Abstract
MoO3 has become a very promising energy storage material owing to its high theoretical capacity and layered structure. However, MoO3 suffers from low specific capacitance and fast degradation performance due to pulverization caused by volume change during discharge and charge process. Here, we report the MoO3 nanoplates (MoO3 NPs) from Mo-based metal–organic frameworks (Mo-MOFs) via a facile heating treatment. When used as an anode in sodium-ion batteries (SIBs), the material showed 154 mAh g−1 superior discharge capacity at 50 mA g−1 after 1200 cycles. Even at 500 mA g−1, it also showed 217 mAh g−1 high specific capacity after 500 cycles. This specific MoO3 material design strategy offers suitable conditions for relieving the volume expansion and provides multiple channels for Na+ transport and electron transfer in MoO3 during discharge and charge process. This work highlights the importance of MoO3 nanoplates in preventing the pulverization caused by volume expansion in SIBs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lithium-ion batteries are widespread, but their price will likely increase due to limited lithium resources [1]. Thus, it is necessary to find new elements that can substitute for lithium to reduce costs. Sodium is abundant with similar electrochemical properties as lithium [2]. However, the choice of electrode materials for sodium-ion batteries (SIBs) is limited because of the larger ionic radius of sodium. This leads to slow Na+ kinetic reactions and significant pulverization [3]. Therefore, finding suitable materials with a stable structure remains a challenge for SIBs.
Transition metal oxides such as Fe3O4 [4], Co3O4 [5], NiO [6], ZnO [7] and MoO3 [8] have been widely studied as electrode materials for SIBs because of their suitable reaction potential with sodium ion and high theoretical capacity. Of these, MoO3 is an environmentally friendly and low-cost anode material delivering a high capacity (1117 mAh g−1) by insertion and conversion reactions [9]. More importantly, the layered structure of MoO3 offers insertion locations and Na+ diffusion channels [10, 11]. However, like most other high-capacity anode materials, MoO3 suffers from a low specific capacitance and fast degradation performance due to the pulverization caused by significant dimensional and volume changes during repeat cycling [12, 13]. To overcome these problems, MoO3 can be composited with a protective layer (carbon [14], graphene [15] or glucose [16]). Researchers have also designed different nanomorphologies (nanobelt [17, 18], nanoparticle [19], nanotube [20], nanowire [21] and nanoplate [13, 22]). In our previous work [16], nanocomposites composed of MoO3−x and glucose were used as a lithium anode. These materials facilitate a pulverized particle reaction in a confined space to ensure electrical contact between the pulverized particles. Unfortunately, this method does not fundamentally solve the pulverization problem. However, the nanomorphology design may fundamentally alleviate the pulverization problem caused by stress. Currently, the design of nanoplate structures has attracted much attention in stress relief due to their great structural flexibility and surface properties [13, 22, 24]. Wu et al. [13] prepared MoO3 nanoplate arrays via MoS2 as a template. This material showed 2.1 mAh cm−2 high specific capacity at 0.2 mA cm−2 after 200 cycles in SIBs. Cao et al. [22] reported MoO3 nanoplates within foam-like carbon nanoflakes via calcining precursor as an anode for lithium-ion batteries. This material delivered 791 mAh g−1 after 100 cycles at 500 mA g−1. The MoO3 NPs were synthesized with a relatively uniform size by MoS2 templates. However, the use of MoS2 templates generates noxious gases such as H2S and NH3. This is not conducive to large-scale commercial production and is environmentally dangerous. Therefore, the environmentally friendly templates are still needed.
Recently, metal–organic frameworks (MOFs) have received intense interest as self-templates for the synthesis of material with different morphologies [24]. Here, we report MoO3 nanoplates (MoO3 NPs) from Mo-based metal–organic frameworks (Mo-MOFs) by facile calcination treatment. The MoO3 NPs can relieve the volume expansion and maintain structural stability during repeated cycling. To better illustrate the advantages of the nanoplate structure in preventing pulverization, MoO3 nanobelts (MoO3 NBs) were synthesized by hydrothermal method for comparison. When two electrodes were used as an anode for SIBs, the MoO3 NPs offered a higher discharge capacity of 154 mAh g−1 at 50 mA g−1 after 1200 cycles than MoO3 NBs (49 mAh g−1). To further investigate the structural stability of MoO3 NPs, the micromorphology of both electrodes was evaluated after discharge–charge. This work offers new insight into the design of structural morphologies that prevent pulverization in anode materials during repeated cycling.
Materials and methods
Preparation of molybdenum-based metal–organic frameworks (Mo-MOFs)
Mo-MOFs were prepared based on the previous literature [25]. 3.5 g commercial MoO3 and 1.66 g imidazole were dispersed into 250 mL deionized (DI) water with string for 10 min. The compound was refluxed under sequential string at 75°C for 12 h. After the reaction finished, the white precipitates of Mo-MOFs were washed and centrifuged several times with DI water. The white powders were obtained after drying overnight in air at 60 °C.
Preparation of MoO3 nanoplates
The MoO3 nanoplates (MoO3 NPs) were obtained from the Mo-MOFs by calcination at 450 °C for 2 h (air atmosphere) with the heating rate of 5 °C min−1. In order to study the change process of MoO3 NPs with the temperature increase, the Mo-MOFs were heated at 300 °C and 600 °C; followed by the aforementioned process, the products were denoted as Mo-MOFs-C300 and Mo-MOFs-C600.
Preparation of MoO3 nanobelts
Preparation of MoO3 nanobelts: The MoO3 nanobelts (MoO3 NBs) were prepared by a hydrothermal treatment method following our previous report [16]. In a typical procedure, 2 g ammonium molybdate ((NH4)6Mo7O24·4H2O) was dissolved into 60 mL DI water to form homogeneous solution with continuous stirring. Then, adding 12 mL HNO3 to the above solution by dropper. After stirring for 1 h, the homogeneous solution was transferred into a 100-mL Teflon-lined autoclave, and the autoclave was placed in an oven at 180 °C for 24 h. After the reaction finished, the products were washed and centrifuged by DI water for several times. The as-synthesized white products were obtained after drying at 60 °C for 12 h in an oven.
Characterizations
The microstructures of samples were tested by field emission scanning electron microscope (FESEM, JEOL JSM 6300, Tokyo, Japan) and transmission electron microscopy (TEM, JEOL, JEM-2010F, 200 kV, Japan). X-ray diffractometer (XRD) measurement of all samples was taken using a PANanalytic X’Pert spectrometer with wavelength of 0.15405 nm. X-ray photoelectron spectroscopy was carried out to confirm the chemical state and bonding situations of all electrode materials (XPS, VG ESCALAB 210 electron spectrometer). The Brunauer–Emmett–Teller (BET, Micromeritics ASAP 2020) was used to obtain nitrogen adsorption–desorption isotherms and pore size distribution of all samples. The Fourier transform infrared spectroscopy (FTIR, Thermo Fisher Scientific Inc., USA) measurement was taken using KBr as the dispersant. The micro-Raman spectroscopy measurement was taken with an excitation wavelength of 532 nm (USA, Thermo Fisher Scientific DXR). The Keithley 4200 SCS and a micro-manipulator 6150 probe station were used to obtain the I–V curves of all samples.
Electrochemical measurements
The working electrode was made by mixing as-prepared samples, conductive additive (acetylene black) as well as binder (PVDF) at a weight ratio of 7:2:1 in N-methyl pyrrolidone (NMP) solvent to form good fluidity slurry with continuous stirring.
After that, the slurry was coated on the copper foil. Then, the copper foil with slurry was placed in vacuum oven at 90 °C for overnight. After drying, the electrode films were cut into a 14-mm-diameter disk with 1–2 mg electrode material on it. The coin cells (CR2032) were assembled in glove box filled with argon. The separator is Celgard 3400 microporous membrane. The electrolyte is 1.0 M NaClO4 with 1:1 ratio ethylene carbonate (EC) and diethyl carbonate as additive (by volume, Novolyte Technologies, USA). The electrochemical performance of as-assembled batteries was tested in an incubator (25 °C). The performance of cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) was tested by electrochemical workstation (CHI 760E, Shanghai, China). The cycling stability and rate capability was obtained by battery testing system (NEWARE).
Results and discussion
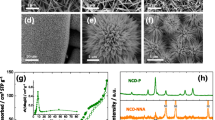
Figure 1a shows the synthesis of MoO3 NPs—their growth changes with increasing temperature. The MoO3 NPs were first synthesized from Mo-MOFs in a facile and green way. The micro-rodlike morphology (Fig. S1a) and XRD pattern (Fig. 1b) of Mo-MOFs are both consistent with the previous literature and confirm the successful preparation of Mo-MOFs [25]. After 300 °C treatment for 2 h (Mo-MOFs-C300), the morphology of Mo-MOFs is destroyed with many ordered nanorods appearing on the damaged surface; the structure of MoO3 is gradually formed with growth of (020), (040) and (060) peaks in the XRD pattern. The nanoplate morphology (Fig. 2) of MoO3 is complete when the temperature increased to 450 °C, and the XRD pattern of the MoO3 NPs (Fig. 1b) nicely agrees with the JCPDS (05-0508). The intensity of (020), (040) and (060) peaks in the XRD pattern of MoO3 NPs is lower than that of MoO3 NBs (hydrothermal method), indicating that the MoO3 NBs have stronger anisotropic characteristics than MoO3 NPs—this may be critical to preparing nanoplates [26, 27]. It is noted that the intensity of (020), (040) and (060) peaks in the XRD pattern of commercial MoO3 is also lower than that of MoO3 NBs. This reveal that the MoO3 NBs also have stronger anisotropic characteristics than commercial MoO3. This result may explain that the commercial MoO3 is not a belt morphology (Fig. S3). At 600 °C, the XRD patterns (Fig. 1b) show that the product is still MoO3 (named as Mo-MOFs-C600), but the micromorphology (Fig. S1c) is not as uniform as with MoO3 NPs (at 450 °C for 2 h). These results suggest that 450 °C is a suitable temperature to synthesize uniform MoO3 NPs. Moreover, the (020), (040) and (060) peaks of the Mo-MOFs-C600 are little discernable shift compared with the MoO3 NPs (450 °C) (Fig. 1c). This suggest that there is no causal relationship between layer spacing and temperature. However, further analyzing the data in Fig. 1c, the (020), (040) and (060) peaks of the MoO3 NPs are shifted toward lower angles in comparison with MoO3 NBs, which indicate that MoO3 NPs have a larger interlayer spacing than MoO3 NBs. The increscent interlayer spacing offers more diffusion channels for sodium ions and electrons [28].
a Diagram of MoO3 NPs synthesis process with the temperature increase and the corresponding FESEM images(inset); b XRD patterns of Mo-MOFs, Mo-MOFs-C300, MoO3 NPs, MOFs-C600, MoO3 NBs and commercial MoO3; c selected XRD pattern insets of the (020), (040) and (060) diffraction peaks that compare MoO3 NPs, MOFs-C600 and MoO3 NBs, respectively
XPS was performed to further study the chemical states of MoO3 NPs (Fig. 3a–d). The XPS survey spectrum reveals the existence of Mo, O and C (Fig. 3a). The high-resolution Mo 3d spectrum (Fig. 3b) revealed two peaks located at 232.80 and 235.94 eV, which is accord well with Mo3d5/2 and Mo3d3/2 of the oxidation state (Mo6+) of MoO3 NPs, respectively. In the O 1s XPS spectrum (Fig. 3c), the Mo–O bond of MoO3 NPs is deconvoluted with binding energies at 530.60 eV [29]. It is well known that carbon can directly enhance the electrical conductivity of the electrode [30]. Moreover, the MoO3 NPs are prepared from Mo-MOFs. Therefore, it is necessary to identify the C 1s spectrum to confirm whether carbon exists in the MoO3 NPs. Figure 3d shows three typical peaks: C–C (284.48, 284.41 eV), C–O (286.07, 285.9 eV) and C=O (288.62, 288.81 eV) [31]. Comparing the peak position of the C 1s XPS spectrum between MoO3 NBs and MoO3 NPs, the difference in the position of the corresponding peak is lower 0.2 V, and the atomic percentage of C 1s in MoO3 NBs and MoO3 NPs is approximate (20.76% vs. 29.12%) (Table S1). Therefore, it is possible that the C 1s of XPS is adventitious carbon. To verify this hypothesis, FTIR and Raman spectra were also analyzed (Fig. S4). The FTIR analysis shows that the spectra of MoO3 NPs and MoO3 NBs are nearly identical (Fig. S4a). However, what is surprising is that the Raman spectrum (Fig. S4b) has an inconspicuous G-band and D-band of the carbon substrate. One possible reason for this is that a small amount of carbon/MoO3 NPs composition may exist in MoO3 NPs. TGA is further analyzed to confirm the carbon content in the MoO3 NPs (Fig. S4c). The results suggest that the content of carbon and H2O is 0.153%. Hence, the carbon content can be ignored in the MoO3 NPs. This reasonably assumes that carbon will not affect the electrochemical performance of MoO3 NPs.
The electrochemical performance of MoO3 NPs has been evaluated as an anode for SIBs. Figure 4a shows the cyclic voltammetry (CV) of the MoO3 NPs within 0.1–3 V. The several reduction peaks observed in the first discharge vanish in the subsequent scans, suggesting an irreversible conversion reaction at the MoO3 NPs electrode. There is one clear peak near 2.4 V due to the formation of a solid electrolyte interphase (SEI) and insertion of MoO3 by Na+ to form NaxMoO3 (MoO3 + x Na+ + x e− → NaxMoO3). The peaks near 1.56 V and 1.01 V correspond to excess sodiation, leading to Nax+yMoO3. The potential below 0.5 V is due to the formation of Mo and Na2O (Nax+yMoO3 + (6 − x) Na+ + (6 − x) e− → Mo + 3Na2O) [8, 11, 32]. An anode peak located at 0.668 V is observed in first three charging cycles, indicating a reversible removal of the sodium ion [33]. Compared with MoO3 NBs (Fig. S5a), the integral area of MoO3 NPs is larger than that of MoO3 NBs, suggesting that MoO3 NPs have better electrochemical performance than MoO3 NBs. Figure 4b shows the discharge–charge curves of the MoO3 NPs. There are several distinct plateaus in the first discharging process. The plateaus disappear in subsequent cycles corresponding to the CV curves (Fig. 4a) [32]. Besides, the discharge capacity of MoO3 NBs are 727, 295, 125, 105, 83 and 49 mAh g−1 at 1st, 2nd, 150th, 300th, 600th and 1200th, respectively (Fig. S5b); the discharge capacity of MoO3 NPs are up to 1222, 529, 247, 224, 185 and 154 mAh g−1, respectively (Fig. 4b). According to the calculations, the MoO3 NPs have a higher initial coulombic efficiency than MoO3 NBs (43.7% vs. 40.35%). Electrochemical impedance spectroscopy (EIS) was performed to compare the electrical conductivity and diffusion dynamics of MoO3 NBs and MoO3 NPs electrodes (Fig. 4c). Each Nyquist plot includes three parts: high-frequency semicircle, middle-frequency semicircle and low-frequency tail—these correspond to the sodium-ion migration impedance, charge transfer impedance and sodium-ion diffusion level, respectively [34]. The first and second semicircles of MoO3 NPs are much small than that of the MoO3 NBs, suggesting a lower ionic resistance and charge transfer resistance of MoO3 NPs. Moreover, the MoO3 NPs still have a lower resistance than MoO3 NBs after one cycle (Fig. S5c). Further I–V tests revealed that the conductivity of MoO3 NPs is preferable to MoO3 NBs (Fig. S6), which corresponds to the EIS results. This suggests that the introduction of the nanoplate structure and the expansion interlayer improves the conductivity of MoO3. Figure 4e compares the cycling performance of MoO3 NBs and MoO3 NPs. The MoO3 NPs electrode delivers 154 mAh g−1 high discharge capacity after 1200 cycles at a current density of 50 mA g−1, which is over threefold higher than MoO3 NBs (ca. 49 mAh g−1). Even at the high current density (500 mA g−1), the MoO3 NPs retain 217 mAh g−1 high discharge capacity after 500 cycles (Fig. S5d)). Particularly revealing is that the MoO3 NPs also have excellent rate performance at different current densities (Fig. 4d). More importantly, the as-synthesized MoO3 NPs have a high capacity and long cycle life compared with other MoO3-based anodes previously reported for SIBs (Fig. 4f, Table 1). In general, a high specific surface area is a key feature to improve electrochemical performance of electrode materials. Thus, Brunauer–Emmett–Teller (BET) analysis (Fig. S7) was performed to confirm whether MoO3 NPs have a large specific surface area. Unfortunately, the MoO3 NPs have a lower specific surface area (4.865 m2 g−1) than MoO3 NBs (7.168 m2 g−1) (Table S2). Therefore, the structural stability of MoO3 NPs is the key to perform excellent electrochemical performance.
Initial three CV curves of MoO3 NPs: a at 0.1 mV s−1; b the discharge–charge curves at 50 mA g−1; the Nyquist plots (c) and the rate capability (d) of MoO3 NBs and MoO3 NPs; e cyclic performance of MoO3 NBs and MoO3 NPs at 50 mA g−1; f electrochemical performance comparison of MoO3 NPs in this work with other MoO3-based anodes previously reported for SIBs
To quantify the kinetics of both samples, scan rate-dependent cyclic voltammetry (Fig. 5a, b) was used to evaluate the capacitive contribution (k1v) and diffusion-controlled reactions (k2v1/2) to the current response (I) at a fixed potential (V) according to the following formula [37]:
where v is the scan rate. The pseudocapacitive effects of the MoO3 NPs are higher than that of MoO3 NBs at a scan rate of 0.1 mV s−1 (21.9% vs. 16.8%) (Fig. 5c). As the scan rate increases, the pseudocapacitive effects of MoO3 NPs further increase up to 55.3% at 1 mV s−1 (Fig. 5d). However, the MoO3 NPs still show a low pseudocapacitive contribution. It is not surprising that the pseudocapacitive effects play a key role for small particles with a high specific surface area [38]. However, the size of MoO3 NPs is a little large with a low surface area (4.8653 m2 g−1) (Table S2). Besides, as shown in discharge–charge curves (Fig. 4b), the MoO3 NPs electrode has several distinct plateaus corresponding to a lower section of pseudocapacitive effects [39].
Cyclic voltammetry of MoO3 NBs (a) and MoO3 NPs (b) at different scan rates within 0.1 and 3 mV s−1; c capacitive contribution of MoO3 NBs (inset) and MoO3 NPs at scan rate of 0.1 mV s−1; d comparison of the capacitive contribution rate and diffusion controlled at various scan rates of MoO3 NBs and MoO3 NPs
The excellent electrochemical performance of MoO3 NPs can be explained by the stability of its electrode morphology during repeated cycles. To verify that the morphology of MoO3 NPs is more stable than that of MoO3 NBs during discharge–charge process, FESEM images of the two electrodes were compared after battery reactions. Figure 6a, b shows that the surface of both electrodes suffers from pulverization due to volume changes after 1 cycle. However, the microstructure of MoO3 NPs (Fig. 6a) is clearly not as seriously damaged. After 10 cycles, the MoO3 NPs electrode retains its nanoplate structure. Surprisingly, even after 1200 cycles, the complete nanoplate structure of MoO3 NPs still can be observed. Except for the structural stability of the MoO3 NPs itself, the MoO3 NPs stability is also related to the high structural flexibility and surface properties of the nanoplate structure. This can buffer the stress caused by volume changes during repeated cycles and then prevent pulverization, which eventually ensures the structural integrity of MoO3 NPs. In contrast, the morphology of MoO3 NBs (Fig. 6b) undergoes a huge change from the 1st to the 10th cycle, suffering tiny cracks at the 1st cycle, extensive cracking on the 2nd cycle and disintegration after 10 cycles. These results show that the MoO3 NPs electrode maintains its integrity during repeated cycles leading to good contact between the electrode and the copper. This improves conductivity and provides multiple channels for Na+ and electron transport [40,41,42]. These features are the keys by which MoO3 NPs exhibit high reversible capacity and long-term stability.
The ex situ XRD (Fig. 7a, b) and XPS (Fig. 7d) spectra also provided insight into the electrochemical reversibility of the two electrodes. At the sodiation state (0.01 V) (Fig. 7a), the crystallinity of NaxMoO3 (JCPDS 12-0773) in MoO3 NPs is higher than that of MoO3 NBs, which may be due to the high transformation of MoO3 NPs electrode during first sodiation process (MoO3 + x Na+ + x e − → NaxMoO3) (Fig. 7c). Moreover, the higher peak of Na2O in MoO3 NPs electrode than that of MoO3 NBs electrode is observed. This suggest that more Na+ move in the NaxMoO3 to form Na2O (JCPDS 02-1288) and Mo (JCPDS 01-1207) (NaxMoO3 + (6 − x) Na+ + (6 − x) e − → Mo + 3Na2O) (Fig. 7c) during the further sodiation processes. At the desodiation state (at 3 V) (Fig. 7b), both MoO3 NBs and MoO3 NPs electrodes appeared weak diffraction peaks of Mo and Na2O. Moreover, the diffraction peaks of Na2O in MoO3 NPs are slightly lower than that of MoO3 NBs. We come to the conclusion that higher transformation of MoO3 NPs electrode compared with MoO3 NBs, according to the conversion reaction equation: Mo + Na2O → NaxMoO3. It is found that the diffraction peaks of NaxMoO3 in MoO3 NBs are higher than that of MoO3 NPs. This may be that the total of as-formed NaxMoO3 is the sum of the NaxMoO3 during the first sodiation and desodiation process. The crystallinity of MoO3 phase in MoO3 NBs electrode is higher than that MoO3 NPs electrode, which provide more experimental evidence for low transformation of MoO3 NBs electrode. In addition, as is shown in Fig. 7d, when desodiation at 3 V, the fraction of Mo4+ (233.2, 230.1 eV) in MoO3 NBs are higher than the Mo4+ in MoO3 NPs electrode (233.2, 230.1 eV), which indicates that more Na+ was trapped in the MoO3 NBs structure than in MoO3 NPs [43]. This leads to trapped sodium ions that cannot participate in subsequent cycles. However, MoO3 NPs can ensure that the embedded sodium ions are removed more smoothly than MoO3 NBs. This may be related to the stable structure and structural integrity of MoO3 NPs during repeated cycling. Taken together, these results suggest that the excellent electrochemical performance of MoO3 NPs is attributed to the high reversible reaction during repeated cycling.
Conclusions
We report a facile and green synthesis method of MoO3 NPs from Mo-MOFs. The nanoplate structure is the result of growth from lower anisotropy. When used as an anode for SIBs, the MoO3 NPs show 154 mAh g−1 superior reversible capacity at a current density of 50 mA g−1 after 1200 cycles. This is nearly three times than that of MoO3 NBs (ca. 49 mAh g−1). The nanoplate structure of MoO3 NPs can relieve the stress caused by expansion of volume during discharge–charge process, which prevents pulverization and keeps the micromorphology stability. This study has also shown that the high transformation made contribution to excellent electrochemical performance of MoO3 NPs. This work offers new insight into the design of structural morphologies to prevent pulverization in other anode materials during repeated cycling.
References
Tarascon JM (2010) Is lithium the new gold? Nat Chem 2:510
Slater MD, Kim D, Lee E, Johnson CS (2013) Sodium-ion batteries. Adv Funct Mater 23:947–958
Li W, Hu S, Luo X, Li Z, Sun X, Li M, Liu F, Yu Y (2017) Confined amorphous red phosphorus in MOF-derived N-doped microporous carbon as a superior anode for sodium-ion battery. Adv Mater 29:1–8
Liu H, Jia M, Zhu Q, Cao B, Chen R, Wang Y, Wu F, Xu B (2016) 3D-0D graphene-Fe3O4 quantum dot hybrids as high-performance anode materials for sodium-ion batteries. ACS Appl Mater Interfaces 8:26878–26885
Liu Y, Cheng Z, Sun H, Arandiyan H, Li J, Ahmad M (2015) Mesoporous Co3O4 sheets/3D graphene networks nanohybrids for high-performance sodium-ion battery anode. J Power Sources 273:878–884
Wang B, Wang G, Cheng X, Wang H (2016) Synthesis and electrochemical investigation of core-shell ultrathin NiO nanosheets grown on hollow carbon microspheres composite for high performance lithium and sodium ion batteries. Chem Eng J 306:1193–1202
Wang Y, Deng Q, Xue W, Jian Z, Zhao R, Wang J (2018) ZnO/rGO/C composites derived from metal–organic framework as advanced anode materials for Li-ion and Na-ion batteries. J Mater Sci 53:6785–6795. https://doi.org/10.1007/s10853-018-2003-3
Sreedhara MB, Santhosha AL, Bhattacharyya AJ, Rao CNR (2016) Composite of few-layer MoO3 nanosheets with graphene as a high performance anode for sodium-ion batteries. J Mater Chem A 4:9466–9471
Riley LA, Lee SH, Gedvilias L, Dillon AC (2010) Optimization of MoO3 nanoparticles as negative-electrode material in high-energy lithium ion batteries. J Power Sources 195:588–592
Xia W, Xu F, Zhu C, Xin HL, Xu Q, Sun P, Sun L (2016) Probing microstructure and phase evolution of α-MoO3 nanobelts for sodium-ion batteries by in situ transmission electron microscopy. Nano Energy 27:447–456
Spahr ME, Novak P, Haas O, Nesper R (1995) Electrochemical insertion of lithium, sodium, and magnesium in molybdenum(Vi) oxide. J Power Sources 54:346–351
McDowell MT, Xia SM, Zhu T (2016) The mechanics of large-volume-change transformations in high-capacity battery materials. Extreme Mech Lett 9:480–494
Wu K, Zhan J, Xu G, Zhang C, Pan D, Wu M (2018) MoO3 nanosheet arrays as superior anode materials for Li- and Na-ion batteries. Nanoscale 10:16040–16049
Ding J, Abbas SA, Hanmandlu C, Lin L, Lai C, Wang P, Li L, Chu C, Chang C (2017) Facile synthesis of carbon/MoO3 nanocomposites as stable battery anodes. J Power Sources 348:270–280
Zhang X, Fu C, Li J, Yao C, Lu T, Pan L (2017) MoO3/reduced graphene oxide composites as anode material for sodium ion batteries. Ceram Int 43:3769–3773
Yang C, Lu H, Li C, Wang L, Wang H (2018) Spatially-confined electrochemical reactions of MoO3 nanobelts for reversible high capacity: critical roles of glucose. Chem Eng J 337:1–9
Wang Z, Madhavi S, Lou XW (2012) Ultralong α-MoO3 nanobelts: synthesis and effect of binder choice on their lithium storage properties. J Phys Chem C 116:12508–12513
Xia Q, Zhao H, Du Z, Zeng Z, Gao C, Zhang Z, Du X, Kulka A, Świerczek K (2015) Facile synthesis of MoO3/carbon nanobelts as high-performance anode material for lithium ion batteries. Electrochim Acta 180:947–956
Lee S-H, Kim Y-H, Deshpande R, Parilla PA, Whitney E, Gillaspie DT, Jones KM, Mahan AH, Zhang S, Dillon AC (2008) Reversible lithium-ion insertion in molybdenum oxide nanoparticles. Adv Mater 20:3627–3632
Jiang Y, Sun M, Ni J, Li L (2019) Ultrastable sodium storage in MoO3 nanotube arrays enabled by surface phosphorylation. ACS Appl Mater Interfaces 11:37761–37767
Meduri P, Clark E, Kim JH, Dayalan E, Sumanasekera GU, Sunkara M (2012) MoO3−x nanowire arrays as stable and high-capacity anodes for lithium ion batteries. Nano Lett 12:1784–1788
Cao D, Dai Y, Xie S, Wang H, Niu C (2018) Pyrolytic synthesis of MoO3 nanoplates within foam-like carbon nanoflakes for enhanced lithium ion storage. J Colloid Interface Sci 514:686–693
Chen J, Lou X (2012) SnO2 and TiO2 nanosheets for lithium-ion batteries. Mater Today 15:246–254
Cao X, Zheng B, Shi W, Yang J, Fan Z, Luo Z, Rui X, Chen B, Yan Q, Zhang H (2015) Reduced graphene oxide-wrapped MoO3 composites prepared by using metal-organic frameworks as precursor for all-solid-state flexible supercapacitors. Adv Mater 27:4695–4701
Martin-Zarza P, Arrieta J, MuAoz-Roca M, Gili P (1993) Synthesis and characterization of new octamolybdates containing imidazole, 1-methyl- or 2-methyl-imidazole co-ordinatively bound to molybdenum. J Chem Soc Dalton Trans. https://doi.org/10.1039/DT9930001551
Lou X, Zeng H (2002) Hydrothermal synthesis of α-MoO3 nanorods via acidification of ammonium heptamolybdate tetrahydrate. Chem Mater 14:4781–4789
Li S, Hou H, Huang Z, Liao H, Qiu X, Ji X (2017) Alternating voltage introduced [001]-oriented α-MoO3 microrods for high-performance sodium-ion batteries. Electrochim Acta 245:949–956
Cai Y, Yang H, Zhou J, Luo Z, Fang G, Liu S, Pan A, Liang S (2017) Nitrogen doped hollow MoS2/C nanospheres as anode for long-life sodium-ion batteries. Chem Eng J 327:522–529
Qiu J, Yang Z, Li Y (2015) N-doped carbon encapsulated ultrathin MoO3 nanosheets as superior anodes with high capacity and excellent rate capability for Li-ion batteries. J Mater Chem A 3:24245–24253
Ji H, Liu X, Liu Z, Yan B, Chen L, Xie Y, Liu C, Hou W, Yang G (2015) In situ preparation of sandwich MoO3/C hybrid nanostructures for high-rate and ultralong-life supercapacitors. Adv Funct Mater 25:1886–1894
Ma F, Yuan A, Xu J, Hu P (2015) Porous alpha-MoO3/MWCNT nanocomposite synthesized via a surfactant-assisted solvothermal route as a lithium-ion-battery high-capacity anode material with excellent rate capability and cyclability. ACS Appl Mater Interfaces 7:15531–15541
Hariharan S, Saravanan K, Balaya P (2013) α-MoO3: a high performance anode material for sodium-ion batteries. Electrochem Commun 31:5–9
Liu Y, Zhang B, Xiao S, Liu L, Wen Z, Wu Y (2014) A nanocomposite of MoO3 coated with PPy as an anode material for aqueous sodium rechargeable batteries with excellent electrochemical performance. Electrochim Acta 116:512–517
Guo J, Sun A, Chen X, Wang C, Manivannan A (2011) Cyclability study of silicon–carbon composite anodes for lithium-ion batteries using electrochemical impedance spectroscopy. Electrochim Acta 56:3981–3987
Xu Y, Zhou M, Wang X, Wang C, Liang L, Grote F, Wu M, Mi Y, Lei Y (2015) Enhancement of sodium ion battery performance enabled by oxygen vacancies. Angew Chem Int Ed Engl 54:8768–8771
Li Y, Wang D, An Q, Ren B, Rong Y, Yao Y (2016) Flexible electrode for long-life rechargeable sodium-ion batteries: effect of oxygen vacancy in MoO3−x. J Mater Chem A 4:5402–5405
Liu T-C, Pell WG, Conway BE, Roberson SL (1998) Behavior of molybdenum nitrides as materials for electrochemical capacitors: comparison with ruthenium oxide. J Electrochem Soc 145:1882–1888
Wang J, Polleux J, Lim J, Dunn B (2007) Pseudocapacitive contributions to electrochemical energy storage in TiO2 (Anatase) nanoparticles. J Phys Chem C 111:14925–14931
Chao D, Zhu C, Yang P, Xia X, Liu J, Wang J, Fan X, Savilov SV, Lin J, Fan HJ, Shen ZX (2016) Array of nanosheets render ultrafast and high-capacity Na-ion storage by tunable pseudocapacitance. Nat Commun 7:12122
Mukhopadhyay A, Sheldon BW (2014) Deformation and stress in electrode materials for Li-ion batteries. Prog Mater Sci 63:58–116
Zhao Y, Ding C, Hao Y, Zhai X, Wang C, Li Y, Li J, Jin H (2018) Neat design for the structure of electrode to optimize the lithium-ion battery performance. ACS Appl Mater Interfaces 10:27106–27115
Yan P, Zheng J, Gu M, Xiao J, Zhang J, Wang C (2017) Intragranular cracking as a critical barrier for high-voltage usage of layer-structured cathode for lithium-ion batteries. Nat Commun 8:14101
Kim H, Cook J, Lin H, Ko J, Tolbert S, Ozolins V, Dunn B (2017) Oxygen vacancies enhance pseudocapacitive charge storage properties of MoO3−x. Nat Mater 16:454–460
Acknowledgements
This study was funded by the National Natural Science Foundation of China (No. 41572034) and the Guangxi Natural Science Foundation (No. 2018GXNSFAA294012).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, C., Xiang, Q., Li, X. et al. MoO3 nanoplates: a high-capacity and long-life anode material for sodium-ion batteries. J Mater Sci 55, 12053–12064 (2020). https://doi.org/10.1007/s10853-020-04788-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-04788-z