Abstract
In this study, we successfully synthesized ZnO/CeO2 composite nanoparticles for efficient ultraviolet (UV) filtering applications using a simple precipitation route. Various ratios of Ce/Ti, 2.5 at.%, 5 at.%, and 10 at.% were used to precipitate ceria onto commercial ZnO nanopowder at pH 9. The calculated mean crystallite sizes of the resultant nanocomposites were ~ 90 nm, ~ 79 nm, and ~ 41 nm for the 2.5 at.%, 5 at.% and 10 at.% ceria amounts, respectively. A stronger and more selective absorbance within the UV range was observed due to precipitation of a small amount of ceria to decorate the commercial ZnO surface. The photocatalyst results show that the addition of ceria, particularly with the precipitation amount increased up to 10 at.%, can effectively reduce crystal violet degradation by about 97% in a period of time from 0 to 30 min when exposed to ultraviolet light over 30 min or by around 99% under solar simulation for 30 min.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to the increased intensity of ultraviolet (UV)-rays that reach the earth’s surface, which is attributed to the collapse of the ozone layer in the past two decades, concerns about skin protection from UV-rays have been raised recently [1]. As is well known, ultraviolet radiation is divided into UVC (270–290 nm), UVB (290–320 nm), and UVA (320–400 nm) [2, 3]. Around 95–99% of the UV radiation that reaches the earth consists of UVA, 1–5% is UVB, while UVC is filtered by ozone in the stratosphere. Hence, UVC does not reach the earth’s surface [2, 4]. The UVB range causes erythema, and it is a well known carcinogen. Recently, however, great attention has been paid to the UVA rays as well, since they have the ability to penetrate deeper into human skin [2, 5,6,7], causing accumulation of higher radiation doses and consequent health problems such as aging acceleration, skin cancer, etc. Therefore, sunscreen agents with efficient filters for UVA are becoming one of the important tools today to protect the human skin from the dangerous effects of the UV-rays. In general, there are organic and inorganic UV blockers incorporated in sunscreen agents [8]. The organic UV blockers are not very efficient or desirable, however, due to their partial degradation under irradiation, which results in an increase in UV absorption, allergy effects, and potential photoallergy [8,9,10]. Materials such as titanium dioxide (TiO2) and zinc oxide (ZnO) [11] that can absorb, reflect, or scatter UV-rays are widely used as efficient inorganic UV blockers. Yet, even though they offer good UV-shielding characteristics, they also have high photocatalytic activity when absorbing UV radiation, leading to high generation of reactive oxygen species (ROS), which are considered to be harmful to the cells and have an impact on the photostability of cosmetics formulations, which is undesirable in sun care agents [12,13,14]. Nano-ZnO is an attractive UVA filter for use in sunscreen formulations due to its transparency across the visible light wavelength region and high absorption [15, 16]. Thus, the UVA blocking properties of ZnO are essential for efficient modern sunscreens. Nevertheless, the generation of ROS under UV light due to the photocatalytic effect of nano-ZnO is still a major issue, which needs to be addressed. Therefore, it is important to develop a novel type of ZnO-based UVA filters with low photocatalytic activity through tailoring with other materials. Several other metal oxide nanomaterials have been investigated for use in sunscreen formulations, including pure ceria (CeO2) [17, 18], alkaline earths, rare earths [19, 20], transition metal doped CeO2 [21], α-Fe2O3 [22] and Ce-doped Fe2O3 [23]. CeO2 nanoparticles and compounds incorporating cerium are also of particular interest for sunscreen application [20]. CeO2 is an attractive rare earth oxide due to its ROS scavenging properties [24, 25]. It also features good UV absorption and transparency in the visible domain [26,27,28], and it is a large band gap semiconductor as well, which makes it suitable for sunscreen application [29,30,31]. Much research has been carried out to create cerium or ceria doped ZnO with different nanostructures as well as nanofilms [32,33,34,35,36,37], nanopowders [38,39,40,41], nanorods [42,43,44,45,46], nanoneedles [47], nanofibers [48], and nanoplatelets [49]. Synthesis of a CeO2–ZnO composite, ZnO/CeO2, or Ce/CeO2 nanostructures has been rarely reported. Recently a new concept was used to reduce the photocatalytic activity of nano-TiO2, by incorporating quantum dots of bismuth carbonate ((BiO2)CO3) on its surface. The photocatalytic activity of TiO2 was successfully reduced by up to 60% through addition of (BiO2)CO3 to form a new nanocomposite material using a precipitation method [50]. In a similar way, another work reported that a nanocomposite of iron oxide (Fe2O3)/CeO2 fabricated via a precipitation route showed strong, selective absorption in the UV range and a significant reduction in crystal violet dye degradation that was achieved by scavenging the photogenerated hydroxyl (OH˙) radicals [51]. Based on these reports, using CeO2 with ZnO as a core–shell nanocomposite is expected to reduce the photocatalytic activity of ZnO. In this work, nanocomposites of ZnO/CeO2 were prepared via a simple precipitation method. The effects of the precursor, pH, and amount of ceria on the ZnO photocatalytic activity were investigated.
Experimental section
Chemicals
Commercial ZnO Nanopowder (< 100 nm), cerium acetate hydrate (C2H3O2)3Ce.XH2O (99.9%), ammonium hydroxide (NH4OH, 28–30% NH3 basis), hydrogen peroxide (H2O2) were purchased from SIGMA ALDRICH and used to synthesize ZnO-based CeO2 (ZnO/CeO2 nanocomposite).
Sample preparation
We assert a cost-effective technique, which can be adopted for manufacturing application. In this article, pure commercial ZnO nanoparticles and CeO2 coated ZnO nanoparticles were produced by the precipitation method.
Precipitation method
To formulate ZnO/CeO2 nanocomposite, an amount of ZnO nanopowder of about 0.2 g was suspended in deionized water, while a solution of (C2H3O2)3Ce.XH2O (Sigma Aldrich) was separately prepared. The two solutions were mixed to obtain desired stoichiometric ZnO/CeO2 ratios of 2.5 at.%, 5 at.%, and 10 at.% CeO2. These different amounts of cerium were then precipitated from the solutions using concentrated (NH4OH) (28–30%) in a dropwise addition method for a total of 2 mL to reach the desirable pH 9 at 80 °C. After sometime, an amount of concentrated (H2O2 30%) was added to crystallize the precipitated products. After that, the resultant products were washed and dried over night at 80 °C. Finally, the products were ground for characterization. This was following the same process reported in [51]. Figure 1 presents a schematic diagram of the precipitation technique.
Characterization
X-ray diffraction (XRD; MMA diffractometer, GBC Scientific Equipment) was used to examine the crystallinity and purity of the prepared nanocomposites products as well as the commercial ZnO nanoparticles. This was performed with an XPERT-PRO (9PW 3050/60) equipped with Cu Kα radiation (λ = 1.54060 Å) operated at 40 kV and 30 mA at room temperature. The samples were scanned over a 20°–80° 2θ angular range with a 0.2° min−1 scan rate. In addition, a scanning electron microscope (SEM, JEOL JSM-7500FA) and a transmission electron microscope (TEM, JOEL ARM- 200F) were used to confirm the product morphologies. Furthermore, the dye photodegradation and UV absorbance of the fabricated nanocomposites were tested through UV–visible spectroscopy (Shimadzu UV-1800). The FT-IR spectrum of the sample was recorded using a Shimadzu 8400S spectrometer by the KBr pellet technique in the range 400–4500 cm−1. X-ray photoelectron spectroscopy (XPS) measurements of the elemental compositions were also conducted via a SPECS PHOIBOS 100 analyzer, with a high vacuum chamber and base pressure under 10-8 mbar. XPS also was used to analyze the Zn valence state to understand the surface chemical configuration of the tested products. X-ray excitation was delivered with Al Kα radiation and photon energy, hν = 1486.6 eV at 12 kV and 120 W. The XPS spectra to determine binding energies were collected at 20 eV pass energy in the fixed analyzer transmission mode. The final step in XPS is analysis by a commercial software package (CasaXPS 2.3.15), which was used to analyze the data that comes from the spectroscope analyzer.
Photocatalytic and sun simulator tests
Commercial naked ZnO and our fabricated ZnO/CeO2 nanocomposites with different amounts of ceria were used with crystal violet as a chemical indicator. The experiment was conducted as follows: firstly, to prepare the dye stock solution, 2.61 g of crystal violet powder dye (Tris (4-(dimethylamino) methylium chloride, 99% anhydrous, Sigma Aldrich) was suspended in 1L of distilled water using a volumetric flask at the concentration of 6.4 mmol L−1. Secondly, a 5 mg amount of naked commercial ZnO and 5 mg amounts of the as-prepared samples were, respectively, mixed under magnetic stirring in crystal violet solution with a volume of 100 ml. This was performed in the dark for 30 min. Moreover, an RPR-200 photocatalytic reactor (Rayonet) reactor lined with a set of 300 nm and 350 nm Hg lamps 20 cm away from the reaction mixture was used as the irradiation source. An exposure period of 30 min was used, with aliquots of the reaction mixture collected every 5 min. Finally, a UV-3600 Shimadzu was used to collect the UV- Vis spectra of the irradiated aliquots obtained and to assess the degree of dye degradation at its major absorbance peak at λ = 590 nm. The same procedure was used to test the materials for their response to an LCS—100™ solar simulator, with 1 sun solar irradiation.
Results and discussion
Materials characterization
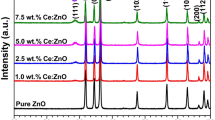
X-ray diffraction (XRD) was used to investigate the crystallinity of the nanocomposites, which resulted from the precipitation procedure. Figure 2 presents the diffraction patterns of the synthesized nanocomposites. The patterns were indexed and were assigned to the hkl planes (100), (002), (101), (110), (102), (103), (200), (112), (201), (004), (202) which match very well with hexagonal wurtzite ZnO. These peaks show a favorable zinc oxide space group [P63 mc, (186)/amd, JCPDS No. 96-230-0116]. The peak at 28° in the ZnO/CeO2 nanocomposites was assigned to the cubic fluorite phase of CeO2, and it is closely matched with the corresponding PDF card (96–900–9009). This peak of CeO2 represents the (111) plane reflection for this phase. The CeO2 peak intensity varied according to the ceria content whereby the nanocomposite with a low amount of ceria (2.5 at.%) presented a very small peak of CeO2, the 5 at.% sample exhibited a broad peak, and the 10 at.% sample an even broader peak related to ceria. Thus, increasing the cerium acetate (C2H3O2)3Ce·XH2O concentration led to an increased amount of ceria in the nanocomposite, resulting in broad ceria peaks. This was strong evidence that the preparation was successful. According to Fig. 2, the full width at half maximum (FWHM) and intensity of the small CeO2 peak in the XRD patterns of the prepared samples, as compared to the ZnO pattern, indicate the amount of CeO2.
The effects of the ceria loading on the cell parameters and particle size of the core ZnO nanoparticles were also assessed. The XRD patterns reveal that the nanocomposites of ZnO/CeO2 do comprise ZnO and CeO2. The cell parameters, average crystallite size, d-spacing, amount of ceria, peaks, and corresponding phase angles are presented in Table 1.
Figure 3 shows the ultraviolet–visible absorbance of the manufactured nanocomposites and the commercial uncoated ZnO. A suspension concentration of 25 mg mL−1 was used to perform the experiments. In general, the samples show selective absorbance across the UVA wavelength region. The absorbance increased gradually when the precipitated amount of ceria increased up to 10 at.%. Thus, the improvement in the absorption features of the new ZnO/CeO2 nanocomposite is due the CeO2 added to the commercial ZnO, in particular with the increasing amount of ceria up to 10 at.%.
The charge transfer O 2p to Ce 3dguarantees that there are some ions of Ce4+ with several coordination states, which can result in switching between 4 and 8 in the fluorite cubic system of CeO2 [26].
FE-SEM was used to confirm the morphologies of the fabricated ZnO/CeO2 nanocomposites as well as that of pure commercial ZnO. Figure 4 clearly shows the respective surface morphologies at different magnifications. The corresponding images of uncoated ZnO, which are represented by Fig. 4 (a and b), show the irregular morphology of pure ZnO. Figure 4c–h shows that the particle sizes and morphology of the prepared nanocomposites were affected by CeO2 addition to the commercial ZnO, because the size was reduced with increasing amounts of ceria. With the high amount of 10 at.%, agglomerated spherical nanoparticles were produced, composed of smaller ZnO crystallites. Figures 5 and 6 also demonstrate this change, which might be related to the nucleation and synergetic impact between CeO2 and ZnO [52,53,54].
Figure 5 presents TEM images of the commercial uncoated ZnO and the synthesized nanocomposites. High-resolution TEM analysis was performed using a JEOL-JEMARM200F microscope. The results confirm that the commercial uncoated ZnO and resulted ZnO/CeO2 (2.5 at.%) nanocomposite have exposed ZnO (100) and CeO2 (111) planes, with d-spacings of 2.635 Å for ZnO and 3.17 Å for CeO2, respectively. The images in Fig. 5(a, c) demonstrate these results. Generally, the practical values of the d-spacings related to ZnO and CeO2 phases are 2.614 Å and 3.17 Å, respectively. Therefore, the high-resolution (HR)-TEM analysis is in agreement with the XRD patterns, although a hexagonal structure is displayed by ZnO and a cubic structure by CeO2, as well as the different lattice planes. Throughout the formation of the nanocomposites, nucleation and synergetic effects between ZnO and CeO2 were corroborated, and the nanocomposites were defect-rich.
Other TEM images related to commercial ZnO and the fabricated ZnO/CeO2 nanocomposites are presented in Fig. 6, from which the morphology and particle sizes of the samples could be investigated. It was found that ~ 93 nm is the average size for pure ZnO, while the averages sizes for the nanocomposites with different amounts of ceria were ~ 90 nm for 2.5 at.%, ~ 79 nm for 5 at.%, and ~ 41 nm for 10 at.%. The sample of ZnO/CeO2 10 at.% shows agglomeration and aggregated particles as in the SEM images. Some of these particles were relatively big spherical agglomerates around 200–300 nm in size, and there were also small spherical particles with an average size of 41 nm. This morphology may have occurred due to the synergetic effects mentioned above. Additionally, the ZnO size was reduced effectively with increasing ceria content.
High-resolution energy dispersive spectroscopy (EDS) mapping was used to measure the elemental composition of the synthesized nanocomposite samples to confirm the presence of ceria. The EDS mapping for the commercial ZnO and the nanocomposites can be seen in Figs. 7 and 8 . The images in Fig. 7 show the composition of pure commercial ZnO, which contains only Zn and O. Furthermore, the images in Fig. 8 can show the compositions of the prepared ZnO/CeO2 nanocomposites (2.5, 5, and 10), and they reveal that the resultant materials consist of Zn, Ce, and O. A secondary Ce phase was identified via TEM analysis, and it proves that there was a perfect dispersion of ceria on top of the pure commercial ZnO. There were no impurities detected in the naked ZnO or the nanocomposites, according to the EDS analysis. Finally, EDS analysis confirmed the successful fabrication of our ZnO/CeO2 nanocomposites with different amounts of ceria.
High-resolution TEM and EDS maps of the synthesized ZnO/CeO2 nanocomposites. Each column corresponds to a set of images for the following given ceria loadings: a, b 2.5 at.%, c, d 5 at.% and e, f 10 at.%. The distribution of Zn (pink), Ce (green) and O (red) are shown below each corresponding gray-scale TEM image
The elemental composition of the prepared nanocomposites was also investigated using X-ray photoelectron spectroscopy (XPS) survey spectra, as shown for ZnO/CeO2 (10 at.%) in Fig. 9. The ZnO/CeO2 nanocomposite XPS survey spectra consist of Zn, Ce, O, and C regions. The Zn 2p3/2 and Zn 2p1/2 orbitals are centered at peaks with binding energy values of 1021.5 eV and 1044.6 eV, respectively, as shown in Fig. 9b. The spectra show that Zn is in the 2 + oxidation state. Furthermore, the high-resolution XPS spectrum of Ce in Fig. 9c shows that the Ce peaks consist of a mixture of Ce3+ and Ce4+ ions, as has previously been shown for nanocrystalline CeO2. Generally, the Ce oxidation states look alike and are close to each other, as shown in the XPS spectrum. Some Ce3+ defects can be formed around the nanocomposite surface, particularly as the size of the CeO2 nanoparticles decreases due to an increase in lattice strain and the formation of defects [55]. The ratio of Ce3+/Ce4+ is also important in regard to its ROS scavenging properties and has been shown to be closely linked to the size of the nanoparticles [56, 57] and can be formed due to the synergetic interaction between CeO2 and ZnO. The presence of Ce3+ is generally accompanied by the localized formation of amorphous Ce2O3, which might not be detected by XRD because its concentration or amount is too small in the nanocomposite relative to both ZnO and CeO2. The peaks with energies of 532.5, 531.6, 530.4, and 534.2 eV represents O 1s, and it can be found that these four binding energies are related to and linked with the ZnO and CeO2 lattice oxygen (Zn2+, Ce3+, Ce4+) and the hydroxyl group OH˙ [52], as shown in Fig. 9d. Therefore, the XPS analysis confirms the presence of Ce3+ in the ZnO/CeO2 nanocomposite.
FT-IR spectroscopy was used to examine the chemical composition of pristine CeO2, ZnO and ZnO coated CeO2 nanostructures, as shown in Fig. 10. The existence of the nanostructures of the metal oxides in the sample can be elucidated in the lower wavelength region of the FT-IR spectra. The peak at 870 cm−1 is attributed to Zn-OH bending vibrations [58, 59]. Absorption bands from 800 to 4000 cm−1 were observed in the FT-IR spectrum of the prepared CeO2 nanoparticles, whereby, bands positioned at 450 cm−1 and 824 cm−1 correspond to the Ce–O metal–oxygen bond [60,61,62], and significant vibration band ranging from 400 to 500 cm−1 assigned to the characteristic stretching mode of the Zn–O bond according to [63], were not covered in our experimental setup due to equipment range limitation. Additionally, the ZnO coated CeO2 nanoparticles showed peaks at 1390 and 1514 cm−1 [64] which are correlated to the acetate ion residuals of the starting materials [59]. Furthermore, the 1630 cm−1 and 3440 cm−1 bands correspond to the hydroxyl (O–H) group stretching and bending vibrations, respectively, which are associated with the residual water molecules and/or physically adsorbed OH groups on the surfaces of the CeO2 and ZnO coated with CeO2 samples [65]. In general, for the ZnO coated CeO2 samples it can be seen that the intensity and the broadening of the metal oxides peaks (band from 800 to 900 cm−1) decreases with decreasing CeO2 loading.
UV photocatalytic characterization
Figure 11 shows the photocatalytic measurements for the prepared nanocomposites as well as the commercial naked ZnO. The measurements were replicated three times to establish the error margins and standard deviation. Crystal violet dye was used as a colorimetric indicator for photodegradation based upon the production of ROS sufficient in reactivity to mineralize the dye and O⋅−2 superoxide radical indicator. These superoxide radicals might be created because of ZnO exposure to UV-rays. As a result, a colorless derivative of crystal violet will be produced [57, 66]. In comparing the commercial naked ZnO with the samples capped with ceria, it is clear that the ceria loading in the nanocomposite samples drastically reduces the degree of dye degradation over the exposure period tested.
It can be seen that the highest level of dye degradation was achieved with the uncoated commercial ZnO, while a substantial decrease in dye degradation was achieved for the nanocomposite samples. ZnO is a semiconductor material that nominally displays a wide band gap value of 3.27 eV for the wurtzite phase [67] and is primarily an absorber of UVA radiation, as evidenced in Fig. 3. Illumination of the uncoated ZnO nanoparticles with wavelengths of light greater than this band gap is sufficient in generating photoexcited charge carriers. Excitation results in the promotion of an electron (e−) from the valence band to the conduction band, which in turn leaves behind a positively charged hole (h+). These photoexcited charge carriers can migrate to the surface of particle and interact with chemically adsorbed species. In this instance, the aqueous medium of the reaction mixture enables the generation of ROS through surface mediated redox reactions between photoexcited charge carriers and adsorbed water (H2O). The resulting ROS, which typically includes the powerful hydroxyl radical, can then go on to oxidize the crystal violet dye and cause irreparable damage to its chemical structure. Over the exposure period tested, the continual generation of ROS gradually results in the de-colorization of the reaction mixture due to degradation of the colored dye molecules to colorless photoproducts, as evident by the gradual decrease in relative absorbance (C/Co) (Fig. 10). In the case of the nanocomposite samples, a substantial reduction in the photocatalyzed degradation of the crystal violet dye while under UV irradiation is observed as compared to the uncoated commercial ZnO. With increasing ceria loading, up to 10 at.% it can be seen that the dye degradation is reduced by 97% in UV light. Considering that no significant change was observed in the absorption band position of the nanocomposite samples relative to the uncoated ZnO, and that the absorption properties improved with increasing ceria loaded, it would have ordinarily been expected that some improvement in the photocatalysis would be achieved. One possible reason this is not observed could be due to the deposition of CeO2 on the surface of the ZnO nanoparticles, resulting in an inhibition of the free radical production or scavenging of the ROS produced. CeO2 nanoparticles have been previously reported to display prominent antioxidant properties, particularly when a large number of surface defect sites exist [56]. These surface defect sites are often increased with decreasing particle size and enable the interchangeable redox of Ce3+/Ce4+ with chemically adsorbed species [51, 57]. Thus, it could be suggested that the application of such fine CeO2 nanoparticles with existing surface defects in the nanocomposite samples could impart some form of ROS scavenging property, resulting in the gradual decrease in dye degradation with increased ceria loading as observed. Furthermore, the performance of the 10 at.% nanocomposite in particular is a vast improvement on our previous work with Na-doped ZnO [68], in terms of reducing photocatalysis while also maintaining UV absorption.
The suppression of dye photo degradation is also higher compared to the effect of Co and Mn doping of ZnO reported in [69, 70] where the decomposition of Rhodamine-B dye under simulated sunlight is from 10% (for Co doping) to 30% in case of Mn doping and similar exposure time. In addition, both elements are applied in higher doping concentrations. Similarly, the suppression of photodegradation through Ni doping of ZnO films [71] reveals lower values—in 30 min the degradation C/C0 values are typically between 70% and 80%.
Solar simulated light photocatalytic characterization
To verify the photostability of the synthesized ZnO/CeO2 nanocomposite particles, a solar simulator LCS-100™ light source was used to replicate real environmental conditions these materials may face if incorporated into a sunscreen formulation. A (1 sun) solar simulator for visible and UV light was provided to conduct this measurement. Figure 12 highlights the results of these solar simulated light photodegradation experiments. It can be seen that, as with the UV photodegradation results, the uncoated commercial ZnO induced the highest level of dye degradation, followed by the 2.5 at.%, 5 at.% and 10 at.% nanocomposite samples. The dye degradation is reduced by 99% for ceria amount of 10 at.% in the composite. In a similar manner to that outlined for the UV photodegradation experiments, the deposition of CeO2 in the nanocomposite samples provides dye protection by mitigating dye degradation through efficient scavenging of generated ROS by the core ZnO. The low degree of photocatalytic activity demonstrated by the nanocomposite samples under solar simulated light irradiation is particularly important if applied in sunscreen formulations as a UV filter. Normally, sunscreen formulations consist of a combination of both organic (various including oxybenzone and octocrylene) and inorganic UV filters (TiO2 or ZnO). Incorporation of uncoated ZnO into such formulations can impact the efficacy of the cream when applied to the skin due to the potential photodegradation of the organic components by photoexcited ZnO and ROS generation. In the case of the ZnO/CeO2 nanocomposites produced in this work, the low photocatalytic performance of the materials under simulated conditions highlights their effective potential for application in such products.
Conclusion
In this article, we have demonstrated that photocatalytically active commercial ZnO was encrusted with CeO2 nanodots to build nanocomposite particles using cost-effective simple precipitation procedure. The composite materials show excellent absorbance capabilities with low photocatalytic activity, as well as high photostability with increasing amounts of ceria up to 10 at.%, whereas the photocatalytic activity of commercial nano-ZnO was reduced by around 97% under UV and visible light, and 99% under solar simulation in 30 min. These novel nanocomposites can be used as efficient inorganic UV filters in sunscreens.
References
Furusawa T et al (2008) The microwave effect on the properties of silica-coated TiO2 fine particles prepared using sol–gel method. Mater Res Bull 43(4):946–957
Kullavanijaya P, Lim HW (2005) Photoprotection. J Am Acad Dermatol 52(6):937–958
Liu X, Yin S, Sato T (2009) Synthesis of broad-spectrum UV-shielding plate-like titanate/calcia-doped ceria composite in different pH solution. Mater Chem Phys 116(2–3):421–425
Antoniou C et al (2008) Sunscreens–what’s important to know. J Eur Acad Dermatol Venereol 22(9):1110–1119
Im YM et al (2015) Effect of ZnO nanoparticles morphology on UV blocking of poly (vinyl alcohol)/ZnO composite nanofibers. Mater Lett 147:20–24
Pinnell SR et al (2000) Microfine zinc oxide is a superior sunscreen ingredient to microfine titanium dioxide. Dermatol Surg 26(4):309–314
Schaefer H, Moyal D, Fourtanier A (1998) Recent advances in sun protection. Protection of the skin against ultraviolet radiations. John Libbey Eurotext, Paris, pp 119–129
Schauder S, Ippen H (1997) Contact and photocontact sensitivity to sunscreens: review of a 15-year experience and of the literature. Contact Dermat 37(5):221–232
Roscher NM et al (1994) Photodecomposition of several compounds commonly used as sunscreen agents. J Photochem Photobiol, A 80(1–3):417–421
Serpone N et al (2002) An in vitro systematic spectroscopic examination of the photostabilities of a random set of commercial sunscreen lotions and their chemical UVB/UVA active agents. Photochem Photobiol Sci 1(12):970–981
Dodd A et al (2010) Optical and photocatalytic properties of nanoparticulate (TiO2) x (ZnO) 1–x powders. J Alloy Compd 489(2):L17–L21
Cai R et al (1991) Photokilling of malignant cells with ultrafine TiO2 powder. Bull Chem Soc Japan 64(4):1268–1273
Serpone N, Salinaro A, Emeline A (2001) Deleterious effects of sunscreen titanium dioxide nanoparticles on DNA: efforts to limit DNA damage by particle surface modification. In: Nanoparticles and nanostructured surfaces: novel reporters with biological applications. 2001. International society for optics and photonics
Yang H, Zhu S, Pan N (2004) Studying the mechanisms of titanium dioxide as ultraviolet-blocking additive for films and fabrics by an improved scheme. J Appl Polym Sci 92(5):3201–3210
Chen H-C et al (2006) Effects of temperature on columnar microstructure and recrystallization of TiO2 film produced by ion-assisted deposition. Appl Opt 45(9):1979–1984
Senatova S et al (2015) Optical properties of stabilized ZnO nanoparticles, perspective for UV-protection in sunscreens. Curr Nanosci 11(3):354–359
Zholobak N et al (2011) UV-shielding property, photocatalytic activity and photocytotoxicity of ceria colloid solutions. J Photochem Photobiol, B 102(1):32–38
Boutard T et al (2013) Comparison of photoprotection efficiency and antiproliferative activity of ZnO commercial sunscreens and CeO2. Mater Lett 108:13–16
Yabe S, Sato T (2003) Cerium oxide for sunscreen cosmetics. J Solid State Chem 171(1–2):7–11
Truffault L et al (2010) Application of nanostructured Ca doped CeO2 for ultraviolet filtration. Mater Res Bull 45(5):527–535
Truffault L et al (2011) Synthesis and characterization of Fe doped CeO. Nanosci Nanotechnol 11:1–10
Truffault L et al (2011) Synthesis of nano-hematite for possible use in sunscreens. J Nanosci Nanotechnol 11(3):2413–2420
Cardillo D, Konstantinov K, Devers T (2013) The effects of cerium doping on the size, morphology, and optical properties of α-hematite nanoparticles for ultraviolet filtration. Mater Res Bull 48(11):4521–4525
Heckert EG et al (2008) The role of cerium redox state in the SOD mimetic activity of nanoceria. Biomaterials 29(18):2705–2709
Celardo I et al (2011) Ce3 + ions determine redox-dependent anti-apoptotic effect of cerium oxide nanoparticles. ACS Nano 5(6):4537–4549
He G, Fan H, Wang Z (2014) Enhanced optical properties of heterostructured ZnO/CeO2 nanocomposite fabricated by one-pot hydrothermal method: fluorescence and ultraviolet absorption and visible light transparency. Opt Mater 38:145–153
Li R et al (2002) UV-shielding properties of zinc oxide-doped ceria fine powders derived via soft solution chemical routes. Mater Chem Phys 75(1–3):39–44
Yabe S et al (2001) Synthesis and UV-shielding properties of metal oxide doped ceria via soft solution chemical processes. Int J Inorg Mater 3(7):1003–1008
Bi L et al (2008) Structural, magnetic, and magneto-optical properties of Co-doped Ce O2−δ films. J Appl Phys 103(7):07D138
He Y, Yang B, Cheng G (2003) Controlled synthesis of CeO2 nanoparticles from the coupling route of homogenous precipitation with microemulsion. Mater Lett 57(13–14):1880–1884
Yamashita M et al (2002) Synthesis and microstructure of calcia doped ceria as UV filters. J Mater Sci 37(4):683–687. https://doi.org/10.1023/A:1013819310041
Ge C, Xie C, Cai S (2007) Preparation and gas-sensing properties of Ce-doped ZnO thin-film sensors by dip-coating. Mater Sci Eng, B 137(1–3):53–58
Li C et al (2011) Electrospinning of CeO2–ZnO composite nanofibers and their photocatalytic property. Mater Lett 65(9):1327–1330
Yayapao O et al (2013) Sonochemical synthesis, photocatalysis and photonic properties of 3% Ce-doped ZnO nanoneedles. Ceram Int 39:S563–S568
Yousefi M et al (2011) Enhanced photoelectrochemical activity of Ce doped ZnO nanocomposite thin films under visible light. J Electroanal Chem 661(1):106–112
Fangli D et al (2010) Preparation, characterization and infrared emissivity study of Ce-doped ZnO films. J Rare Earths 28(3):391–395
Anbia M, Fard SEM (2012) Humidity sensing properties of Ce-doped nanoporous ZnO thin film prepared by sol–gel method. J Rare Earths 30(1):38–42
Morinaga Y et al (1997) Effect of Ce doping on the growth of ZnO thin films. J Cryst Growth 174(1–4):691–695
Mahmoud WE (2010) Synthesis and optical properties of Ce-doped ZnO hexagonal nanoplatelets. J Cryst Growth 312(21):3075–3079
de Lima JF et al (2009) ZnO: CeO2-based nanopowders with low catalytic activity as UV absorbers. Appl Surf Sci 255(22):9006–9009
Panda N et al (2013) Thermoluminescence and decay studies on cerium doped ZnO nanopowders. Mater Lett 95:205–208
Yang J et al (2008) Low-temperature growth and optical properties of Ce-doped ZnO nanorods. Appl Surf Sci 255(5):2646–2650
Dar G et al (2012) Ce-doped ZnO nanorods for the detection of hazardous chemical. Sens Actuators B Chem 173:72–78
Tan WK et al (2013) Photoluminescence properties of rod-like Ce-doped ZnO nanostructured films formed by hot-water treatment of sol–gel derived coating. Opt Mater 35(11):1902–1907
Rezaei M, Habibi-Yangjeh A (2013) Simple and large scale refluxing method for preparation of Ce-doped ZnO nanostructures as highly efficient photocatalyst. Appl Surf Sci 265:591–596
Xia C, Hu C, Zhou P (2013) Low-temperature growth and optical properties of Ce-doped ZnO nanorods. J Exp Nanosci 8(1):69–76
Sofiani Z et al (2006) Optical properties of ZnO and ZnO: Ce layers grown by spray pyrolysis. Opt Commun 267(2):433–439
George A et al (2011) Detailed of X-ray diffraction and photoluminescence studies of Ce doped ZnO nanocrystals. J Alloy Compd 509(20):5942–5946
Karunakaran C, Gomathisankar P, Manikandan G (2010) Preparation and characterization of antimicrobial Ce-doped ZnO nanoparticles for photocatalytic detoxification of cyanide. Mater Chem Phys 123(2–3):585–594
Bogusz K et al (2018) TiO2/(BiO)2 CO3 nanocomposites for ultraviolet filtration with reduced photocatalytic activity. J Mater Chem C 6(21):5639–5650
Cardillo D et al (2016) Multifunctional Fe2 O3/CeO2 nanocomposites for free radical scavenging ultraviolet protection. RSC Adv 6(70):65397–65402
Rajendran S et al (2016) Ce 3 + -ion-induced visible-light photocatalytic degradation and electrochemical activity of ZnO/CeO2 nanocomposite. Sci Rep 6:31641
Saravanan R et al (2018) Line defect Ce3 + induced Ag/CeO2/ZnO nanostructure for visible-light photocatalytic activity. J Photochem Photobiol, A 353:499–506
Saravanan R, et al. (2012) Photocatalytic degradation of organic dyes using ZnO/CeO2 nanocomposite material under visible light. In Advanced materials research. Trans Tech Publications Ltd
Ying JY, Tschöpe A (1996) Synthesis and characteristics of non-stoichiometric nanocrystalline cerium oxide-based catalysts. Chem Eng J Biochem Eng J 64(2):225–237
Nelson BC et al (2016) Antioxidant cerium oxide nanoparticles in biology and medicine. Antioxidants 5(2):15
Xue Y et al (2011) Direct evidence for hydroxyl radical scavenging activity of cerium oxide nanoparticles. J Phys Chem C 115(11):4433–4438
Lamba R et al (2015) CeO2ZnO hexagonal nanodisks: efficient material for the degradation of direct blue 15 dye and its simulated dye bath effluent under solar light. J Alloy Compd 620:67–73
Ye Z et al (2016) Well-dispersed nebula-like ZnO/CeO2@ HNTs heterostructure for efficient photocatalytic degradation of tetracycline. Chem Eng J 304:917–933
Zamiri R et al (2015) Dielectrical properties of CeO2 nanoparticles at different temperatures. PLoS ONE 10(4):e0131851
Zuas O, Abimanyu H, Wibowo W (2014) Synthesis and characterization of nanostructured CeO2 with dyes adsorption property. Process Appl Ceram 8(1):39–46
Kumar E, Selvarajan P, Muthuraj D (2013) Synthesis and characterization of CeO2 nanocrystals by solvothermal route. Mater Res 16(2):269–276
Nagaraju G et al (2017) Electrochemical heavy metal detection, photocatalytic, photoluminescence, biodiesel production and antibacterial activities of Ag–ZnO nanomaterial. Mater Res Bull 94:54–63
Selvi N, Sankar S, Dinakaran K (2014) Size controlled synthesis of pure CeO2 and ZnO COATED CeO2 core-shell nanoparicles for opto-electronic applications. In: 2014 International conference on science engineering and management research (ICSEMR). IEEE
Selvi N et al (2014) Effect of ZnO, SiO2 dual shells on CeO2 hybrid core–shell nanostructures and their structural, optical and magnetic properties. RSC Adv 4(99):55745–55751
Suhail FSA, Mashkour MS, Saeb D (2015) The study on photo degradation of crystal violet by polarographic technique. Int J Basic Appl Sci 15:12–21
Lee G, Kawazoe T, Ohtsu M (2002) Difference in optical bandgap between zinc-blende and wurtzite ZnO structure formed on sapphire (0001) substrate. Solid State Commun 124(5–6):163–165
Mueen R et al (2020) Na-doped ZnO UV filters with reduced photocatalytic activity for sunscreen applications. J Mater Sci 55(7):2772–2786. https://doi.org/10.1007/s10853-019-04122-2
Tsuzuki T et al (2012) Reduction of the photocatalytic activity of ZnO nanoparticles for UV protection applications. Int J Nanotechnol 9(10–12):1017–1029
He R, Hocking RK, Tsuzuki T (2012) Co-doped ZnO nanopowders: location of cobalt and reduction in photocatalytic activity. Mater Chem Phys 132(2–3):1035–1040
Kaneva NV, Dimitrov DT, Dushkin CD (2011) Effect of nickel doping on the photocatalytic activity of ZnO thin films under UV and visible light. Appl Surf Sci 257(18):8113–8120
Acknowledgements
This work is part of the University of Wollongong Global Challenges project “NEXT GENERATION SUNSCREENS: Designed and tested for Australian conditions, with global implications for sun safety.” Furthermore, the authors acknowledge the use of the facilities within the Electron Microscopy Centre at the University of Wollongong. The authors would also like to acknowledge the support provided by the University of Diyala and the Iraqi Ministry of Higher Education and Scientific Research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mueen, R., Morlando, A., Qutaish, H. et al. ZnO/CeO2 nanocomposite with low photocatalytic activity as efficient UV filters. J Mater Sci 55, 6834–6847 (2020). https://doi.org/10.1007/s10853-020-04493-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-04493-x