synergy using a 3D g-C3N4/TiO2 free-separation photocatalyst. Chem Eng J 370:287–294
Acknowledgement
This study is supported by the National Natural Science Foundations of China (No. 21906072 and No. 22006057), the Natural Science Foundation of Jiangsu Province (BK20190982), “Doctor of Mass entrepreneurship and innovation” Project in Jiangsu Province, and Doctoral Scientific Research Foundation of Jiangsu University of Science and Technology (China) (1062931806 and 1142931803).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Handling Editor: Joshua Tong.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhu, X., Guo, F., Pan, J. et al. Fabrication of visible-light-response face-contact ZnSnO3@g-C3N4 core–shell heterojunction for highly efficient photocatalytic degradation of tetracycline contaminant and mechanism insight. J Mater Sci 56, 4366–4379 (2021). https://doi.org/10.1007/s10853-020-05542-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-05542-1