Abstract
The solid oxide fuel cells (SOFCs) are promising electrochemical conversion devices and have been studied for several decades. While numerous achievements have been obtained in different types of electrolyte materials, respectively, systematic reviews based on cell performance have been notably rare. In this review, the overall research progress and highlights of the performance of solid oxide fuel cells based on three different types of electrolytes are described in detail. This review can provide overall perspective and useful guidance for the further development and application of SOFCs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the last two decades, solid oxide fuel cells (SOFCs) have developed in the research area through a variety of methods to improve cell performance. The development of SOFCs is always the current focus of the community of fuel cell with the relevant articles of more than 1000 per year in recent decades as shown in Fig. 1. With several prominent features when compared with the current commercial fuel cells, SOFCs have become the focus of the research community. The main characters of SOFCs are listed below:
- (1)
Relatively high current density and power density compared to other types of fuel cells;
- (2)
Electrolyte resistivity is usually negligible, and in most cases cell performance is mainly determined by cathode loss [1];
- (3)
Hydrogen, methane and alcohol can be used directly as fuel without precious metals as catalysts;
- (4)
Sealing and corrosion problems can only be avoided in certain designs, such as tubular designs, since most developers do not use tubular designs and should be given sufficient attention;
- (5)
All-solid-state structure with ceramic material as electrolyte and electrode.
Even though many advantages, factors which hinder the commercialization of SOFCs are the high cost and the stability which lead to a short lifespan of the SOFCs. The community has taken efforts to address issues and an amount of achievements has been obtained. The research on the theory and application of SOFCs has been studied in the past decade, and the promotion in cell performance has been studied continually by several methods, such as the thin-film formation techniques, doping techniques and alternative nanomaterials [2,3,4,5,6,7,8]. Nanomaterials and related nanotechnologies, as one of the thin-film formation techniques, have attracted increased attention because they enable cell performance improvements by reducing the cell polarization resistance and engender newly emerged functionalities, which significantly evolve current SOFC technology [9]. This review presents the highlights and study progress of cell performance of SOFCs all over the world.
The working principles and performance factors
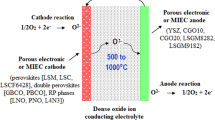
According to types of conducting ions indifferent electrolyte materials, SOFCs can be classified into three categories: oxygen-ion-conducting SOFCs, proton-conducting SOFCs and mixed-ion-conducting SOFCs, all of which will be defined and reviewed in the later chapters. As the result of different electrolyte materials, the reaction product is created on the anode side of anions-conducting SOFC, the reaction product is created on the cathode side of cations-conducting SOFC, and the reaction product is created on both the anode side and the cathode side of anions- and cations-conducting composite SOFC, as is shown in Fig. 2.
As can be seen from the following schematics, the reactions inside the cell depend on the material of the electrolyte. Initially, most researchers focused on the breakthroughs in ionic conductivity and peak power density, and few of them reported cell stability and comparisons of each promotion phase, which could not lead to a clear developmental trend of SOFC. It must be pointed out that SOFC is no longer always the above-mentioned oxygen ion transmission mode, and the composite electrolyte usually brings higher ionic conductivity and longer working time. [10,11,12,13,14,15,16].
In the traditional oxygen ion transportation mode, oxygen from the air is reduced to oxygen ions in cathode and O2− is transferred to anode through the oxygen vacancy channel by the drive of concentration difference and potential difference. The reactions can be subscribed as follows:
When the fuel is H2,
When the fuel is CO,
When the fuel is CnHmOl,
Thus, the overall reactions are:
When the fuel is H2,
When the fuel is CO,
When the fuel is CnHmOl,
In the proton transportation mode, the oxidation reactions of hydrogen molecules occur in the anode. Driven by the concentration difference and the potential difference, hydrogen ions are transferred to the cathode through the interface transfer based on the proposed “swing model”. The reactions can be subscribed as follows:
The fuel is H2:
Thus, the overall reaction is:
In the composite electrolyte, some researchers have demonstrated that the introduction of different types of electrolytes can extensively increase ion conductivity. Besides, the materials of fluorite structure, perovskite structure and apatite structure are used as the components of solid oxide fuel. Such electrolyte material is typically of the category controversial terms, as they are based on an improved solid oxide electrolyte and excellent cell performance, and therefore information worth mentioning [17, 18]. Generally, they include ceria-based carbonate composite electrolyte, ceria-based tungstate complex electrolyte and so on. It is reported that the total ionic conductivity of the composite material is higher than the sum of each raw material; that is, the behavior of the composite material changes and promotes the ionic conduction mechanism, resulting in an increase in the ionic conductivity. In some certain cases, proton conduction has been found to be predominated in the fluoride over the oxygen ion conduction [19, 20]. One of the improved fuel cells based on general SOFCs, ceria-based carbonate composite electrolyte, is taken as an example to explain the principle of composite electrolyte. The reactions can be subscribed as follows:
The anode side:
The cathode side:
Thus, the overall reaction is:
As can be concluded from the above reaction formulas, the ions conduction within different conducting medium occurs at the same time. However, the mechanism of H+ conduction is unclear and the effects of composites on cell stability and longevity of the cell are required to be studied.
The factors for cell performance are usually the requirements which are crucial properties for electrolyte to meet. As an advanced fuel cell device, the electrolyte material for low-temperature SOFCs must meet following requirements:
- (1)
Ion conductivity could not be lower than 0.01 S/cm, or the resistivity of the membrane will be reduced.
- (2)
Ion mobility should be more than 0.99, that is, the electrolyte material should be a conductor of ions and an insulator for electrons.
- (3)
The activation energy should be below 0.5 eV in order to make it easy to promote ions mobility.
- (4)
Airtightness must be ensured, and the density of the membrane should reach 95% of the theoretical density to separate fuel and O2.
- (5)
Relatively stable physical and chemical properties can provide long-term stability for fuel cells.
- (6)
Low costs and relatively uncomplicated fabrication techniques should be required.
Otherwise, the type of fuel used in SOFCs also has an impact on the cell performance. Generally, H2 provides the most energy per unit mass, and only H2O is produced, while other gases (such as CH4, C3H8 and C2H6O) release CO and CO2 that are harmful to our living environment. The ratio of the fuel utilized in the experiments is well shown in Fig. 3. We can conclude from the figure that H2 is utilized commonly, while methane also takes up a large part of the pie.
However, economic techniques of hydrogen production are still under development, and the problem of storing and transporting hydrogen should be resolved to the development of SOFCs commercialization. Storing liquid hydrogen in a pressure vessel is considered very tricky, and using excessive pressure to condense hydrogen into a liquid is still potentially dangerous. Condensing the hydrogen into a liquid is a viable approach many Japanese researchers are studying.
Performance of oxygen-ion-conducting electrolyte materials for SOFCs
As the initially found conducting mode, the conducting theory has been studied. As the number of oxygen vacancies and the structure of the lattice determine the conducting of O2− mainly, the feasible approaches can be discovered by analyzing the fundamental theory formula. According to Random Walk theory, the oxygen vacancy diffusion coefficient in the oxides lattice can be expressed as follows [46]:
where z is the number of oxygen vacancies adjacent to equivalent oxygen lattice positions; a is the oxygen vacancy jump distance; υ0 is the oxygen vacancy trial transition frequency; ΔSm is the entropy change of oxygen vacancy migration; ΔHm is the enthalpy change of oxygen vacancy migration (also named activation energy); f is the related factor.
According to the Nernst–Einstein formula, the relationship between oxygen vacancy mobility (μv), oxygen vacancy diffusion coefficient (D) and the number of oxygen vacancy charges (qV) can be expressed as follows:
Assumed:
Therefore:
It can be seen from the above formula that it is feasible to increase the oxygen vacancy mobility by increasing z (the number of oxygen vacancies adjacent to equivalent oxygen lattice positions), reducing ΔHm (activation energy) and maintain \( V_{\text{o}}^{..} \) at a suitable level. One of the most effective methods is to introduce low-valent cationic oxides to generate a proper amount of oxygen vacancies. The researchers have been using doping methods for decades to increase ion conductivity and peak power density.
YSZ
As the most commonly used electrolyte for oxygen-ion-conducting SOFCs, YSZ (yttrium-stabilized zincite) has been already well studied, although many challenges remain to be solved [47]. Fernández et al. [48] have compared three different sintered commercial YSZ powders tapes under strict control by electrochemical impedance spectroscopy, and the results showed that there are no big differences between these powders after the colloidal processing. The thickness of YSZ tape, which is also a key factor for performance of SOFCs, can be controlled by altering the scale of YSZ grain size to some extent. Park et al. [49] investigated YSZ-deposited films by electrostatic spray deposition, and they found that the 5-μm-thick YSZ film is obtained as the optimal condition resulted from the optimal experimental circumstance. Omid et al. [50] investigated electrolyte coating of solid oxide fuel cells (SOFCs) based on yttria-stabilized zirconia (YSZ) on Ni-YSZ cermet fuel cells anode in ethanol by using pulsed constant voltage and DC electrophoretic deposition, and the results indicated that the optimal thickness of YSZ coating by PC and DC was 20 and 88 ± 1 μm, respectively.
One worth being mentioned is that Yi and Anil [51] fabricated anode-supported solid oxide fuel cells with a thin film of yttria-stabilized zirconia (YSZ) as the electrolyte, and the maximum power density was measured to be 1.7 W/cm2 with hydrogen, 1.3 W/cm2 with CH3OH, and 0.8 W/cm2 with alcohol–water (1:1 in volume) at 800 °C, respectively. The investigation revealed that Tb-doped YSZ and Ti-doped YSZ could promote the peak power density up to 50%.
As for the sintering aid, which can reduce sintering temperature while maintaining high conductivity for SOFC electrolyte, Pradnyesh et al. [52] considered Fe as sintering aid for YSZ and relevant studies have been conducted and the results showed that with the increasing of Fe concentration, ionic conductivity decreases. Besides, sintering atmospheres have been also studied and the results indicated that as compared with the samples sintered in air, the samples sintered in Ar are characterized by low conductivity, large lattice parameter, large grain, higher density and better “sinterability”. Sannan and Eric [53] utilized copper as a sintering aid for samarium-doped ceria and pointed that copper content as low as 0.5 mol% leads to a significant reduction in sintering temperature, the smallest decrease in total conductivity and does not affect the thermal expansion coefficient of the electrolyte.
CeO2
As another alternative electrolyte for SOFCs, cerium oxide (CeO2)-based electrolyte shows several times to several orders of magnitude higher oxygen ion conductivity than conventional YSZ electrolyte, and the lower the temperature, the more obvious the advantage. However, Ce4+ is partially reduced to Ce3+ inside the electrolyte, which not only causes the increase in electron conductivity in the electrolyte, but also causes lattice expansion of the electrolyte, which on the one hand causes a decrease in the open-circuit voltage of the cell and on the other hand causes a decrease in mechanical properties. Fu et al. [54] analyzed the effect of Sm doping content on the ionic conduction of CeO2 in SOFCs from first principles, and the results showed that doping content brings several impacts on ionic conductivity. The association energy, activity of oxygen migration and the band structure of SDC (Sm-doped ceria) can be controlled by varying the Sm concentration. Lee et al. [55] prepared and investigated ZrO2 doped with Sc2O3 and CeO2, and the results showed that the ionic conductivity and long-term stability of ZrO2 doped with Sc2O3 and CeO2 are much better than YSZ and other Sc-ZrO2-based electrolytes in the temperature range of 300–1000 °C. It is proved that ZrO2 doped with Sc2O3 and CeO2 can be a promising alternative material for SOFCs, and the multi-site doping is also a promising research direction. The use of two-element or multi-element co-doping can not only hinders the ordering of oxygen vacancies but also flexibly regulates the lattice distortion of the cerium oxide co-solvent. Li et al. [56] fabricated and optimized a SOFC device with the configuration of BaO/Ni-GDC//GDC//GDC-LSCF. The peak power density of 824 mW/cm2 was reached at 750 °C. It can be observed that the presence of BaO can greatly depress the formation of carbon decomposition. Zha et al. [34] fabricated anode-supported SOFCs with 20-μm-thick GDC thin film prepared by co-pressing as the electrolyte and Ni/Cu-based thin films prepared by solution impregnation process as anodes. The results indicated that the cell performance can be impacted on by the composition of anodes, thickness of the electrolyte and the microstructure. Typically, the peak power density of SOFC fueled by hydrogen always indicates the highest value of 602 mW/cm2 at 600 °C, compared with the SOFCs fueled by methane and propane. It can be concluded that if the problem that Ce4+ can be easily reduced into Ce3+ can be solved, CeO2-based materials will be promising SOFC electrolyte materials.
In addition, bilayer electrolytes have attracted researchers’ attentions. Solovyev et al. [57] prepared and investigated several SOFCs with single-layer YSZ or CGO and YSZ/CGO bilayer electrolyte, and the results showed that single-layer CGO is the most effective electrolyte at lower operating temperatures of 650–700 °C. The peak power density of 1250 mW/cm2 can be obtained at 800 °C for the 4-μm YSZ/1.5-μm CGO bilayer electrolyte. The peak power density of 440 mW/cm2 can be reached at 900 °C. Although YSZ has a low conductivity at low and intermediate temperatures, considering its comprehensive performance and cost, many researchers still try to make it thin film and apply it to intermediate temperature fuel cells. Dong et al. [58] developed a functionally graded Bi1.6Er0.4O3 (ESB)/Y0.16Zr0.84O1.92(YSZ) bilayer electrolyte via a cost-effective screen-printing process using nanoscale ESB powders on the tape-cast NiO-YSZ anode support. The novel bilayer solid oxide fuel cell (SOFC) yields extremely high power density of 2.1 W/cm2 at 700 °C, which is 2.4 times higher compared to that of the YSZ single-electrolyte SOFC.
Perovskite electrolyte
In addition to the above two materials characterized by fluorite structure, some materials with the ABO3-type perovskite structure have been found to have very excellent chemical stability in oxidizing and reducing atmosphere, and their ionic conductivity can be dominical over a large range of oxygen partial pressures. Among all materials of the ABO3-type perovskite structure, some materials have oxygen-ion conductivity, while others have proton conductivity. As a typical oxygen-ion-conducting electrolyte material for SOFCs, LSGM (La1 − xSrxGa1 − yMgyO3) showed an excellent performance on ionic conductivity and long-term stability. Raghvendra et al. [59] have studied the electrical conductivity of barium-substituted LSGM electrolyte materials for IT-SOFC. Another metal element Ba was doped with LSGM in the experiment, and the results showed that secondary phases increase with increasing Ba content, which is adverse to the formation of pure phase of the thin film. In addition, few Ba content leads to a slight increment in DC conductivity, while the DC conductivity would decrease once the Ba content takes more than 0.07 for the formation of impure phase of Ba. In other words, elements doping of excessive type might not develop the whole performance of materials if the dopant content exceeds a certain level. Sun et al. [60] utilized the glycine nitrate process to synthesize LSGM (La0.9Sr0.1Ga0.8Mg0.2O3-δ) and LSCM (La0.7Sr0.3Ga0.5Mg0.5O3-δ) powders, and utilized the slurry spin coating technology to fabricate the LSGM/LSCM electrolyte thin film on a porous substrate. The entire fuel cell device was optimized, and the optimal setting is 5 wt% content of ethyl cellulose as binder, 5 wt% content of terpineol as modifier, 9 times of the coating cycle number, and 1400 °C and 4 h for best-deposition sintering. The technical parameters of the preparation process have a significant impact on the performance of electrolyte and even the entire fuel cell device. It can be confirmed that LSGM could be a promising alternative if the preparation process could be optimized properly. However, LSGM contains at least four metal ions, and the preparation process is prone to the occurrence of other phase, which will reduce the conductivity of the electrolyte. On the other hand, thin-film preparation of LSGM electrolyte materials is more difficult than that of YSZ and DCO materials, so the development of more mature thin-film technology will promote the further application of LSGM.
Direct fuels other than hydrogen
Due to the theory of O2−-conducting SOFC, oxygen ions will be transferred to anode through the electrolyte. Therefore, any environmentally friendly fuel that can be oxidized by oxygen ions may be a potential alternative fuel for O2−-conducting SOFC. Ma et al. [61] found that ammonia could be a potential alternative fuel for SOFCs, and fabricated an anode-supported SOFC with YSZ as electrolyte by the dry-pressing technique. The peak power density of this ammonia-fueled SOFC approached 526 mW/cm2 at 850 °C, which is only slightly lower than the common hydrogen-fueled ones. Besides, the ammonia-fueled fuel cell shares the same electrolyte resistance with the hydrogen-fueled one, but larger polarization resistances, which can essentially determine the performance of fuel cells, which is presented by impedance spectra. Under the same research subject, Fuerte et al. [62] made further study on the ammonia-fueled SOFC. Commercial microtubule SOFC with Ni/YSZ as anode, LSM (La0.8Sr0.2MnO3-δ) as cathode and YSZ as electrolyte was fabricated and tested. The mechanism for ammonia conversion was elucidated by the cell behavior, and it was pointed that the potentially available energy obtained from oxidizing ammonia is twice that from oxidizing hydrogen, whereas the higher operating temperature is required when ammonia is used as fuel. The slower process of decomposing ammonia into hydrogen and nitrogen at a relatively low operating temperature can be utilized to explain a nonlinear relationship between voltage and current density, which is the crucial determining factor for the performance of fuel cell. Ammonia-fueled SOFC has not been studied generally, and the research on that only takes a few parts of the entire research community, which can be observed in Fig. 3. The similar condition can also be found in the research fields of methanol- and hydrogen sulfide-fueled SOFCs.
Performance trends of oxygen-ion-conducting SOFCs
Figure 4 depicts a tendency of performance of selected SOFCs literature published since the year 2000. Obviously, the best peak power density of more than 1200 mW/cm2 occurred in 2016, which indeed presents the remarkable progression made during the last several decades. The emergence frequency of low open-circuit voltage is low, and the open-circuit voltage remains at a relatively stable level while the peak power density keeps a chronological progression. The main reason for the tendency is that the preparation methods of thin films have been developed and the ion conductivity has been increased. The data of Figs. 4 and 5 are adapted from Ref. [30, 33, 35, 39, 43, 55, 63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176].
What must be reminded of are the significant developments of ion conductivity which can be seen from Fig. 5 that the tendency of conductivity is flatter while the figures gather between 2010 and 2019 and the highest value over 700 mS/cm has occurred in 2016.
Each of materials has two coins as electrolyte for solid oxide fuel cell, and the disadvantages can be clearly illuminated as follows: YSZ has a low conductivity at low and intermediate temperatures, the raw material cost of Sc2O3 for preparing ScSZ is too high, the long-term high-temperature service performance is severely aged, Ce4+ of CeO2 can be easily reduced into Ce3+, which can impact on the performance of fuel cell to a great extent, and the presence of other phases when preparing LSGM thin film would influence the purity of electrolyte materials which is a critical factor for the performance of SOFCs.
Performance of proton-conducting electrolyte materials for SOFCs
Proton-conducting material is an important functional material with protons as charge carrier for the small diameter, lightweight and the relatively high mobility of the particle. As the oxygen defects are also required in the lattice and water vapor or hydrogen will be absorbed quickly to form proton defects, the feasible approaches can be found by analyzing the fundamental theoretical formulas. As a relatively small particle, it is deemed that it is not possible for the particle to occupy the entire lattice position when forming OH− with the surrounding oxygen ions. In the lattice of perovskite, the proton diffusion coefficient can be expressed as follows [177]:
where \( x_{{{\text{H}}^{ + } }} \) is the ratio of lattice positions of oxygen ions combined with protons; a is a geometry constant; Z is the number of proximal oxygen ions for protons; \( S_{\text{OH-O}} \) is the transition distance; \( v_{\text{H}}^{ + } \) is the effective proton transition frequency; \( \Delta S_{{m,{\text{H}}^{ + } }} \) is the change of migration activation entropy; \( \Delta H_{{m,{\text{H}}^{ + } }} \) is change of migration activation enthalpy.
According to the Nernst–Einstein formula, the proton conductivity is proportional to the number of charges, proton defects concentration and mobility, and the relation can be expressed as follows [178]:
It can be seen from the above formula that the proton concentration is related to the nature of the material and the preparation method, and generally does not change much. Therefore, as temperature changes, conductivity changes with activation enthalpy.
BaCeO3
Except from the ordinary ABO3-type perovskite structure which could be the electrolyte materials of either oxygen-ion-conducting SOFCs or proton-conducting SOFCs, a novel A2B’B”O6 (AB1/2’B1/2”O3)-type dual-perovskite structure was found to be a promising alternative electrolyte material for proton-conducting SOFCs. Bhide and Virkar [179] utilized calcining requisite mixtures of precursors in air to fabricate several mixed dual-perovskites, where A is Ba+2 and B’ and B” are trivalent and pentavalent ions, respectively. The mixed perovskites are characterized by higher stability compared with ordinary perovskites of the type of ABO3. The effect of A-site doping has been studied and as well as B-site doping, and Zuo et al. [180] prepared and investigated BZCY (Ba (Zr0.1Ce0.7Y0.2) O3–δ) as an electrolyte material for low- temperature SOFCs with the configuration of Ni-BZCY7/BZCY7/BCPY4. Compared with the ordinary YSZ, GDC and LSGM, BZCY is characterized by the highest ionic conductivity in a humid 4% H2/Ar atmosphere at 300–700 °C. Impedance analysis of single cells indicated that, at low temperatures, the performance of BZCY7-based cells was primarily limited by the relatively high polarization resistance of the electrodes. Tao and Irvine [181] utilized solid-state reaction method to prepare the nominal BaZr0.8Y0.2O2.9, Ba0.97Zr0.77Y0.16Zn0.04O2.88, and BaCe0.5Zr0.3Y0.16Zn0.04O3–δ powders. The highest known total conductivity of stable single-phase ceramic proton-conducting oxides has been found as 3.14 mS/cm at 400 °C and 10 mS/cm above 600 °C in this experiment. It was also found that effective sintering without firing at ultra-high temperatures of doped barium zirconates is demonstrated to be insufficient to achieve very high grain proton conductivity. Iwahara [182] prepared the Nd-doped BaCeO3 ceramic electrolyte exhibiting a mixed conduction of proton and oxide ions. As affected greatly by ohmic resistance of the electrolyte thin film, it is essential to reduce ohmic resistance of the electrolyte thin film to promote OCV (open-circuit voltage) which is a significant indication of the performance of solid oxide fuel cells. Besides, the results of the experiment indicated that the porous nickel could be another promising anode material for the Nd-doped BaCeO3 electrolyte to replace the ordinary ones except for platinum.
Bilayer electrolyte has been also applied into proton-conducting SOFCs to promote the cell performance. Basbus et al. [183] studied the BaCe0.4Zr0.4Y0.2O3-δ/BaCe0.8Pr0.2O3-δ (BCZY/BCP) bilayer membrane for protonic conductor solid oxide fuel cells, and addressed the blocking of the electronic conductivity. Measurements confirm that the BCZY film blocks the electronic conductivity of BCP under wet synthetic air and protects it from CO2-containing atmospheres. Due to this set of properties, the BCZY/BCP bilayer membrane represents a possible candidate as the electrolyte for SOFC operating between 400 and 600 °C.
The original proton-conducting SOFCs are generally based on electrolyte support, which means a huge thickness of the electrolyte with relatively high ohmic resistance. In 1990, the peak power density of the assembled by Iwahara [182] with a BaCe0.9Nd0.1O3 of 500 μm as electrolyte and Pt as electrode reached 150 mW/cm2 at 600 °C. Hibino et al. [184] made the peak power density of 500 μm BaCe0.75Y0.25O3 supporting SOFC approach 134 mW/cm2 at 600 °C through devising the novel Pd-loaded FeO anode materials and BaPr0.5CoO3 cathode materials, implying that it is vital for improving the performance of proton-conducting SOFCs to devise novel electrodes materials.
Researchers also attempted to improve the preparation technique of thin film to promote the performance of proton-conducting SOFCs. Ito et al. [185] prepared a 0.7-μm-thick BaCe0.8Y0.2O3 thin film on the Pd substrate and assembled a single cell which reached the peak power density of 900 and 1500 mW/cm2 at 400 and 600 °C, respectively. However, Pt is not suitable for commercialization production for its expensive cost. Therefore, replacing Pt with Ni has become a focus of the study. Then Peng et al. [94] fabricated a 50-μm-thickn BaCe0.8Sm0.2O3 thin film on the Ni substrate successfully in 2006, and the peak power density of 340 mW/cm2 was obtained at 700 °C, which was increased by an order of magnitude than the proton-conducting SOFCs with thick film. Bi et al. [186] assembled a single cell with La0.7Sr0.3FeO3 as anode and BaCe0.8Sm0.2O3 as electrolyte. The thickness of the film is only 10 μm due to the advanced preparation technique, so as to lead a low resistivity of 0.86 Ω cm2, leading to a peak power density of 535 mW/cm2.
Semiconductor
Excepted from the conventional proton-conducting electrolyte materials, the electrolytes adapted and promoted from semiconductors could be considered as another approach of proton conductors for SOFCs. Lan and Tao [187] obtained a good proton conductor in intercalation oxides such as LixAl0.5Co0.5O2 by suppressing the electronic conduction, and the specific method is to change the oxidation states of transition elements. An open-circuit voltage of 1.0–1.1 V for hydrogen/air fuel cell and a peak power density of 179 mW/cm2 were observed at 475–575 and 525 °C. The ionic conductivity of LiAl0.5Co0.5O2 was 0.1 S cm−1 at 500 °C, deduced from the series resistance of the hydrogen/air fuel cell. Zhou et al. [188] suppress the electronic conduction through a filling-controlled Mott transition induced by spontaneous hydrogen incorporation. Mott transition was introduced to make the original semiconductor transmitted into an electronic insulator to suppress the electronic leakage. Dong et al. [189] devised a novel approach to suppress the electronic conduction by properly selecting the band structure of the anode and cathode material. TiO2 electrolyte fuel cell was designed using the energy band theory and has a typical combination of semiconductor and ion properties with fuel cells. The TiO2 thin film fuel cell has manifested a notable yield of 364 mW/cm2 at 550 °C. All above typical publications came up with diverse methods to make it possible that semiconductor can be the electrolyte for SOFCs, which heavily advanced the development of novel directions for SOFCs.
Performance trends of proton-conducting SOFCs
Even though research on proton-conducting SOFCs is not as much as that of O2−-conducting SOFCs, the performance of proton-conducting SOFCs is remarkable as well. The proton conductivity and chemical stability of several types of materials are shown in Table 1 [177, 179,180,181,182, 190,191,192].
Summary of the reported representative maximum power density of proton-conducting SOFCs fabricated using various electrolytes, cathode and anode materials is shown in Fig. 6 [94, 177,178,179,180,181,182, 184, 186, 190,191,192,193].
As can be seen from Fig. 6, only a little research on proton-conducting SOFCs was notable before 2009 and the number of researches on proton-conducting SOFCs gradually increased from 2009 on. The highest value of peak power density of 840 mW/cm2 has occurred in 2018. There is obviously a raising tendency over not only the number of researches but also the peak power density of the assembled fuel cell during the last decade.
Among all the proton conductors as electrolytes for SOFCs, the BaCeO3-based electrolytes exhibit the highest proton conductivity. However, BaCeO3-based electrolytes are characterized by poor resistance to carbon dioxide and water corrosion, which could lower proton conductivity, cause thermal expansion of the materials, and seriously reduce the performance of the SOFCs device. Otherwise, BaCeO3-based electrolytes exhibit multi-type ions-conducting property above 600 °C, which makes it difficult to do research and application on the material. For another alternative electrolyte material, BaZrO3 exhibits a relatively high stability in the atmosphere of water or carbon dioxide and excellent chemical and mechanical strength. A low proton conductivity hinders the research of BaZrO3, which can be put forward by elements doping. Proton conductors are expected to play a greater role if the effects of CO2 can be eliminated and made to work in a hydrogen environment. There are still more progresses to be made for the reason that proton-conducting SOFCs have a promising future to converse fuel into energy under a relatively low operation temperature.
Performance of mixed-ion-conducting electrolyte materials for SOFCs
The composite electrolyte refers to the electrolyte which is comprised by two or more materials with different charge conduction characteristics, mainly including oxides and salts. These composite materials have shown tremendously high capability of conducting charges, and the ions conductivity can approach 0.01–1 S/cm at 400–600 °C, which is dozens to hundreds of times of the value of traditional single-phase electrolyte materials. It was found that the two types of charge carrier coexist inside the composite electrolyte and the protons, the conductivity of which is one to two times that of oxygen ions, are the dominant carriers [14,15,16].
Inside the composite electrolyte, all of oxygen ions, hydrogen ions, carbonate ions and even alkali metal ions take part in carrying charges; that’s to say, it is a mixed multi-ion reaction circumstance which makes it tough to realize the transportation processing of ions conducting. The conducting theory of hydrogen ions between the two-phase boundaries of the composite electrolyte is not clear yet. It is generally believed that oxygen ions are conducted through the oxygen vacancy as they do in ordinary O2−-conducting SOFCs, and so do carbonate ions. While interface transmission theory is utilized for hydrogen ions conducting, a model called “swing model”, which is illustrated vividly in Fig. 7 and written as Formula 24, was devised to explain and prove that boundaries conducting is the main path for ions transportation [194].
Although the multi-ionic conduction can help in rationalizing the conductivity enhancement, transport mechanisms at play still are still largely unconfirmed. Michel et al. [195] used an interface model of yttria-stabilized zirconia-LiKCO3 as a case to investigate transport properties and consider different mechanistic assumptions suggested in the literature over the years. There are two main transport mechanisms being considered: cogwheel mechanism and interfacial swing model expressed as follows, respectively. This literature provided important information on the electrochemical processes and transport mechanisms in these composite electrolytes, paving the way toward a deeper understanding of the origin of their enhanced electrochemical properties and suggesting general guidelines for their improvement.
Cogwheel mechanism:
Interfacial swing model:
Under fuel cell conditions, cerium oxide-molten carbonate composite electrolyte exhibits multi-ion conduction characteristics such as oxygen ion, proton and even carbonate ion, which contributes to improving the reaction kinetics at the electrode interface and increase the output power density of the cell. Tetravalent cerium ions are easily converted to trivalent cerium ions in the reduction atmosphere, which makes the open-circuit voltage of cerium-doped single-phase dielectric fuel cells at 400–600 °C often only 0.8–0.9 V, far below the theoretical voltage value, thus limiting the energy conversion efficiency of the cells. In the doped cerium oxide-molten carbonate composite electrolyte, carbonate as the protective layer of doped cerium oxide can reduce the tetravalent cerium ion to a certain extent. Therefore, the open-circuit voltage of doped cerium oxide-molten carbonate composite electrolyte is generally between 1.0 and 1.2 V in the low temperature range.
Ceria-carbonate composite
One thing we must mention is that Benamira et al. [8] investigated composite materials based on gadolinia-doped ceria (GDC) and alkali carbonates (Li2CO3–K2CO3 or Li2CO3–Na2CO3) and measured the stability of such composites over 6000 h. A study of the conductivity of GDC/LiK30 over 6000 h showed a relative stability, beginning with 9 × 10−2 S cm−1 and declining after 3500 h before stabilizing at 7 × 10−2 S cm−1. Zhang et al. [196] reported the stable performance of a fuel cell based on doped ceria–carbonate composite electrolyte and Sm0.5Sr0.5Fe0.8Cu0.2O3−δ cathode. At 550 °C, current output of about 400 mAcm−2 was continually generated during the non-stop 100-hour measurement under constant voltage of 0.7 V although further investigation is needed for longer term stability.
A ceria-based composite electrolyte based on SDC–25 wt% K2CO3 was prepared and investigated by Zuo et al. [197], and the fuel device was configured with 0.3-mm-thick electrolyte, NiO as anode and SSC (Sm0.5Sr0.5CoO2) as cathode. Peak power density and OCV (open-circuit voltage) of hydrogen-fueled SOFCs reached 600 mW/cm2 and 1.05 V at 700 °C, respectively. It was found that the transition temperature of the composite electrolyte is a key factor in the performance of composite electrolyte SOFCs. Generally, composite electrolyte is characterized by low ionic conductivity below a certain temperature, whereas once operating temperature breakthrough the point, the ionic conductivity of the composite electrolyte will be increased significantly. It could be feasible to promote the performance of the SOFCs with composite electrolyte by decreasing the transition temperature. Khan et al. [198] comparatively investigated three types of ceria-based nanocomposite electrolytes, including LNK-SDC (lithium, sodium and potassium composite carbonates–ceria-doped samaria), LN-SDC (lithium and sodium composite carbonates–ceria-doped samaria) and NK-SDC (sodium and potassium composite carbonates–ceria-doped samaria). It was found that the maximum power density was achieved 484 mW/cm2 of LNK-SDC electrolyte at 570 °C using the LiCuZnNi oxide electrodes, which can be concluded that the multi-element doping can be more efficient to promote ionic conductivity than single- or double-element doping at some definite circumstance.
Perovskite-carbonate composite
The phenomenon of composite electrolyte (mixed ions conducting) was proved to be more efficient than single-type ions-conducting electrolyte; however, the ionic transport mechanism can be investigated in more details. Wu et al. [199] investigated the stability of conductivity of LSGM-30 wt% (Li/Na)2CO3 composite electrolyte, and it is the first time to study the conduction behaviors. The results showed that the conduction of oxygen anions, hydrogen cations and carbonate anions occur inside the LSGM-(Li/Na)2CO3 composite electrolyte at the same time, and the dominant factor is the conduction of carbonate anions at high temperature. A more significant discovery is that the wet atmosphere can lower transition temperature at a low-temperature environment, and the presence of carbon dioxide is characterized by the positive impact on the ionic conductivity. Hei et al. [200] devised and investigated a novel doped barium cerate-carbonate composite electrolyte prepared by dry-pressing technique. Peak power densities achieved 957 mW/cm2 and 701 mW/cm2 at 600 °C and 550 °C, respectively.
Single-layer SOFCs
Besides the conventional three-layer (anode layer, electrolyte layer and cathode layer) SOFCs, single-layered nanocomposite fuel cell has also been devised. From the perspective of materials and technologies, there are several publications which can indicate the advanced progress in this novel subject. Zhu et al. [201] fabricated a solid oxide fuel cell which employs only one homogenous layer comprised of a mixture of metal oxide, Li0.15Ni0.45Zn0.4 oxide, and an ionic conductor, ion-doped ceria. The layer exhibits both ionic and semiconducting properties, and the fuel cell device has a peak power density of 600 mW/cm2 with H2 and air as fuel at 500 °C. Asghar et al. [202] used a composite of lithium-nickel-zinc-oxide (electrode material) and gadolinium-doped-cerium-oxide (ionic conductor) to explain the operating principle of a single-component nanocomposite fuel cell. The resistance to the transport of charge carriers and leakage currents would be the dominant performance limiting factors of the 1-layer fuel cell. Particularly, a peak power density of 801 mW/cm2 was obtained using Ni0.8Co0.15Al0.05LiO2 (NCAL)-coated Ni foam as the current collector at 550 °C. Shao et al. [203] utilized diverse cell configurations and compositions to confirm that appropriate selection of material and cell structure design could be a vital factor for stable operation of single-layer solid oxide fuel cells. They found that the metal/semiconductor contact, combining with the in situ formed P–N junction, not only promotes the anode hydrogen oxidation reaction kinetics but also benefits the charge separation to improve the voltage efficiency and the ionic transport through the internal built-in electric fields. Ganesh et al. [204] doped LiCoO2 with Mg to develop ionic transport property by tuning the energy band structure with Mg doping. The results showed that the polarization loss was reduced significantly and power output of the fuel cell devices was improved. The results have demonstrated that designing and changing the band structure can make a great impact on material conductivity and the fuel cell device performance, exhibiting great potential and advances of new-generation LT-SOFCs.
Electrolyte-free SOFCs
From the perspective of new scientific working principle, there are several publications that Zhu et al. [205] devised electrolyte-free fuel cell which consists of a single-component of nanocomposite material which works as a one-layer fuel cell device contrary to the traditional three-layer anode–electrolyte–cathode structure. Electrochemical reaction sites and charge transport paths can be provided by the nanocomposite, which can enable redox reactions to occur on nanoparticles. Then Zhu et al. [206] made use of the alignment of semiconductor energy bands and junction properties to achieve ion transport and prevent electronic short-circuiting. A novel fuel cell with nanocomposite functional layer was designed by perovskite solar cell principle and the new device achieved a stable power output of 1080 mW/cm2 with hydrogen as fuel at 550 °C. Zhu et al. [207] reported a semiconductor-ionic heterostructure La0.6Sr0.4Co0.2Fe0.8O3-δ (LSCF) and Sm–Ca co-doped ceria (SCDC) material possessing unique properties for new generation fuel cells using semiconductor-ionic heterostructure composite materials which displayed promising performance in terms of OCV (above 1.0 V) and enhanced power density (1000 mW/cm2 at 550 °C). The associated benefits of the semiconductor-ionic heterostructure composite include cost-effective materials, simple technology and cheap fabrication processes, which along with deep scientific understanding, may in turn realize and accelerate fuel cell commercialization, and open up hitherto unidentified applications by using the new semiconductor-ionic functionalities and principles. Wang et al. [208] designed a double-layer solid oxide fuel cell (DLSOFC) without using the electrolyte (layer) integrating advantages of positive electrode material of lithium ion battery (LiNi0.8Co0.15Al0.05O2) and oxygen-permeable membranes material (trace amount cobalt incorporated terbium doped ceria, TDC + Co) based on the semiconductor physics principle. This device with two layers can realize the function of SOFC by introducing a depletion layer to replace the electrolyte layer to realize the fuel cell function and at the same time avoids the electronic short-circuiting problem. Zhu et al. [209] designed a novel fuel cell device based on integrating the Schottky junction effect on the electrochemical principle. It can be found that the Schottky junction has a significant effect on the greatly enhanced device performance, and the fuel cell device incorporating the Schottky junction effect reaches a power output of 1000 mW/cm2 at 550 °C.
Performance trends of mixed-ion-conducting SOFCs
The composite electrolyte has not been studied as well as the other types of electrolyte. Fewer published literatures of that can be selected to reveal the truth. The peak power density and peak conductivity of composite electrolyte SOFCs fabricated during the year 2000 and the present are shown in Fig. 8 [17, 18, 197,198,199,200, 210,211,212,213,214,215,216,217,218]. As can be seen from the below figure, the higher values concentrated on the period of 2008 to 2014, which means the researches on composite electrolyte SOFCs have become a focus of the community from 2008 on. The fabricated cells haven’t always shown an excellent chemical stability, which seriously hinders the application of SOFCs, and research data on the life span of the composite electrolyte are limited.
Composite electrolytes present multi-ion co-conduction and the high output power at medium and low temperature can be obtained. It can be certain that the composite electrolyte materials will be the research focus in the future, and multiphase electrolyte materials with high conductivity will contribute to the realization of SOFC at low temperature. However, the underlying problems of ion conduction mechanism and conduction path are yet to be further explored.
Conclusion
SOFCs technology promises to solve the barriers of resistivity, conductivity and operation temperature, while the hydrogen preparation and storage technology promise to be advanced. The exceedingly rapid and promising achievements in SOFCs have been reviewed and discussed. This article reviews the state-of-the-art data on cell performance reported in the literature published during the last two decades. The main observations can be summarized as follows [219]:
- (1)
With the number of researches on SOFCs increasing during the last twenty years, the conductivity and peak power density have been ascending continuously. The highest value of conductivity of the electrolyte reached 1 S/cm, and the peak power density of the cell approached 1700 mW/cm2 which definitely adapted from composite electrolyte (multi-ions-conducting) SOFCs, respectively.
- (2)
A wide range of fuels were utilized in several kinds of SOFCs, and the fuels with carbon elements were also well studied and could be a promising alternative for replacing hydrogen as the main fuel of SOFCs if the preparation cost and storage technology meet the obstacles which could not be solved as soon as possible.
- (3)
The cell performance of O2−-conducting SOFCs, proton-conducting SOFCs and composite electrolyte (mixed-ions-conducting) SOFCs is reviewed, respectively. O2−-conducting SOFCs possess higher power density with higher temperature which is not friendly to proton-conducting SOFCs, and the chemical stability of the latter is much worse than that of the former. Composite electrolyte (mixed-ions-conducting) SOFCs have not been studied well, and the main barrier is still the ionomer stability which should be regarded as imperative.
All three aspects of SOFCs discussed above might face different challenges, although the ultimate target is to promote the power density within a longer life span at a relatively low temperature. High power density and long-term stability are required at the same time, and it is necessary to devise novel alternative electrolyte and electrode materials to approach the above target. By disentangling these issues, low-temperature SOFCs can be an excellent alternative for power generator applied in numerous fields.
References
Steele BCH, Heinzel A (2001) Materials for fuel-cell technologies. Nature 414(6861):345–352
Pelegrini L, João NR, Hotza D (2016) Process and materials improvements on Ni/Cu-YSZ composites towards nanostructured SOFC anodes: a review. Rev Adv Mater Sci 46:6–21
Reddy AA, Tulyaganov DU, Kharton VV, Ferreira José M F (2015) Development of bilayer glass-ceramic SOFC sealants via optimizing the chemical composition of glasses—a review. J Solid State Electrochem 19(10):2899–2916
Zhou G, Li B, Long Z, Zeng H, Li H (2016) Review of preparation technologies for thin electrolyte film of solid oxide fuel cells. Chin J Power Source 40(305):255–258
Pandey A (2018) Progress in solid oxide fuel cell (SOFC) research. JOM J Miner Metals Mater Soc 71(6396):88–89
Xifeng D, Ling G, Han C, Lucun G (2006) Recent research progress of lacro_3 in solid oxide fuel cell. Mater Rev 20(9):29–33
Lyu Y, Wang F, Wang D, Jin Z (2019) Alternative preparation methods of thin films for solid oxide fuel cells: review. Mater Technol. https://doi.org/10.1080/10667857.2019.1674478
Lee YH, Chang I, Cho GY et al (2018) Thin film solid oxide fuel cells operating below 600 °C: a review. Int J Precis Eng Manuf Green Technol 5:441–453. https://doi.org/10.1007/s40684-018-0047-0
Benamira M, Ringuedé A, Hildebrandt L, Lagergren C, Vannier RN, Cassir M (2012) Gadolinia-doped ceria mixed with alkali carbonates for SOFC applications: II—an electrochemical insight. Int J Hydrog Energy 37(24):19371–19379
Stambouli AB, Traversa E (2002) Solid oxide fuel cells (SOFCs): a review of an environmentally clean and efficient source of energy. Renew Sustain Energy Rev 6(5):433–455
Lee M, Park G, Radisavljevic-Gajic V (2013) Modeling of solid oxide fuel cells (SOFCs): an overview. In: 2013 5th international conference on modeling, simulation and applied optimization, ICMSAO 2013, pp. 1–6. https://doi.org/10.1109/icmsao.2013.6552683
Thangadurai V, Kan WH, Mirfakhraei B, Bhella SS, Trinh TT (2012) ChemInform abstract: materials for proton conducting solid oxide fuel cells (H-SOFCs). ChemInform 43(38):483–492. https://doi.org/10.1002/chin.201238217
Hajimolana SA, Hussain MA, Daud WMAW, Soroush M, Shamiri A (2011) Mathematical modeling of solid oxide fuel cells: a review. Renew Sustain Energy Rev 15(4):1893–1917
Ali SAM, Muchtar A, Muhamad N, Sulong AB (2011) A review on preparation of SDC-carbonate as composite electrolyte material for intermediate temperature solid oxide fuel cells (IT-SOFC). Clean Energy Technol, IEEE
Huang J, Mao Z, Liu Z, Wang C (2007) Development of novel low-temperature SOFCs with co-ionic conducting SDC-carbonate composite electrolytes. Electrochem Commun 9(10):2601–2605
Zhong HT, Ai DS, Lin XP (2014) Lsgm-carbonate composite electrolytes for intermediate-temperate SOFCs. Key Eng Mater 602–603:862–865
Ma Y, Singh M, Wang X, Yang F, Huang Q, Zhu B (2014) Study on gdc-kznal composite electrolytes for low-temperature solid oxide fuel cells. Int J Hydrog Energy 39(30):17460–17465
Liu C, Zhang H, Xia J, Li Z (2012) Synthesis and characterization of zr0.85y0.15o1.925-la9.33si6o26composite electrolyte for application in SOFCs. J Adv Ceram 1(4):327–335
Zhu B (2001) Advantages of intermediate temperature solid oxide fuel cells for tractionary applications. J Power Sources 93(1–2):82–86
Zhu B (2001) Proton and oxygen ion-mixed-conducting ceramic composites and fuel cells. Solid State Ionics Diffus React 145(1–4):371–380
Lu C, Worrell W, Vohs J, Gorte R (2003) A comparison of Cu-Ceria-SDC and Au-Ceria-SDC composites for SOFC anodes. ECS Proc. https://doi.org/10.1149/200307.0773PV
Tao Z, Bi L, Yan L, Sun W, Zhu Z, Peng R et al (2009) A novel single phase cathode material for a proton conducting SOFC. Electrochem Commun 11(3):688–690
Fuerte A, Valenzuela RX, Escudero MJ, Daza L (2009) Ammonia as efficient fuel for SOFC. J Power Sources 192(1):170–174
Koh JH, Yoo YS, Park JW, Lim HC (2002) Carbon deposition and cell performance of Ni-YSZ anode support SOFC with methane fuel. Solid State Ionics Diffus React 149(3–4):157–166
Carollo G, Garbujo A, Bedon A, Ferri D, Natile MM, Glisenti A (2018) Cu/CGO cermet-based electrodes for symmetric and reversible solid oxide fuel cells. Int J Hydrog Energy. https://doi.org/10.1016/j.ijhydene.2018.01.201
Ze L, Qing-Shan Z, Min-Fang H (2010) Fabrication and performance of direct methane sofc with a cu-ceo2-based anode. Acta Phys Chim Sin 26(3):583–588
Kim T, Ahn K, Vohs JM, Gorte RJ (2007) Deactivation of ceria-based sofc anodes in methanol. J Power Sources 164(1):42–48
Bogolowski N, Iwanschitz B, Drillet J-F (2015) Development of a coking-resistant nisn anode for the direct methane sofc. Fuel Cells 15(5):711–717
Yang BC, Koo J, Shin JW, Go D, Shim JH, An J (2017) Direct alcohol-fueled low-temperature solid oxide fuel cells: a review. Energy Technol 7(1):5–19
Sasaki K, Watanabe K, Teraoka Y (2004) Direct-alcohol SOFCs: current-voltage characteristics and fuel gas compositions. J Electrochem Soc 151:A965–A970. https://doi.org/10.1149/1.1756884
Huang J, Gao Z, Mao Z (2010) Effects of salt composition on the electrical properties of samaria-doped ceria/carbonate composite electrolytes for low-temperature SOFCs. Int J Hydrog Energy 35(9):4270–4275
Handal HT, Thangadurai V (2014) Electrochemical characterization of multi-element-doped ceria as potential anodes for SOFCs. Solid State Ionics 262:359–364
Chen X, Ni W, Wang J, Zhong Q, Han M, Zhu T (2018) Exploration of co-fe alloy precipitation and electrochemical behavior hysteresis using lanthanum and cobalt co-substituted srfeo 3-δ, SOFC anode. Electrochim Acta 277:226–234
Zha S, Moore A, Abernathy H, Liu M (2004) GDC-based low-temperature SOFCs powered by hydrocarbon fuels. J Electrochem Soc 151:A1128–A1133. https://doi.org/10.1149/1.1764566
Wei G-L, Luo J-L, Sanger A, Chuang K (2004) High-performance anode for H2S-Air SOFCs. J Electrochem Soc 151:A232–A237. https://doi.org/10.1149/1.1636177
Huang QA, Wang B, Qu W, Hui R (2009) Impedance diagnosis of metal-supported SOFCs with sdc as electrolyte. J Power Sources 191(2):297–303
Yin Y, Zhu W, Xia C, Gao C, Meng G (2004) Low-temperature SOFCs using biomass-produced gases as fuels. J Appl Electrochem 34(12):1287–1291
Gorte RJ, Kim H, Vohs JM et al (2002) Novel SOFC anodes for the direct electrochemical oxidation of hydrocarbons. ChemInform 106:10–15
Chen S, Tu H, Li S, Yu Q (2018) Fabrication and performance of Pr2-xLaxNiO4 + δ based cathode for intermediate temperature solid oxide fuel cells. Chin J Power Sources 42(2):219–222
Sun LL, Hu ZM, Luo LH et al (2017) Application of Ru nano flowers doped ni-ysz anode in ethanol-fueled SOFC. Rare Metal Mater Eng 46(08):2322–2326
Lu C, Worrell W, Gorte R, Vohs J (2003) SOFCs for direct oxidation of hydrocarbon fuels with samaria-doped ceria electrolyte. J Electrochem Soc 150(3):354–358. https://doi.org/10.1149/1.1553765
Hong-Xin Y, Hong-Jie G, Run-Jie L, Gang C, Abuliti A, Xin-Wei D (2014) The effect of dry methane flux on the methane reactions in solid oxide fuel cell at ni-ysz anode. J Chem Eng Chin Univ 28(5):1004–1009
You H-X, Gao H-J, Chen G, Abudula A, Ding X-W (2013) Effects of dry methane concentration on the methane reactions at Ni-YSZ anode in solid oxide fuel cell. J Fuel Chem Technol 41:374–379
Fushao L, Shubiao X, Yuxing Y, Feixiang C (2018) Thermal stability and electrochemical performance of LaSrCoO_(4 ± δ) as the cathode material for solid-oxide fuel cells. J Kunming Univ Sci Technol (Natural Science) 43(5):14–21
Xiu-Xia M, Gong X, Tan XY et al (2013) Preparation and properties of direct-methane solid oxide fuel cell based on a graded Cu-CeO2-Ni-YSZ composite anode. Acta Physico-Chimica Sinica 29:1719–1726
Kilner JA (2000) Fast oxygen transport in acceptor doped oxides. Solid State Ionics Diffus React 129(1–4):13–23
Tikkanen H, Suciu C, Wærnhus I, Hoffmann AC (2011) Dip-coating of 8ysz nanopowder for sofc applications. Ceram Int 37(7):2869–2877
Fernández-González R, Molina T, Savvin S, Moreno R, Makradi A, Núñez P (2014) Fabrication and electrical characterization of several YSZ tapes for SOFC applications. Ceram Int 40(9):14253–14259
Park I, Ahn J, Im J, Choi J, Shin D (2012) Influence of rheological characteristics of YSZ suspension on the morphology of YSZ films deposited by electrostatic spray deposition. Ceram Int 38(supp-S1):S481–S484
Oskouyi OE, Maghsoudipour A, Shahmiri M, Hasheminiasari M (2019) Preparation of YSZ electrolyte coating on conducting porous Ni–YSZ cermet by DC and pulsed constant voltage electrophoretic deposition process for SOFCs applications. J Alloy Compd 795:361–369. https://doi.org/10.1016/j.jallcom.2019.04.334
Jiang Y, Virkar A (2001) A high performance, anode-supported solid oxide fuel cell operating on direct alcohol. J Electrochem Soc 148(7):706–709
Satardekar P, Montinaro D, Sglavo V (2015) Fe-doped YSZ electrolyte for the fabrication of metal supported-SOFC by co-sintering. Ceram Int 41(8):9806–9812
Toor S, Croiset E (2019) Reducing sintering temperature while maintaining high conductivity for SOFC electrolyte: copper as sintering aid for Samarium Doped Ceria. Ceram Int 46:1148–1157. https://doi.org/10.1016/j.ceramint.2019.09.083
Fu Z, Sun Q, Ma D, Zhang N, An Y, Yang Z (2017) Effects of Sm doping content on the ionic conduction of CeO 2 in SOFCs from first principles. Appl Phys Lett. https://doi.org/10.1063/1.4993897
Lee D-S, Kim W, Choi S-H, Lee H, Lee JH (2005) Characterization of ZrO2 Co-doped with Sc2O3 and CeO2 electrolyte for the application of intermediate temperature SOFCs. Solid State Ionics 176:33–39. https://doi.org/10.1016/j.ssi.2004.07.013
Li X, Shao G-Q, Luo J, Lu J, Xue M-s, Hou Y, Deng L (2013) Fabrication and characterization of GDC electrolyte/electrode integral SOFC with BaO/Ni-GDC anode. Mater Res Bull 50:337–340. https://doi.org/10.1016/j.materresbull.2013.11.034
Solovev A, Shipilova A, Ionov I, Kovalchuk A, Rabotkin S, Oskirko V (2016) Magnetron-sputtered YSZ and CGO electrolytes for SOFC. J Electron Mater 45:3291–3928. https://doi.org/10.1007/s11664-016-4462-0
Joh D, Park J, Kim D, Wachsman E, Lee K (2017) Functionally graded bismuth oxide/zirconia bilayer electrolytes for high performance intermediate-temperature solid oxide fuel cells (IT-SOFCs). ACS Appl Mater Interfaces 9(10):8443–8449
Singh RK, Singh P (2014) Electrical conductivity of barium substituted LSGM electrolyte materials for IT-SOFC. Solid State Ionics 262:428–432. https://doi.org/10.1016/j.ssi.2014.01.044
Sun H, Sen W, Ma W-H, Yu J, Yang J-J (2014) Fabrication of LSGM thin films on porous anode supports by slurry spin coating for IT-SOFC. Rare Met 34:797–801. https://doi.org/10.1007/s12598-014-0346-8
Ma Q, Ma J, Zhou S, Yan R, Gao J, Meng G (2007) A high-performance ammonia-fueled SOFC based on a YSZ thin-film electrolyte. J Power Sources 164:86–89. https://doi.org/10.1016/j.jpowsour.2006.09.093
Fuerte A, Valenzuela R, Escudero M, Daza L (2009) Ammonia as efficient fuel for SOFC. J Power Sources 192:170–174. https://doi.org/10.1016/j.jpowsour.2008.11.037
Lei Z, Zhu Q-S, Han M-F (2010) Fabrication and performance of direct methane SOFC with a Cu-CeO2-based anode. Acta Phys Chim Sin 26:583–588. https://doi.org/10.3866/PKU.WHXB20100323
Worrell W, Vohs J, Gorte R (2003) A comparison of Cu-ceria-SDC and Au-ceria-SDC composites for SOFC anodes. J Electrochem Soc 150:A1357–A1359. https://doi.org/10.1149/1.1608003
Yang L, Zuo C, Wang S, Cheng Z, Liu M (2008) A novel composite cathode for low-temperature SOFCs based on oxide proton conductors. Adv Mater 20:3280–3283. https://doi.org/10.1002/adma.200702762
Tao Z, Bi L, Yan L, Sun W, Zhu Z, Peng R, Liu W (2009) A novel single phase cathode material for a proton conducting SOFC. Electrochem Commun 11:688–690. https://doi.org/10.1016/j.elecom.2009.01.012
Demin A, Tsiakaras P, Gorbova E, Hramova S (2004) A SOFC based on a co-ionic electrolyte. J Power Sources 131:231–236. https://doi.org/10.1016/j.jpowsour.2003.10.016
Zhu Y, Zhou W, Chen Y, Shao Z (2016) An aurivillius oxide based cathode with excellent CO2 tolerance for intermediate-temperature solid oxide fuel cells. Angew Chem 128:8988–8993. https://doi.org/10.1002/ange.201604160
Huang Y, Vohs J, Gorte R (2006) An examination of LSM-LSCo mixtures for use in SOFC cathodes. J Electrochem Soc 153:A951–A955. https://doi.org/10.1149/1.2186183
Cherng J, Ho M, Yeh TH, Chen WH (2012) Anode-supported micro-tubular SOFCs made by aqueous electrophoretic deposition. Ceram Int 38:S477–S480. https://doi.org/10.1016/j.ceramint.2011.05.057
Wang Z, Cheng M, Dong Y, Zhang M (2006) Anode-supported SOFC with 1Ce10ScZr modified cathode/electrolyte interface. J Power Sources 156:306–310. https://doi.org/10.1016/j.jpowsour.2005.06.035
Yuan K, Zhu J, Dong W, Yu Y, Lu X, Xiaojuan J, Wang X (2017) Applying low-pressure plasma spray (LPPS) for coatings in low-temperature SOFC. Int J Hydrog Energy 42:22243–22249. https://doi.org/10.1016/j.ijhydene.2017.04.215
Jaiswal A, Wachsman E (2005) Bismuth-ruthenate-based cathodes for IT-SOFCs. J Electrochem Soc 152:A787–A790. https://doi.org/10.1149/1.1866093
Koh J-H, Yoo Y-S, Park J, Lim H (2002) Carbon deposition and cell performance of Ni-YSZ anode support SOFC with methane fuel. Solid State Ionics 149:157–166. https://doi.org/10.1016/S0167-2738(02)00243-6
Kharton V, Figueiredo F, Navarro L, Naumovich E, Kovalevsky A, Yaremchenko A, Viskup A, Carneiro A, Marques F, Frade J (2001) Ceria-based materials for solid oxide fuel cells. J Mater Sci 36:1105–1117. https://doi.org/10.1023/A:1004817506146
Kupecki J, Kluczowski R, Papurello D, Lanzini A, Kawalec M, Krauz M, Santarelli M (2018) Characterization of a circular 80 mm anode supported solid oxide fuel cell (AS-SOFC) with anode support produced using high-pressure injection molding (HPIM). Int J Hydrog Energy. https://doi.org/10.1016/j.ijhydene.2018.02.143
Im H-N, Jeon S-Y, Choi M-B, Kim H-S, Song S-J (2012) Chemical stability and electrochemical properties of CaMoO 3 − δ for SOFC anode. Ceram Int Ceram Int 38:153–158. https://doi.org/10.1016/j.ceramint.2011.05.155
Ma Q, Tietz F (2012) Comparison of Y and La-substituted SrTiO3 as the anode materials for SOFCs. Solid State Ionics 225:108–112. https://doi.org/10.1016/j.ssi.2012.03.048
Carollo G, Garbujo A, Bedon A, Ferri D, Natile M, Glisenti A (2018) Cu/CGO cermet based electrodes for symmetric and reversible solid oxide fuel cells. Int J Hydrog Energy. https://doi.org/10.1016/j.ijhydene.2018.01.201
Marcucci A, Luisetto I, Zurlo F, Licoccia S, Bartolomeo E (2019) Pd-doped perovskite-based SOFC anodes for biogas. J Solid State Electrochem. https://doi.org/10.1007/s10008-019-04473-5
Lang M, Henne R, Schaper S, Schiller G (2001) Development and characterisation of vacuum plasma sprayed thin film SOFCs. J Therm Spray Technol 10:618–625. https://doi.org/10.1361/105996301770349141
Bogolowski N, Iwanschitz B, Drillet J-F (2015) Development of a coking-resistant NiSn anode for the direct methane SOFC. Fuel Cells 15:711–717. https://doi.org/10.1002/fuce.201400187
Akikusa J, Adachi K, Hoshino K, Ishihara T, Takita Y (2001) Development of a low temperature operation solid oxide fuel cell. J Electrochem Soc 148:A1275–A1278. https://doi.org/10.1149/1.1409972
Yang B, Koo J, Shin J, Go D, Shim J, An J (2017) Direct alcohol-fueled low-temperature solid oxide fuel cells: a review. Energy Technol 7:5–19. https://doi.org/10.1002/ente.201700777
Xie Z-X, Chen T, Wang Z-M, Shen Z-Y, Liao R, Li Y-M (2016) Effect of a-site deficiency on the performance of La0.6Sr1.4MgMoO6 for solid oxide fuel cell. Front Energy Res 45:1180–1185
Li C-J, Li C-X, Wang M (2005) Effect of spray parameters on the electrical conductivity of plasma-sprayed La1 − xSrxMnO3 coating for the cathode of SOFCs. Surf Coat Technol 198:278–282. https://doi.org/10.1016/j.surfcoat.2004.10.083
He H, Vohs J, Gorte R (2003) Effect of synthesis conditions on the performance of Cu-CeO2-YSZ anodes in SOFCs. J Electrochem Soc 150:A1470–A1475. https://doi.org/10.1149/1.1614268
Zhu W, Xia C, Ding D, Shi X, Meng G (2006) Electrical properties of ceria-carbonate composite electrolytes. Mater Res Bull 41:2057–2064. https://doi.org/10.1016/j.materresbull.2006.04.001
Fu C, Sun K, Zhang N, Xinbing C, Zhou D (2007) Electrochemical characteristics of LSCF–SDC composite cathode for intermediate temperature SOFC. Electrochim Acta 52:4589–4594. https://doi.org/10.1016/j.electacta.2007.01.001
Handal H, Thangadurai V (2013) Electrochemical characterization of multi-element-doped ceria as potential anodes for SOFCs. Solid State Ionics 262:359–364. https://doi.org/10.1016/j.ssi.2013.11.037
Ramos T, Sogaard M, Mogensen M (2014) Electrochemical characterization of Ni/ScYSZ electrodes as SOFC anodes. J Electrochem Soc 161:F434–F444. https://doi.org/10.1149/2.045404jes
Lyskov N, Kaluzhskikh M, Leonova L, Mazo G, Istomin S, Antipov E (2012) Electrochemical characterization of Pr2CuO4 cathode for IT-SOFC. Int J Hydrog Energy 37:18357–18364. https://doi.org/10.1016/j.ijhydene.2012.09.099
Hwang H, Moon J-W, Lee S, Lee E (2005) Electrochemical performance of LSCF-based composite cathodes for intermediate temperature SOFCs. J Power Sources 145:243–248. https://doi.org/10.1016/j.jpowsour.2005.02.063
Peng R, Wu Y, Lizhai Y, Zongqiang M (2006) Electrochemical properties of IT-SOFC based on proton conducting Sm-doped BaCeO3 electrolyte thin film. Solid State Ionics 177:389–393. https://doi.org/10.1016/j.ssi.2005.11.020
Zha SW, Li HB, Xia C, Liu XQ, Meng GY (2003) Electrochemical properties of La0.8Sr0.2Ga0.8Mg0.2O2.8 electrolyte and its application to SOFCs. Chem J Chin Univ Chinese Edn 24:506–508
Chen X, Ni W, Du X, Zhong Q, Zhu T, Han M (2018) Electrochemical property of multi-layer anode supported solid oxide fuel cell fabricated through sequential tape-casting and co-firing. J Mater Sci Technol 35:695–701. https://doi.org/10.1016/j.jmst.2018.10.015
Jafari M, Salamati H, Zhiani M, Shahsavari E (2018) Enhancement of an IT-SOFC cathode by introducing YSZ: electrical and electrochemical properties of composites. Int J Hydrog Energy. https://doi.org/10.1016/j.ijhydene.2018
Yang C, Li W, Shangquan Z, Bi L, Peng R, Chen C, Liu W (2009) Fabrication and characterization of an anode-supported hollow fiber SOFC. J Power Sources 187:90–92. https://doi.org/10.1016/j.jpowsour.2008.10.069
Xu D, Liu X, Wang D, Yi G, Gao Y, Zhang D, Su W (2007) Fabrication and characterization of SDC–LSGM composite electrolytes material in IT-SOFCs. J Alloy Compd 429:292–295. https://doi.org/10.1016/j.jallcom.2006.04.009
Somalu M, Yufit V, Cumming D, Lorente E, Brandon NP (2011) Fabrication and characterization of Ni/ScSZ cermet anodes for IT-SOFCs. Fuel Energy Abstr 36:5557–5566. https://doi.org/10.1016/j.ijhydene.2011.01.151
Li Y, Yu C, Fang M, Zhuan X, Zhongyang L, Kefa C (2006) Fabrication and characterization of SOFC anode, Ni-SDC cermet. J Chin Rare Earth Soc 24(1):32–36
Holtappels P, Bagger C (2002) Fabrication and performance of advanced multi-layer SOFC cathodes. J Eur Ceram Soc 22:41–48. https://doi.org/10.1016/S0955-2219(01)00238-2
Wang C, Worrell W, Park S, Vohs J, Gorte R (2001) Fabrication and performance of thin-film YSZ solid oxide fuel cells. J Electrochem Soc 148:A864–A868. https://doi.org/10.1149/1.1382588
Hansen KK, Hansen KV, Mogensen M (2010) High-performance Fe–Co-based SOFC cathodes. J Solid State Electrochem 14:2107–2112. https://doi.org/10.1007/s10008-010-1052-6
Wang W, Mogensen M (2005) High- performance lanthanum-ferrites-base cathode for SOFC. Solid State Ionics 176:457–462. https://doi.org/10.1016/j.ssi.2004.09.007
Huang Q-A, Wang B, Qu W, Hui SR (2009) Impedance diagnosis of metal-supported SOFCs with SDC as electrolyte. J Power Sources 191:297–303. https://doi.org/10.1016/j.jpowsour.2009.02.004
Yang Y-C, Zhang J, Lin K-Y, Sun P-K, Tseng H-C (2017) Influence of feedstocks on processes and microstructure of flame-sprayed SOFC anode. Ceram Int 43:S723–S728. https://doi.org/10.1016/j.ceramint.2017.05.283
Zhang X, Ohara S, Okawa H, Maric R, Fukui T (2001) Interactions of a La0.9Sr0.1Ga0.8Mg0.2O3 − δ electrolyte with Fe2O3, Co2O3 and NiO anode materials. Solid State Ionics 139:145–152. https://doi.org/10.1016/S0167-2738(00)00833-X
Liu C-Y, Tsai S-Y, Ni C-T, Fung K-z (2018) Interfacial reaction between YSZ electrolyte and La0.7Sr0.3VO3 perovskite anode for application. J Aust Ceram Soc 55:1–6. https://doi.org/10.1007/s41779-018-0215-2
Zheng Y, Shi Y, Gu H, Gao L, Chen H, Guo L (2009) La and Ca co-doped ceria-based electrolyte materials for IT-SOFCs. Mater Res Bull 44:1717–1721. https://doi.org/10.1016/j.materresbull.2009.03.017
Liping S, Lihua H, Zhao H, Qiang L, Pijolat C (2008) Substituted Sr2MnO4 as a possible cathode material in SOFC. J Power Sources 179:96–100. https://doi.org/10.1016/j.jpowsour.2007.12.090
Leng Y, Chan SH, Jiang S, Khor K (2004) Low-Temperature SOFC with thin film GDC electrolyte prepared in situ by solid-state reaction. Solid State Ionics 170:9–15. https://doi.org/10.1016/j.ssi.2004.02.026
Xia C, Liu M (2001) Low-temperature SOFCs based on Gd0.1Ce0.9O1.95 fabricated by dry pressing. Solid State Ionics 144:249–255. https://doi.org/10.1016/S0167-2738(01)00980-8
Yin Y, Zhu W, Xia C, Gao C, Meng G (2004) Low-temperature SOFCs using biomass-produced gases as fuels. J Appl Electrochem 34:1287–1291. https://doi.org/10.1007/s10800-004-2053-x
Richter J, Holtappels P, Graule T, Nakamura T, Gauckler L (2009) Materials design for perovskite SOFC cathodes. Monatshefte für Chemie 140:985–999. https://doi.org/10.1007/s00706-009-0153-3
Ralph J, Schoeler A, Krumpelt M (2001) Materials for lower temperature solid oxide fuel cells. J Mater Sci 36:1161–1172. https://doi.org/10.1023/A:1004881825710
Xia C, Liu M (2014) 2002 Microstructures, conductivity, and electrochemical properties of Ce0.9Gd0.1O2 and GDC–Ni anodes for low-temperature SOFCs. Solid State Ionics 152–153:423–430
Zha S, Rauch W, Liu M (2004) NiCe 0.9Gd 0.1O 1.95 anode for GDC electrolyte-based low-temperature SOFCs. Solid State Ionics 166:241–250. https://doi.org/10.1016/j.ssi.2003.11.012
Reich C, Kaiser A, Irvine J (2001) Niobia based rutile materials as SOFC anodes. Fuel Cells 1:249–255. https://doi.org/10.1002/1615-6854(200112)1:3/4%3c249:AID-FUCE249%3e3.0.CO;2-A
Jadhav S, Puri V, Jadhav L (2016) NiO-GDC-BCY composites as an anode for SOFC. J Alloy Compd. https://doi.org/10.1016/j.jallcom.2016.05.243
Gorte R, Vohs J (2003) Novel SOFC anodes for the direct electrochemical oxidation of hydrocarbons. J Catal 216:477–486. https://doi.org/10.1016/S0021-9517(02)00121-5
Haanappel V, Mertens J, Rutenbeck D, Tropartz C, Herzhof W, Sebold D, Tietz F (2005) Optimisation of processing and microstructural parameters of LSM to improve the electrochemical performance of anode-supported SOFCs. J Power Sources 141:216–226. https://doi.org/10.1016/j.jpowsour.2004.09.016
Fukui T, Ohara S, Naito M, Nogi K (2003) Performance and stability of SOFC anode fabricated from NiO/YSZ composite particles. J Eur Ceram Soc 23:2963–2967. https://doi.org/10.1016/S0955-2219(03)00311-X
Wang S, Kato T, Nagata S, Honda T, Kaneko T, Iwashita N, Dokiya M (2002) Performance of a La0.6Sr0.4Co0.8Fe0.2O3-Ce0.8Gd0.2O1.9-Ag cathode for ceria electrolyte SOFCs. Solid State Ionics 146:203–210. https://doi.org/10.1016/S0167-2738(01)01015-3
Stöver D, Hathiramani D, Vaßen R, Damani R (2006) Plasma-sprayed components for SOFC applications. Surf Coat Technol 201:2002–2005. https://doi.org/10.1016/j.surfcoat.2006.04.039
Jun C, Jianjun H, Xinjun L, Ying L, Bing Q, Shishou J, Xisheng W (2013) Plasma-sprayed coating of an apatite-type lanthanum silicate electrolyte for intermediate temperature solid oxide fuel cells (IT-SOFCs). Plasma Sci Technol 15:673–676. https://doi.org/10.1088/1009-0630/15/7/13
Amaya DM, Estrada D, Hotza D, Neto JBR, Escobar J (2017) Porous Cu/YSZ anodes processed by aqueous tape casting for IT-SOFC. J Eur Ceram Soc. https://doi.org/10.1016/j.jeurceramsoc.2017.05.002
Yang Y-C, Chang T-H, Wu Y-C, Wang S-F (2012) Porous Ni/8YSZ anode of SOFC fabricated by the plasma sprayed method. Int J Hydrog Energy 37:13746–13754. https://doi.org/10.1016/j.ijhydene.2012.03.080
Liu J-W, Sun L-P, Zhao H, Huo L-H (2019) Synthesis and electrochemical properties of Pr1-xSrCo0.5Ni0.5O4 + δ cathode materials. Chin J Inorganic Chem 35(2):245–253
Zhang X, Chen X, Xiang J, Ma W, Yu J (2010) Preparation and properties of CDC-LSCMCo composite anodes for IT-SOFC. Rare Metal Mater Eng 39(01):177–181
Jung S, Vohs J, Gorte R, Jung W-S (2007) Preparation of SOFC anodes by electrodeposition. J Electrochem Soc. https://doi.org/10.1149/1.2790280
Hao E, Rao P, Liu S (2016) Preparation of the La0.4Sr0.6Co0.4Fe0.6O3 cathode powder by EDTA and citric acid compound complexing method. Foshan Ceram 1:1006–8236
Chang H, Yan J, Chen H, Yang G, Shi J, Zhou W, Cheng F, Li S-D, Shao Z (2019) Preparation of thin electrolyte film via dry pressing/heating/quenching/calcining for electrolyte-supported SOFCs. Ceram Int. https://doi.org/10.1016/j.ceramint.2019.02.026
Jia L, Lü Z, Huang X, Liu Z, Chen K, Sha X, Li G, Su W (2006) Preparation of YSZ film by EPD and its application in SOFCs. J Alloy Compd 424:299–303. https://doi.org/10.1016/j.jallcom.2005.12.065
Oskouyi OE, Shahmiri M, Maghsoudipour A, Hasheminiasari M (2019) Pulsed constant voltage electrophoretic deposition of YSZ electrolyte coating on conducting porous Ni–YSZ cermet for SOFCs applications. J Alloy Compd 785:220–227. https://doi.org/10.1016/j.jallcom.2019.01.166
Dekel D (2017) Review of cell performance in anion exchange membrane fuel cells. J Power Sources. https://doi.org/10.1016/j.jpowsour.2017.07.117
Rashid NL, Samat AA, Jais AA, Somalu MR, Muchtar A, Baharuddin NA, Isahak WN (2019) Review on zirconate-cerate-based electrolytes for proton conducting solid-oxide fuel cell. Ceram Int 45:6605–6615. https://doi.org/10.1016/j.ceramint.2019.01.045
Khan M, Azad A, Savaniu C, Hing P, Irvine J (2017) Robust doped BaCeO3-δ electrolyte for IT-SOFCs. Ionics. https://doi.org/10.1007/s11581-017-2086-x
Sun L, Hu Z, Luo L, Wu Y, Shi J, Cheng L, Xu X (2017) Application of Ru nano flowers doped Ni-YSZ anode in ethanol-fueled SOFC. Rare Metal Mater Eng 46:2322–2326
He Z (2015) Preparation and properties of SmBaCoCuO5 + δ material as cathode for IT-SOFC. China Ceram Indus 6:15–19
Stankevičiūtė A, Kalyk F, Budrytė G, Abakevičienė B (2017) Combustion synthesis and characterization of samaria-doped ceria electrolyte for IT-SOFCs
Hong-xin Y et al (2014) The effect of dry methane flux on the methane reactions in solid oxide fuel cell at Ni-YSZ anode. J Chem Eng Chin Univ 28(05):1004–1009
Songbo L, Yanru Y, Yingjie Z, An S (2013) Preparation and properties of cathode material La0.7Sr0.3CuxCoyMn(1-x-y)O3 for SOFC. Rare Metal Mater Eng 42:487–490
Li F, Xia S, Yan Y, Cheng F (2018) Thermal stability and electrochemical performance of LaSrCoO4 ± δ as the cathode material for solid-oxide fuel cells. J Kunming Univ Sci Technol (Natural Science) 43(05):14–21
Wang H-X, Tu H-Y (2010) Synthesis and characterization of SOFC cathode materials La0.6Sr0.4CoO3-δ by glycine-nitrate process. J Funct Mater 41:397–400
Zhang L, Bai Y, Liu J (2013) Performance of LSM prepared by different methods. J Chin Rare Earth Soc 31(4):473–481
Shaula A, Oliveira J, Kolotygin V, Kharton V, Cavaleiro A (2010) Sputtered YSZ based protective thin films for SOFCs. Surf Eng 26:584–589. https://doi.org/10.1179/174329409X455430
Singh S, Priyanka P, Presto S, Viviani M, Sinha ASK, Varma S, Singh P (2018) Structural and electrical conduction behaviour of yttrium doped strontium titanate: anode material for SOFC application. J Alloy Compd 748:637–644. https://doi.org/10.1016/j.jallcom.2018.03.170
Castro-Robles JD, Chávez-Carvayar J (2018) Structural, morphological and transport properties of nanostructured La1-Sr Co0.2Fe0.8O3-thin films, deposited by ultrasonic spray pyrolysis. Mater Chem Phys 225:50–54. https://doi.org/10.1016/j.matchemphys.2018.12.053
Ahmed S, Sarker MS, Rahman MM, Kamruzzaman M, Khan MK (2018) Effect of yttrium(Y) on structural, morphological and transport properties of CdO thin films prepared by spray pyrolysis technique. Heliyon. https://doi.org/10.1016/j.heliyon.2018.e00740
Ermiş İ, Shaikh S (2018) Study of crystallographic, thermal and electrical properties of (Bi 2 O 3) 1-x-y (Tb 4 O 7) x (Gd 2 O 3) y electrolyte for SOFC application. Ceram Int. https://doi.org/10.1016/j.ceramint.2018.07.109
Valluri VL, Bauri R, Gandhi A, Paul S (2011) Synthesis and characterization of nanocrystalline ScSZ electrolyte for SOFCs. Fuel Energy Abstr 36:14936–14942. https://doi.org/10.1016/j.ijhydene.2011.02.139
Martins RF, Brant M, Domingues R, Paniago R, Sapag K, Matencio T (2009) Synthesis and characterization of NiO-YSZ for SOFCs. Mater Res Bull 44:451–456. https://doi.org/10.1016/j.materresbull.2008.04.017
Meng X, Yang N, Song J, Tan X, Ma Z-F, Li K (2011) Synthesis and characterization of terbium doped barium cerates as a proton conducting SOFC electrolyte. Fuel Energy Abstr 36:13067–13072. https://doi.org/10.1016/j.ijhydene.2011.07.075
Chen M, Kim B-H, Xu Q, Nam O, Ko J (2008) Synthesis and performances of Ni–SDC cermets for IT-SOFC anode. J Eur Ceram Soc 28:2947–2953. https://doi.org/10.1016/j.jeurceramsoc.2008.05.009
Zhou M, Zheng Y, Ge L, Li S, Guo L (2011) Synthesis of Gd2O3 and Y2O3 co-doped ceria-based electrolyte materials for intermediate temperature SOFC. J Chin Ceram Soc 39:1012–1016
Zhang K, Ge L, Ran R, Shao Z, Liu S (2008) Synthesis, characterization and evaluation of cation-ordered LnBaCo 2O 5 + δ as materials of oxygen permeation membranes and cathodes of SOFCs. Acta Mater 56:4876–4889. https://doi.org/10.1016/j.actamat.2008.06.004
Park S, Gorte R, Vohs J (2001) Tape cast solid-oxide fuel cells for the direct oxidation of hydrocarbons. J Electrochem Soc 148:A443–A447. https://doi.org/10.1149/1.1362538
Kolodiazhnyi T, Petric A (2005) The applicability of Sr-deficient n-type SrTiO3 for SOFC anodes. J Electroceram 15:5–11. https://doi.org/10.1007/s10832-005-0375-7
Tao Z, Ding H, Chen X, Hou G, Zhang Q, Tang M, Gu W (2015) The co–doping effect of Sm and In on ceria for electrolyte application in IT–SOFC. J Alloy Compd. https://doi.org/10.1016/j.jallcom.2015.12.164
Silva ER, Curi M, Furtado JG, Ferraz HC, Secchi AR (2019) The effect of calcination atmosphere on structural properties of Y-doped SrTiO3 perovskite anode for SOFC prepared by solid-state reaction. Ceram Int. https://doi.org/10.1016/j.ceramint.2019.02.011
Gao H, Liu J, Chen H, Li S, He T, Ji Y, Zhang J (2008) The effect of Fe doping on the properties of SOFC electrolyte YSZ. Solid State Ionics 179:1620–1624. https://doi.org/10.1016/j.ssi.2008.01.040
Fan B, Ren X, Cong Y, Liang W (2015) The influence of A-site composition change of LSCF cathode materials on the transmittability of oxygen ion and electrical performance of SOFC. J Funct Mater 46(A2):125–128, 134
Hui SR, Wang Z, Kesler O, Rose L, Jankovic J, Yick S, Maric R, Ghosh D (2007) Thermal plasma spraying for SOFCs: applications, potential advantages, and challenges. J Power Sources 170:308–323. https://doi.org/10.1016/j.jpowsour.2007.03.075
Xu X, Xia C, Huang S, Peng D (2005) YSZ thin films deposited by spin-coating for IT-SOFCs. Ceram Int 31:1061–1064. https://doi.org/10.1016/j.ceramint.2004.11.005
Coddet P, Amany M-L, Vulliet J, Caillard A, Thomann A-L (2018) YSZ/GDC bilayer and gradient barrier layers deposited by reactive magnetron sputtering for solid oxide cells. Surf Coat Technol. https://doi.org/10.1016/j.surfcoat.2018.09.085
Liu M, Dong D, Peng R, Gao J, Diwu J, Liu X, Meng G (2008) YSZ-based SOFC with modified electrode/electrolyte interfaces for operating at temperature lower than 650°C. J Power Sources 180:215–220. https://doi.org/10.1016/j.jpowsour.2008.01.066
Xiao H, Sun L-P, Zhao H, Huo L-H, Bassat J, Rougier A, Fourcade S, Grenier J (2015) Preparation and electrochemical properties of LaBiMn2O6 cathode for IT-SOFCs. Chin J Inorg Chem 31:1139–1144. https://doi.org/10.11862/cjic.2015.153
Wang C, Wu G, Xie F, Wang X, Wang J (2017) Preparation and properties of LSGM-(Li/Na)2CO3 composite electrolytes for LT-SOFC. Rare Earth Mater Eng 46:257–261
Sun L, Luo L, Wu Y, Xu X, Cheng L, Shi J, Huang Z (2014) LSM composite powder preparation for SOFC cathode by ultrasonic spray pyrolysis. China Ceram 50(10):29–32
Zhu Z, Xia C (2015) The influence of doping bismuth on the property of intermediate temperature solid oxide fuel cell cathode material La1.75Sr0.25NiO4 + δ. Heat Treatment Technol Equip 36(5):92–98
Sun L, Lu J, Yin J, Yin Y, Ma Z (2013) Synthesis and characterization of La0.75Sr0.25Mn0.5Cr0.5-xCuxO3-δ for SOFC anode material. J Inorg Mater 28(9):925–930
Meng XX, Gong X, Yang NT, Tan XY, Ma ZF (2013) Preparation and properties of direct-methane solid oxide fuel cell based on a graded Cu-CeO2-Ni-YSZ composite anode. Acta Phys Chim Sin. https://doi.org/10.3866/PKU.WHXB201305151
Zhang J, Song S (2014) Preparation and performance of NiCu-CeO2 anode for direct methane SOFC. Chin J Power Sources 38(12):2270–2273
Bu Y, Guan W, Liu W, Wang J, Pei W, Yang J, Huang K, Zhong Z (2018) Electrochemical properties of solid oxide fuel cell based on double-sided cathodes under rarefied reduced atmosphere. J Chin Ceram Soc 46:867–872
Fan Y-H, Sun L-P, Zhao H, Huo L-H, Bassat J-M, Rougier A, Fourcade S, Jean Grenier (2016) Synthesis and high temperature electrochemical properties of Co-doped. Chin J Inorg Chem. https://doi.org/10.11862/cjic.2016.207
Norby T (2009) Proton conductivity in perovskite oxides. Springer, Boston, pp 217–241. https://doi.org/10.1007/978-0-387-77708-5_11
Norby T, Larring Y (1997) Concentration and transport of protons in oxides. Curr Opin Solid State Mater Sci 2:593–599. https://doi.org/10.1016/S1359-0286(97)80051-4
Bhide S, Virkar A (1999) Stability of AB ‘ B-1/2 ‘‘ O-1/2(3)-type mixed perovskite proton conductors. J Electrochem Soc 146:4386–4392. https://doi.org/10.1149/1.1392648
Zuo C, Zha S, Liu M, Hatano M, Uchiyama M (2006) Ba(Zr0.1Ce0.7Y0.2)O3–δ as an electrolyte for low-temperature solid-oxide fuel cells. Adv Mater 18:3318–3320. https://doi.org/10.1002/adma.200601366
Tao S, Irvine J (2006) A stable, easily sintered proton-conducting oxide electrolyte for moderate-temperature fuel cells and electrolyzers. Adv Mater 18:1581–1584. https://doi.org/10.1002/adma.200502098
Iwahara H, Uchida H, Morimoto K (1990) Cheminform abstract: high temperature solid electrolyte fuel cells using perovskite-type oxide based on BaCeO3. J Electrochem Soc 137(2):462–465
Basbus J, Arce MD, Troiani H, Su Q, Wang H, Caneiro A, Mogni L (2019) Study of BaCe0.4Zr0.4Y0.2O3-δ/BaCe0.8Pr0.2O3-δ (BCZY/BCP) bilayer membrane for protonic conductor solid oxide fuel cells (PC-SOFC). Int J Hydrog Energy. https://doi.org/10.1016/j.ijhydene.2019.06.164
Hibino T, Hashimoto A, Suzuki M, Sano M (2002) A solid oxide fuel cell using Y-doped BaCeO3 with Pd-loaded FeO anode and Ba0.5Pr0.5CoO3 cathode at low temperatures. J Electrochem Soc 149:A1503–A1508. https://doi.org/10.1149/1.1513983
Ito N, Iijima M, Kimura K, Iguchi S (2005) New intermediate temperature fuel cell with ultra-thin proton conductor electrolyte. J Power Sources 152:200–203. https://doi.org/10.1016/j.jpowsour.2005.01.009
Bi L, Shangquan Z, Fang S, Zhang L, Xie K, Xia C, Liu W (2008) Preparation of an extremely dense BaCe 0.8Sm 0.2O 3 − δ thin membrane based on an in situ reaction. Electrochem Commun 10:1005–1007. https://doi.org/10.1016/j.elecom.2008.04.035
Lan R, Tao S (2014) Novel proton conductors in the layered oxide material LixlAl0.5Co0.5O2. Adv Energy Mater. https://doi.org/10.1002/aenm.201301683
Zhou Y, Guan X, Zhou H, Ramadoss K, Adam S, Liu H, Lee S, Shi J, Tsuchiya M, Fong D, Ramanathan S (2016) Strongly correlated perovskite fuel cells. Nature. https://doi.org/10.1038/nature17653
Dong W, Tong Y, Zhu B, Xiao H, Wei L, Huang C, Wang B, Wang X, Kim J-S, Wang H (2019) Semiconductor TiO 2 thin film as an electrolyte for fuel cells. J Mater Chem A. https://doi.org/10.1039/C9TA01941C
Du Y, Nowick A (1996) Galvanic cell measurements on a fast proton conducting complex perovskite electrolyte. Solid State Ionics 91:85–91. https://doi.org/10.1016/S0167-2738(96)00409-2
Matzke T, Cappadonia M (1996) Proton conductive perovskite solid solutions with enhanced mechanical stability. Solid State Ionics 86:659–663. https://doi.org/10.1016/0167-2738(96)00231-7
Fukui T, Ohara S, Kawatsu S (1998) Conductivity of BaPrO3 based perovskite oxides. J Power Sources 71:164–168. https://doi.org/10.1016/S0378-7753(97)02813-9
An H, Lee HW, Kim BK, Son JW, Yoon KJ, Kim H, Shin D, Ji HI, Lee JH (2018) A 5 × 5 cm2 protonic ceramic fuel cell with a power density of 1.3 W cm−2 at 600°C. Nature Energy 3(10):870–875
Wang X, Ma Y, Li S, Kashyout A, Zhu B, Muhammed M (2011) Ceria-based nanocomposite with simultaneous proton and oxygen ion conductivity for low-temperature solid oxide fuel cells. J Power Sources 196:2754–2758. https://doi.org/10.1016/j.jpowsour.2010.11.033
Ricca C, Ringuedé A, Cassir M, Adamo C, Labat F (2018) Conduction mechanisms in oxide-carbonate electrolytes for SOFC: highlighting the role of the interface from first-principles modelling. J Phys Chem C. https://doi.org/10.1021/acs.jpcc.8b02174
Zhang L, Lan R, Kraft A, Tao S (2011) A stable intermediate temperature fuel cell based on doped-ceria–carbonate composite electrolyte and perovskite cathode. Electrochem Commun 13:582–585. https://doi.org/10.1016/j.elecom.2011.03.015
Zuo N, Zhang M, Mao Z, Xie F (2011) Fabrication and characterization of composite electrolyte for intermediate-temperature SOFC. J Eur Ceram Soc 31:3103–3107. https://doi.org/10.1016/j.jeurceramsoc.2011.04.030
Khan I, Asghar MI, Lund P, Basu S (2017) High conductive (LiNaK) 2 CO 3 Ce 0.85 Sm 0.15 O 2 electrolyte compositions for IT-SOFC applications. Int J Hydrog Energy. https://doi.org/10.1016/j.ijhydene.2017.05.152
Wu G, Wang C, Xie F, Wang X, Mao Z, Liu Q, Zhan Z (2016) Ionic transport mechanism of composite electrolyte for low temperature SOFCs. Int J Hydrog Energy. https://doi.org/10.1016/j.ijhydene.2016.05.021
Hei Y, Huang J, Wang C, Mao Z (2014) Novel doped barium cerate–carbonate composite electrolyte material for low temperature solid oxide fuel cells. Int J Hydrog Energy. https://doi.org/10.1016/j.ijhydene.2014.04.031
Zhu B, Raza R, Abbas G, Singh M (2011) An electrolyte-free fuel cell constructed from one homogenous layer with mixed conductivity. Adv Funct Mater 21:2465–2469. https://doi.org/10.1002/adfm.201002471
Asghar MI, Jouttijärvi S, Jokiranta R, Valtavirta A-M, Lund P (2018) Wide bandgap oxides for low-temperature single-layered nanocomposite fuel cell. Nano Energy. https://doi.org/10.1016/j.nanoen.2018.08.070
Shao K, Li F, Zhang G, Zhang Q, Maliutina K, Fan L (2019) Approaching durable single-layer fuel cells: promotion of electroactivity and charge separation via nanoalloy redox exsolution. ACS Appl Mater Interfaces. https://doi.org/10.1021/acsami.9b08448
Kapu SG, Wang B, Kim J-S, Zhu B (2019) Ionic conducting properties and fuel cell performance developed by band structures. J Phys Chem C. https://doi.org/10.1021/acs.jpcc.8b11914
Zhu B, Lund P, Raza R, Patakangas J, Huang Q-A, Fan L, Singh M (2013) A new energy conversion technology based on nano-redox and nano-device processes. Nano Energy 2:1179–1185. https://doi.org/10.1016/j.nanoen.2013.05.001
Zhu B, Huang Y, Fan L, Ma Y, Wang B, Xia C, Afzal M, Zhang B, Dong W, Wang H, Lund P (2015) Novel fuel cell with nanocomposite functional layer designed by perovskite solar cell principle. Nano Energy. https://doi.org/10.1016/j.nanoen.2015.11.015
Zhu B, Wang B, Wang Y, Raza R, Tan W, Kim J, Aken P, Lund P (2017) Charge separation and transport in La 0.6 Sr 0.4 Co 0.2 Fe 0.8 O 3-δ and ion-doping ceria heterostructure material for new generation fuel cell. Nano Energy. 37:195–202. https://doi.org/10.1016/j.nanoen.2017.05.003
Wang G, Wu X, Cai Y, Ji Y, Yaqub A, Zhu B (2016) Design, fabrication and characterization of a double layer solid oxide fuel cell (DLFC). J Power Sources 332:8–15. https://doi.org/10.1016/j.jpowsour.2016.09.011
Zhu B, Lund P, Raza R, Ma Y, Fan L, Afzal M, Patakangas J, He Y, Zhao Y, Tan W, Huang Q-A, Zhang J, Wang H (2015) Schottky junction effect on high performance fuel cells based on nanocomposite materials. Adv Energy Mater. https://doi.org/10.1002/aenm.201401895
Jing Y, Patakangas J, Lund P, Zhu B (1970) An improved synthesis method of ceria-carbonate based composite electrolytes for low-temperature SOFC fuel cells. Int J Hydrog Energy 38:16532–16538. https://doi.org/10.1016/j.ijhydene.2013.05.136
Khan I, Tiwari P, Basu S (2017) Analysis of gadolinium-doped ceria-ternary carbonate composite electrolytes for solid oxide fuel cells. Ionics 24:211–219. https://doi.org/10.1007/s11581-017-2184-9
Khan A, Raza R, Limaae R, Chaudhry M, Ahmed E, Abbas G (2013) Comparative study of the nano-composite electrolytes based on samaria-doped ceria for low temperature solid oxide fuel cells (LT-SOFCs). Int J Hydrog Energy 38:16524–16531. https://doi.org/10.1016/j.ijhydene.2013.05.060
Xia C, Wang B, Cai Y, Zhang W, Afzal M, Zhu B (2016) Electrochemical properties of LaCePr-oxide/K2WO4 composite electrolyte for low-temperature SOFCs. Electrochem Commun 77:44–48. https://doi.org/10.1016/j.elecom.2016.12.013
Yu J, Tian N, Deng Y, Li G, Liu L, Cheng L, Gao P, Pan Q, Wang Y, Chen X, Qi K (2014) Fabrication and characterization of BaCe0.8Y0.2O2.9-Ce0.85Sm0.15O1.925 composite electrolytes for IT-SOFCs. Science China Chem 58:473–477. https://doi.org/10.1007/s11426-014-5176-x
Chockalingam R, Basu S (2011) Impedance spectroscopy studies of Gd-CeO 2-(LiNa)CO 3 nano composite electrolytes for low temperature SOFC applications. Fuel Energy Abstr 36:14977–14983. https://doi.org/10.1016/j.ijhydene.2011.03.165
Fan L, Wang Y, Jia Z, Xiong Y, Brito M (2015) Nanofiber-structured SSC–GDC composite cathodes for a LSGM electrolyte based IT-SOFCs. Ceram Int. https://doi.org/10.1016/j.ceramint.2015.01.104
Xie F, Wang C, Mao Z, Zhan Z (2013) Preparation and characterization of La0.9Sr0.1Ga0.8Mg0.2O2.85–(Li/Na)2CO3 composite electrolytes. Int J Hydrog Energy 38:11085–11089. https://doi.org/10.1016/j.ijhydene.2013.02.082
Tan W, Fan L, Raza R, Khan A, Zhu B (2013) Studies of modified lithiated NiO cathode for low temperature solid oxide fuel cell with ceria-carbonate composite electrolyte. Int J Hydrog Energy 38:370–376. https://doi.org/10.1016/j.ijhydene.2012.09.160
Yamamoto T, Morita H, Yoshikawa M, Yoshiba F, Asano K, Yasumoto K et al (2009) Development of SOFC performance and durability evaluations technology. ECS Trans 25(2). https://doi.org/10.1149/1.3205560
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled, “Review of cell performance in solid oxide fuel cells.”
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lyu, Y., Xie, J., Wang, D. et al. Review of cell performance in solid oxide fuel cells. J Mater Sci 55, 7184–7207 (2020). https://doi.org/10.1007/s10853-020-04497-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-04497-7