Abstract
Metal oxide semiconductor sensors based on nanocrystalline In2O3 and its composites are found to be very sensitive in detecting low-concentration (~ 5 ppm) gases such as ozone, nitrogen dioxide, formaldehyde and butane. Here, we successfully obtained fiber-shaped In2O3 crystalline nanofibers via electrospun and calcination routes. The gas sensing properties of the In2O3 nanofibers were studied by exposing them to the acetic acid vapor with different concentrations from 500 ppb to 2000 ppm at the optimum operating temperature (250 °C). The device possesses ultra-high response of 66.7 toward 2000 ppm acetic acid vapor, low response and recovery times of 25 s and 37 s (100 ppm), respectively, and significant selectivity to acetic acid at 100 ppm. In particular, the sensor based on In2O3 nanofibers has very low detection limit and can reach 500 ppb. Therefore, the presented In2O3 nanofiber sensor can be used in practice in acetic acid detection area in the future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acetic acid is colorless, flammable and easy volatile and is extensively used in plastics, pharmaceuticals, dyes, insecticides and photographic chemicals industries [1]. However, inhalation of a certain amount of acetic acid gas can induce irritation in skin, eyes and respiratory system. A long-term exposure in the ambient of acetic acid leakage may induce some kinds of diseases [2, 3]. The maximum permitted concentrations of acetic acid vapor in workplace are 7.5 ppm in China (GBZ 2-2002) and 9.3 ppm in USA (D3620-04). So, it is very urgent to have a effective way to probe acetic acid concentration in the laboratory and domestic utilities. Unfortunately, the detection toward acetic acid is still unsatisfied [4,5,6,7,8]. For example, the sensing properties, especially sensitivity and working temperature, need to be improved further [1, 4]. In recent decades, gas sensors are highly required in medical, environmental and domestic utilities for detecting toxic and combustible gases. In this respect, metal oxide semiconductors (MOSCs) such as ZnO [1], Bi2O3 [9], SnO2 [8, 10], α-Fe2O3 [7], MoO3 [11], In2O3 [12,13,14,15] and their nanocomposites are promising candidates owing to their thermal stability, excellent response, reversibility and effective cost [16, 17]. In order to increase sensitivity and decrease operating temperature of the gas sensor, noble metals and transition metals are favorite dopants, which can significantly enhance the sensing properties of MOSCs [18,19,20,21]. But, these types of sensors have the disadvantages of complicated synthesis process, narrow range of detection limit and high working temperature [19, 20]. The sensors based on semiconductor oxides can work via the variation in the device resistance due to the surface processes of sensing materials [18, 19]. In general, the absorbed acetic acid gas molecule could decompose into two CO2, two H2O molecules and eight electrons (8 e−1) due to the lower bond energy of C–C in CH3COOH, and then the released electrons result in an abrupt alteration of device’s resistance [2, 3, 18].
In a wide variety of MOSC nanomaterials, In2O3 and its composites exhibit higher sensitivity and shorter response/recovery times than other sensing materials [9, 22]. For example, the sensing performance of Bi2O3-decorated In2O3 nanorods is stronger (1.5–4.9 times) than that of the pristine In2O3 nanorod at the corresponding concentrations [9]. And the nanosheet-based In2O3 microflowers gas sensor exhibited excellent sensing properties toward formaldehyde [22]. In 2018, the broken In2O3 microtubes were obtained by chemical conversion method, and these broken In2O3 microtubes exhibited significant response toward triethylamine at 1–100 ppm, and the detection limit is 0.1 ppm [23]. Very recently, Co-doped In2O3 nanorods were fabricated by hydrothermal strategy, and the In2O3/1%Co nanorods exhibited a high response of 23.2 for 10 ppm to HCHO at 130 °C [24]. Lee et al. reported that the CuO-loaded In2O3 nanofibers significantly enhanced the gas response toward 5 ppm H2S from 515 to 1.16 × 105 at 150 °C [25]. What’s more, one-dimensional (1D) structure allows efficient electron transport along the longitudinal direction, is simple and has high volume prepared by electrospinning [25]. To the best of our knowledge, electrospinning can be used to prepare nanofibers with many particles, which can greatly increase the specific surface area [3, 7, 26]. Up to now, few studies have been reported regarding the acetic acid sensing characteristics of In2O3 nanofibers. Therefore, it is of great significance to design and fabricate an acetic acid sensor with good performance based on In2O3 nanofibers.
We have successfully obtained In2O3 nanofibers composed of many nanoparticles. The detection limit of In2O3 nanofibers sensor can reach 500 ppb and has excellent selectivity toward acetic acid. Meanwhile, the homemade devices exhibit good repeatability and stability.
Experimental section
Materials
Polyvinylpyrrolidone (PVP, Mw = 1500000), indium(III) nitrate hydrate (In(NO3)3·H2O) and N,N-dimethylformamide (DMF) were purchased from Aladdin Company and used without any purification.
Preparation of In2O3 nanofibers
In this study, the In2O3 nanofibers were fabricated via electrospinning combined with calcination in air. Typically, 0.5 g of In(NO3)3·H2O was added into a solvent of DMF in a little bottle under vigorous stirring for 6 h. Then, 0.25 g of PVP was dissolved into 3 ml ethanol in another little bottle under vigorous stirring for 6 h. Then, both of them were mixed together under stirring and loaded into a 10-ml injector with a 22-gauge needle tip for electrospinning, and the parameters of electrospinning are the same as the above process. After electrospinning, the nanofiber-shaped In2O3 precursors were preheated at 160 °C for 2 h and then calcined at 600 °C under air for 4 h. Finally, the In2O3 nanofibers composed of many nanoparticles were successfully obtained.
Characterizations
The surface morphology of the nanofibers was observed using a field emission scanning electron microscope (FESEM, Zeiss Gemini 500). The diameter of the electrospun fibers was measured using image analysis software (Adobe Acrobat X Pro 10.1.2.45) according to the SEM images. Energy-dispersive X-ray spectroscopy (EDX) mapping of the samples was performed using an Oxford Link-ISIS 300 EDX attachment at 15 kV. The microstructure of the samples was investigated by X-ray diffraction (XRD), performed on a Bruker D8 diffractometer with Cu Kα radiation (λ = 0.154 nm). XPS measurements were recorded with a Thermo Scientific Escalab 250xi instrument equipped with a monochromatic Al Kα source. And the gas sensing properties were measured by WS-30A gas sensing measurement system. During the tests of gas sensing, a certain volume of the acetic acid liquid was injected into the test chamber. When the resistance of the sensor was stable, the sensor was transferred into another chamber that was full of fresh air and began to recover. The testing chamber was blown and purged by nitrogen (purity: 99.9%) before and after every cycle. In this article, the sensor response to the testing gas is defined as R = Ra/Rg, where Ra and Rg are the resistances of the sensors in air and in target gases, respectively. Here, the response/recovery times were defined as the time taken to achieve 90% of the total resistance changes after the sensor was exposed to the acetic acid vapor and fresh air, respectively.
Results and discussion
Figure 1a illustrates an overall process of material synthesis and device preparation. Here, we firstly obtained In2O3 nanofibers via electrospinning and calcination routes, and then the sensing devices based on In2O3 nanofibers were prepared according to our previous report [27]. In Fig. 1b, it can be seen that the In2O3 precursors formed a multilayered network randomly, which is similar to a nonwoven nanofiber mat (inset in Fig. 1b). As depicted in the high-magnitude SEM in Fig. 1c, the as-prepared In2O3 precursors have uniform shape and smooth surface, without any obvious beads and defects on the surface of the precursors, which implies that the In2O3 electrospinning solution has good spinnability [28, 29]; see Fig. 1c. In addition, the diameter of the In2O3 precursors is roughly distributed at 90 nm, as shown in the inset of Fig. 1c. Then, the In2O3 precursors were calcined in air at 600 °C immediately after preheating at 160 °C. The In2O3 precursors were broken into pieces after calcination process; see Fig. 1d (the low-magnitude SEM image). In order to investigate the details of the calcined In2O3 nanofibers, the high-resolution SEM image was taken (Fig. 1e). Evidently, it can be seen that the surface of the In2O3 nanofibers are uneven, compared to the In2O3 precursors. Besides, some nanoparticles appeared on the surface of In2O3 nanofibers. Such structure plays a significant role in gas sensing enhancement because the rough surface can greatly increase the active sites to the target gas. On the other hand, the roughened surface is benefit to gas adsorption/desorption and diffusion of target gas molecule [22, 27]. Additionally, the diameter tendency observed in the as-spun nanofibers was decreased after calcination [7, 29]. According to the statistical result, the average diameter of In2O3 nanofibers (the inset of Fig. 1e) decreased by approximately 22% compared to the distribution of In2O3 precursors (see the inset of Fig. 1c). This obvious decrease in diameter was attributed to either the evaporation of residual solvent (DMF) absorbed in the fibers or a partial decomposition of PVP, which acts as the polymeric scaffold of In2O3 precursors [29].
a Schematic illustration of the fabrication processes for In2O3 nanofibers and corresponding gas sensor. b The SEM image of In2O3 precursor fibers; the inset is nonwoven In2O3 nanofiber mat. c High-resolution SEM image of In2O3 precursor fibers, and the inset is histograms of the corresponding In2O3 precursor diameters. d Low-magnitude SEM image of the calcined In2O3 nanofibers. e High-resolution SEM image of In2O3 nanofibers, and the inset is histograms of the corresponding In2O3 nanofibers diameters
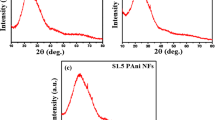
To investigate the purity and crystalline phases of the samples, the XRD patterns of In2O3 precursors and In2O3 nanofibers were scanned from 10° to 90°. Obviously, the XRD pattern of In2O3 precursors (stabilized at 160 °C) did not reveal any crystalline structure (Fig. 2a, dark pattern). In contrast, the calcined In2O3 nanofibers exhibit strong diffraction peaks, as shown in Fig. 2a (red pattern). It can be observed that the peaks of crystal planes (211), (222), (400), (440) and (622) are the main characteristic peaks of In2O3, which correspond to cubic structure of In2O3 (JCPDS 06-0416) [28, 30]. The result suggests that In2O3 nanofibers with excellent crystallinity were successfully synthesized [30].
a XRD pattern of In2O3/PVP composite fibers (In2O3 precursor fibers) and In2O3 nanofibers, respectively. b and c are high-resolution XPS spectra of In 3d and O 1s of In2O3 nanofibers, respectively. d, e and f are the corresponding energy-dispersive X-ray spectroscopic (EDX) elemental mapping images of O e and In f, respectively
The high-resolution XPS measurement was carried out to analyze the chemical constituents and elements valence of the In2O3 nanofibers. Figure 2b shows the XPS spectrum of In 3d state, which indicates the peaks at 444.5 eV and 452.0 eV, corresponding to the In 3d5/2 and In 3d3/2, respectively. The difference value between In 3d3/2 and In 3d5/2 is 7.5 eV, which indicates that In element exists mainly in the state of In3+ in In2O3 [23, 24, 28]. Figure 2c shows two peaks of the oxygen species and the lower energy peak (529.5 eV) can be attributed to lattice oxygen on the surface of In2O3 nanofibers, while the peak located at 531.8 eV may be ascribed to chemisorbed oxygen species. According to the previous reports, the chemisorbed oxygen is good for catalytic activity in gas sensors and to improve the performance of volatile organic compound (VOC) gas sensors [7, 18]. Besides, the elemental mapping images (Fig. 2d–f) further confirmed the composition of the product and the spatial distribution of the elements. Obviously, O (Fig. 2e) and In (Fig. 2f) signals were detected as a fiber-like structure, while In and O signals were detected in the whole region, which indicated the uniform distributions of In and O elements over the whole nanofibers.
In order to ascertain the optimum operating temperature of the In2O3 nanofibers sensor, the response value of the sensor toward 100 ppm acetic acid under different temperatures from 150 to 350 °C was measured and the results are shown in Fig. 3a. We can see that the response increases sharply with the increase in temperature from 150 to 250 °C for the In2O3 nanofibers sensor and reaches the maximum value of 28.4 at 250 °C, and then drops rapidly with further increase in the temperature.
a Sensing performances of the In2O3 nanoparticle fibers toward 100 ppm acetic acid gas at different operating temperatures. b Dynamic response curves of the In2O3 sensor toward acetic acid from 500 ppb to 2000 ppm at 250 °C. c Response versus gas concentrations for In2O3 sensors, and the inset gives the curve of low concentration from 500 ppb to 100 ppm. d Repeatability test (five periods) of the In2O3 nanoparticle fibers sensor to 100 ppm of acetic acid at 250 °C
Above the temperature of 250 °C, the decrease in adsorbed oxygen ions will increase conduction electron density and thus reduce the gas response. Therefore, the optimum working temperature should be 250 °C to proceed with the following measurements. Figure 3b gives the dynamic response and recovery properties of sensors based on In2O3 nanofibers of different acetic acid vapor concentrations (from 500 ppb to 2000 ppm) at 250 °C. We can see that the response increases quickly with the increase in the acetic acid concentration; see Fig. 3c, and the response of the In2O3 nanofibers sensor increases steeply from 500 ppb to 100 ppm (inset in Fig. 3c). Thus, the In2O3 nanofibers sensor can effectively detect acetic acid with the ultra-low concentration due to the nature of one-dimensional (1D) structures, which can facilitate the rapid and efficient adsorption of gas molecules, as well as good sensing performance. It is worth mentioning that the response is 3.4 at 500 ppb, which is larger than that of international criterion of 1.2 [19, 20]. The lower limit of quantitation (LLoQ) of acetic acid vapor is evaluated by linear extrapolation of the response sensitivity as a function of acetic acid concentration, and the calculating formula of the LLoQ is LLoQ = 3 × (standard Deviation/slope) [31], predicting an ultra-low acetic acid detection concentration of 161 ppb for the In2O3 nanofibers sensor operated at 250 °C. The detailed response values of In2O3 nanofiber sensors are listed in Table 1.
When the concentration of acetic acid reaches 1000 ppm, the response value shows the status of saturation and the similar characteristics of other-category VOC sensors have been reported previously [18, 26, 30]. We suggest that the response of gas sensor is determined by the surface reaction rate. Therefore, the large specific surface area is a very important factor to promote the surface reaction rate [32,33,34]. According to Table 1, the maximum response value is 66.7 under 2000 ppm, which is very close to 1000 ppm (66.6). On this aspect, the nanoparticles of In2O3 nanofibers greatly affect the response value of In2O3 nanofibers sensor. Figure 3d shows a typical cycling stability test of the In2O3 sensor to 100 ppm of acetic acid at 250 °C. It can be found that the In2O3 sensor has a consistent response property, and the value is 28.1. In addition, the device exhibits excellent recovery characteristics and repeatability.
Figure 4a gives the response time (τres) and recovery time (τrecov) of the In2O3 nanofibers sensor to 100 ppm acetic acid at 250 °C, which are the vital performance parameters for gas sensors. The τres of pure In2O3 sensor is 25 s, and the measured recovery time (τrecov) is 37 s, which are shorter than those from previous reports [1,2,3,4, 7, 8]. The above results are sufficient to prove that the response and recovery times toward acetic acid gas can be greatly improved by In2O3 nanofibers. The long-term stability of In2O3 nanofibers gas sensor is also investigated and shown in Fig. 4b. The sensor exhibits nearly constant sensor signals to 100 ppm acetic acid for 60 days, confirming the good stability of the In2O3 nanofibers sensor. Again, acetone, methanol, NH3 and benzene sensing characteristics of the In2O3 nanofibers sensors are also measured under the same conditions to evaluate the selectivity of In2O3 nanofibers sensor. Figure 4c shows the sensitivities of In2O3 nanofibers sensor to 100 ppm of different gases at 250 °C. We can see that In2O3 nanofibers sensor show less sensitivity to other gases, indicating the excellent selectivity to acetic acid vapor.
a Response and recovery characteristics of the In2O3 nanoparticle fibers sensor toward 100 ppm acetic acid at 250 °C. b Line chart of the long-term stability of In2O3 sensor of 1 day, 15 days, 30 days, 45 days and 60 days, respectively. c Selectivity of the In2O3 nanoparticle fibers sensor toward different VOCs at 250 °C. d Schematic diagram of the acetic acid sensing mechanism of In2O3 nanoparticle fibers
The comparison of the responses to acetic acid gas among the In2O3 nanofibers sensors and previous reports such as CdSxSe1−x nanoribbons, Y-doped SnO2 nanoparticles, Pr-doped ZnO nanofibers, and SnO2 nanoflowers is shown in Table 2. In contrast to other materials, the In2O3 nanofibers gas sensor has a lower operation temperature and extremely high sensitivity. In short, the In2O3 nanofibers gas sensors showed good sensing properties, especially sensitivity and working temperature.
The gas sensing mechanism can be explained as follows: The gas-sensitive materials react with reducing gas such as acetic acid and lead to the change in electrical resistance of the materials, as shown in Fig. 4d [36, 37]. When gas sensors are exposed to air, oxygen molecules are adhered to the surface of In2O3 nanofibers and electrons transport occurs between these metal oxides and the gas molecules, leading to the formation of adsorbed oxygen O−, O2− and O2− in the following fashions: \( {\text{O}}_{{2({\text{gas}})}} + 2{\text{e}}^{ - } \to 2{\text{O}}_{{({\text{ads}})}}^{ - } ,{\text{ O}}_{{2({\text{gas}})}} + {\text{e}}^{ - } \to {\text{O}}_{{2({\text{ads}})}}^{ - } ,{\text{ O}}_{{2({\text{gas}})}}^{ - } + {\text{e}}^{ - } \to {\text{O}}_{{2({\text{ads}})}}^{ - } \) [38,39,40,41]. As a result, the carrier concentration will decrease, which results in the increase in the device resistance. When the sensor is exposed to acetic acid gas, the acetic acid molecules will react with oxygen ions and release a certain number of electrons, which go back to the conduction band, resulting in the resistance decrease of the device. According to the previous reports, the surface process of charge was greatly influenced by surface defect [32]. Hence, the reaction is:
Therefore, the acetic acid sensitivity toward In2O3 gas sensors is basically above oxidization reaction process, with free electrons flowing into the conduction band. These free electrons will in turn improve the oxygen adsorption on the In2O3 surface [29, 42].
Conclusion
In summary, we have successfully obtained In2O3 nanofibers via a synergetic approach of electrospinning and calcination processes. The In2O3 nanofibers exhibit enhanced sensing performance toward acetic acid at 250 °C. Specifically, the sensor based on In2O3 nanofibers has very low detection limit and can reach 500 ppb. According to our study, it is believed that the sensor based on In2O3 nanofibers is a promising candidate for the efficient detection of acetic acid.
References
Wang C, Ma S, Sun A, Qin R, Yang F, Li X, Li F, Yang X (2014) Characterization of electrospun Pr-doped ZnO nanostructure for acetic acid sensor. Sens Actuators B: Chem 193:326–333
Bhamore JR, Ganguly P, Kailasa SK (2016) Molecular assembly of 3-mercaptopropinonic acid and guanidine acetic acid on silver nanoparticles for selective colorimetric detection of triazophos in water and food samples. Sens Actuators B: Chem 233:486–495
Dedecker K, Pillai RS, Nouar F, Pires J, Steunou N, Dumas E, Maurin G, Serre C, Pinto ML (2018) Metal-organic frameworks for cultural heritage preservation: the case of acetic acid removal. ACS Appl Mater Interfaces 10:13886–13894
Cheng L, Ma SY, Wang TT, Luo J, Li XB, Li WQ, Mao YZ, Gz DJ (2014) Highly sensitive acetic acid gas sensor based on coral-like and Y-doped SnO2 nanoparticles prepared by electrospinning. Mater Lett 137:265–268
Li XB, Zhang QQ, Ma SY, Wan GX, Li FM, Xu XL (2014) Microstructure optimization and gas sensing improvement of ZnO spherical structure through yttrium doping. Sens Actuators B: Chem 195:526–533
Lin S, Swager TM (2018) Carbon nanotube formic acid sensors using a nickel bis(ortho-diiminosemiquinonate) selector. ACS Sens 3(3):569–573
Ma L, Ma SY, Qiang Z, Xu XL, Chen Q, Yang HM, Chen H, Ge Q, Zeng QZ, Wang BQ (2017) Preparation of Co-doped LaFeO3 nanofibers with enhanced acetic acid sensing properties. Mater Lett 200:47–50
Jin WX, Ma SY, Tie ZZ, Li WQ, Luo J, Cheng L, Xu XL, Wang TT, Jiang XH, Mao YZ (2015) Synthesis of hierarchical SnO2 nanoflowers with enhanced acetic acid gas sensing properties. Appl Surf Sci 353:71–78
Park S, Kim S, Sun GJ, Lee C (2015) Synthesis, structure, and ethanol gas sensing properties of In2O3 nanorods decorated with Bi2O3 nanoparticles. ACS Appl Mater Interfaces 7:38–46
Wang D, Tian L, Li HJ, Wan KC, Yu X, Wang P, Chen AY, Wang XY, Yang JH (2019) Mesoporous ultrathin SnO2 nanosheets in situ modified by graphene oxide for extraordinary formaldehyde detection at low temperatures. ACS Appl Mater Interfaces 11:12808–12818
Tian J, Zhang H, Li Z (2018) Synthesis of double-layer nitrogen-doped microporous hollow carbon@MoS2/MoO2 nanospheres for supercapacitors. ACS Appl Mater Interfaces 10:29511–29520
Yan SN, Li ZJ, Li H, Wu ZL, Wang JQ, Shen WZ, Fu YQ (2018) Ultra-sensitive room-temperature H2S sensor using Ag–In2O3 nanorod composites. J Mater Sci 53:16331–16344. https://doi.org/10.1007/s10853-018-2789-z
Li ZJ, Yan SN, Wu ZL, Li H, Wang JQ, Shen WZ, Wang ZG, Fu YQ (2018) Hydrogen gas sensor based on mesoporous In2O3 with fast response/recovery and ppb level detection limit. Int J Hydrog Energy 43:22746–22755
Haiduk YS, Khort AA, Lapchuk NM, Savitsky AA (2019) Study of WO3–In2O3 nanocomposites for highly sensitive CO and NO2 gas sensors. J Solid State Chem 273:25–31
Lee CS, Li HY, Kim BY, Jo YM, Byun HG, Hwang IS, Abdel-Hady F, Wazzan AA, Lee JH (2019) Discriminative detection of indoor volatile organic compounds using a sensor array based on pure and Fe-doped In2O3 nanofibers. Sens Actuators B: Chem 285:193–200
Xu S, Xu Y, Zhao H, Xu R, Lei Y (2018) Sensitive gas-sensing by creating adsorption active sites: coating an SnO2 layer on triangle arrays. ACS Appl Mater Interfaces 10:29092–29099
Dong C, Liu X, Han B, Deng S, Xiao X, Wang Y (2016) Nonaqueous synthesis of Ag-functionalized In2O3/ZnO nanocomposites for highly sensitive formaldehyde sensor. Sens Actuators B: Chem 224:193–200
Miller DR, Akbar SA, Morris PA (2014) Nanoscale metal oxide-based heterojunctions for gas sensing: a review. Sens Actuators B: Chem 204:250–272
Lupan O, Postica V, Hoppe M, Wolff N, Polonskyi O, Pauporte T, Viana B, Majerus O, Kienle L, Faupel F, Adelung R (2018) PdO/PdO2 functionalized ZnO: Pd films for lower operating temperature H2 gas sensing. Nanoscale 10:14107–14127
Qiu HW, Wang MQ, Li L, Li JJ, Cao MH (2018) Hierarchical MoS2-microspheres decorated with 3D AuNPs arrays for high-efficiency SERS sensing. Sens Actuators, B 255:1407–1414
Dong C, Jiang M, Tao Y, Shen Y, Lu Y, Yuan Y, Wang Y (2018) Nonaqueous synthesis of Pd-functionalized SnO2/In2O3 nanocomposites for excellent butane sensing properties. Sens Actuators B: Chem 257:419–426
Dong C, Liu X, Han B, Deng S, Xiao X, Wang Y (2016) Nonaqueous synthesis of Ag-functionalized In2O3/ZnO nanocomposites for highly sensitive formaldehyde sensor. Sens Actuators B: Chem 224:193–200
Ma J, Fan H, Tian H, Ren X, Wang C, Gao S, Wang W (2018) Ultrahigh sensitivity and selectivity chlorine gas sensing of In2O3 hollow microtubules by bio-template method with degreasing cotton. Sens Actuators B: Chem 262:17–25
Wang Z, Hou C, De Q, Gu F, Han D (2018) One-step synthesis of co-doped In2O3 nanorods for high response of formaldehyde sensor at low temperature. ACS Sens 3:468–475
Liang X, Kim TH, Yoon JW, Kwak CH, Lee JH (2015) Ultrasensitive and ultraselective detection of H2S using electrospun CuO-loaded In2O3 nanofiber sensors assisted by pulse heating. Sens Actuators B: Chem 209:934–942
Cheng JP, Wang J, Li QQ, Liu HG, Li Y (2016) A review of recent developments in tin dioxide composites for gas sensing application. J Ind Eng Chem 44:1–22
Zhang FH, Wang YC, Wang L, Liu J, Ge HL, Wang B, Huang XY, Wang XD, Chi ZT, Xie WF (2019) High performance In2(MoO4)3@In2O3 nanocomposites gas sensor with long-term stability. J Alloys Compd 805:180–188
Zhang W, Zhang W, Chen B, Shao R, Guan R, Zhang W, Zhang Q, Hou G, Yue L (2017) Controllable biomolecule-assisted synthesis and gas sensing properties of In2O3 micro/nanostructures with double phases. Sens Actuators B: Chem 239:270–278
Garcia-Marquez A, Glatzel S, Kraupner A, Kiefer K, Siemensmeyer K, Giordano C (2018) Branch-like iron nitride and carbide magnetic fibres using an electrospinning technique. Chem Eur J 24:895–4901
Wu CH, Chou TL, Wu RJ (2018) Rapid detection of trace ozone in TiO2–In2O3 materials by using the differential method. Sens Actuators B: Chem 255:117–124
Hu J, Zou C, Su YJ, Li M, Yang Z, Ge MY, Zhang YF (2017) One-step synthesis of 2D C3N4-tin oxide gas sensors for enhanced acetone vapor detection. Sens Actuators, B 253:641–651
Liu BT, Wu CR, Chen G, Chen WB, Peng LL, Yao YC, Wei Z, Zhu H, Han T, Tang DY, Zhou M (2019) All-in-one surface engineering strategy on nickel phosphide arrays towards a robust electrocatalyst for hydrogen evolution reaction. J Power Sources 429:46–54
Liu BT, Wang SW, Mo QH, Peng LL, Cao SX, Wang J, Wu CR, Li C, Guo J, Liu BQ, Chen WB, Lin Y (2018) Epitaxial MoS2 nanosheets on nitrogen doped graphite foam as a 3D electrode for highly efficient electrochemical hydrogen evolution. Electrochim Acta 292:407–418
Wu CR, Liu BT, Wang J, Su YY, Yan HQ, Ng CT, Li C, Wei JM (2018) 3D structured Mo-doped Ni3S2 nanosheets as efficient dual-electrocatalyst for overall water splitting. Appl Surf Sci 441:1024–1033
Zhang J, ZhuY Sun JP, Shao MW (2015) Visible-light-enhanced gas sensing of CdSxSe1−x nanoribbons for acetic acid at room temperature. Sens Actuators B: Chem 215:497–503
Souissi R, Labidi A (2018) Ethanol sensing properties of sprayed B-In2S3 thin films. Sens Actuators B: Chem 261:522–530
Hu J, Su YJ, Li M, Yang Z, Ge MY, Zhang YF (2017) One-step synthesis of 2D C3N4-tin oxide gas sensors for enhanced acetone vapour detection. Sens Actuators, B 253:641–651
Hu J, Su YJ, Li M, Ye XZ, Cai BF, Kong EW, Yang Z, Zhang YF (2018) Light-assisted recovery for a highly-sensitive NO2 sensor based on RGO-CeO2 hybrids. Sens Actuators, B 270:119–129
Wang T, Hu NT, Hu J, Huang D, Jiang WK, Wang S, Wu SM, Zhang YF, Yang Z (2018) Microwave preparation and remarkable ethanol sensing properties of ZnO particles with controlled morphologies in water-ethylene glycol binary solvent system. Sens Actuators, B 255:1006–1014
Wang D, Wan KC, Zhang ML, Li HJ, Wang P, Wang XY, Yang JH (2019) Constructing hierarchical SnO2 nanofiber/nanosheets for efficient formaldehyde detection. Sens Actuators B: Chem 283:714–723
Zhou TT, Zhang T, Zeng Y, Zhang R, Lou Z, Deng JA, Wang LL (2018) Structure-driven efficient NiFe2O4 materials for ultra-fast response electronic sensing platform. Sens Actuators B: Chem 255:1436–1444
Xu K, Tian S, Zhu J, Yang Y, Shi J, Yu T, Yuan C (2018) High selectivity of sulfur-doped SnO2 in NO2 detection at lower operating temperatures. Nanoscale 10:20761–20771
Acknowledgements
The authors acknowledge the National Natural Science Foundation of China (Grant No. 51227804), the Natural Science Foundation of Jiangsu Province (BK20170330) and the Postdoctoral Scientific Research Foundation of Qingdao. The authors would also like to thank Chemical Experimental Teaching Center of Qingdao University for the measurement help.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, YC., Sun, ZS., Wang, SZ. et al. Sub-ppm acetic acid gas sensor based on In2O3 nanofibers. J Mater Sci 54, 14055–14063 (2019). https://doi.org/10.1007/s10853-019-03877-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-03877-y