Abstract
Chemical and electrochemical functionalization of graphene oxide by o-phenylenediamine with its subsequent polymerization was conducted. Electrochemical functionalization was carried out by anodic oxidation of o-phenylenediamine on the graphene oxide-covered gold electrode, while chemical functionalization was made by incubation of graphene oxide-covered electrode in the monomer solutions of different pHs. Cyclic voltammetry with the simultaneous mass changes monitored by EQCM was used for the testing of the resulting electrodes. The results demonstrate that formation of poly(o-phenylenediamine) takes place not only by electrochemical oxidation of monomer but also as a result of the spontaneous heterogeneous redox reaction between oxygen functionalities on graphene oxide and o-phenylenediamine. Resulting composite of graphene oxide/poly(o-phenylenediamine) showed relatively stable response and increased currents over the wide potential range in comparison with the same polymer formed at bare gold electrode giving rise to the significant pseudocapacitance of this material. The electroactivity of the composite was preserved in neutral medium, and hydration was identified as a rate-limiting process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chemical functionalization by covalent or physical attachments of molecules is a versatile method for the preparation of advanced materials and composites with tailored surfaces and improved properties for specific applications including capacitors [1, 2], sensorics [3, 4], batteries [5, 6], flexible electronics [7, 8], touch screens, light-emitting diodes, solar cells or field-effect transistors [8, 9]. Careful selection of functional molecules enables modification and precise control of the electrochemical properties of the base material.
Graphene, 2D sp2 hybridized carbon sheet, became very popular recently as emerging material possessing remarkable mechanical [10, 11], electrical [12, 13] and thermal [14, 15], optical [16, 17] properties opening up many potential applications in diverse fields of science and technology. Low internal resistance [18] and high charge storage ability [19] attracted a huge interest for the applications of graphene-based materials in the preparation of the electrodes in supercapacitors [20, 21]. However, its widespread application is hampered by the difficulties of obtaining pure defect-free graphene sheets, their low processability and tendency of graphene to agglomerate.
Among many direct methods of the graphene synthesis such as epitaxial growth [22, 23] or chemical vapour deposition [24], chemical oxidation and exfoliation of graphite [25,26,27] with subsequent chemical [26, 28,29,30] or electrochemical reduction [31,32,33] of resulting graphene oxide (GO) stand out due to low price and possibility to produce large amounts of product [34,35,36]. Chemically obtained graphene is termed reduced graphene oxide (rGO) since residual oxygen functional groups still persist in the final product in higher or lesser extent [37,38,39].
Various oxygen functional groups present in rGO and GO disrupt extended π conjugation in graphene which gives unfavourable electronic effect [39,40,41,42]. The conductivity of such materials is significantly decreased compared to the pure defect-free graphene, and it is not desirable in many applications including energy storage. On the other hand, oxygen groups such as carbonyl, hydroxyl and carboxyl provide anchor sites for the chemical derivatization of graphene with various functional molecules enabling chemists to accurately control the properties of new materials by covalent or physical attachment of suitable molecules [43,44,45,46].
Conducting polymers received a lot of attention due to their interesting properties with many potential applications [47,48,49,50,51,52,53]. They usually have good charge storage ability, facile redox reactions and high surface area making them promising candidates for the design of active electrode materials for batteries and supercapacitors [5, 54, 55]. However, their widespread use is now limited by their unfavourable characteristics including poor chemical stability especially at high anodic potentials, dimensional instability due to heavy traffic of counter-ions and solvent molecules across the interface during charging/discharging reactions and sloping discharge profile [56]. To meet these challenges, many attempts have been made to design composite electrode materials consisting of conducting polymer with either dimensionally stable material with large surface area such as graphene or other types of pseudocapacitive materials such as transition metal oxides [6, 57,58,59,60,61].
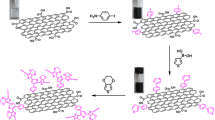
In this work, we focused on the preparation of poly(o-phenylenediamine)/graphene oxide (PoPDA/GO) composite electrodes and on the investigation of their properties especially in the context of their application in supercapacitors. There are a couple of different approaches for the preparation of such electrodes. First, aromatic amine-based polymers can be formed either by their in situ polymerization in the presence of graphene or GO [62,63,64,65] or by anodic oxidation of o-PDA on the GO- or rGO-modified electrodes [62, 63]. Depending on the porosity of underlying GO, the produced electrode would rather have bilayer characteristics.
Another approach is to use various oxygen functionalities of GO for the chemical attachment of o-PDA monomer via its aromatic amine and its subsequent polymerization. Aromatic amines readily react with oxygen functional groups of GO producing various functional species [66,67,68]. o-PDA monomer is particularly of interest for performing these reactions since if one amine group is used for the formation of amide bonds with GO, the remaining amine could preserve extended π aromatic conjugation which is an important requisite for its oxidation followed by the polymerization. However, due to the surface confinement of this process, the resulting chain lengths are expected to be shorter compared to the PoPDA obtained by the direct anodic oxidation of monomers in solution.
Finally, there is a possibility that redox active groups at the surface of GO participate directly in the redox reaction with o-PDA resulting in rGO and PoPDA deposited on the electrode [67, 69, 70]. This route would be favourable from the supercapacitor application standpoint since both rGO providing high surface double layer charge and PoPDA contributing with its pseudocapacitive charge would be formed in situ in a simple one-step process [63].
There have been several attempts in the literature to synthesize GO/PoPDA composite material aimed for different applications [4, 71,72,73]. Lu et al. [71] reported about the preparation and supercapacitor performance of N-doped graphene using o-PDA as the double-N precursor. They obtained good electrochemical behaviour of the resulting material with high specific capacitance and cycling stability.
Experimental
Materials
The graphene oxide (GO—4 mg mL−1) water dispersion was prepared from natural graphite powders by a Hoffman’s method as described elsewhere [33, 74]. o-PDA (Alfa Aesar), methanol (Gram-mol), ethanol (Gram-mol), sulphuric acid (Lach-Ner), perchloric acid (Fisher Chemical) and sodium sulphate (Lach-Ner) were of analytical grade and were used without further purification.
Electrode preparations
GO-PoPDA was prepared on the quartz crystal gold electrode (RenLux Crystal Co., Ltd, China) in order to be simultaneously investigated by cyclic voltammetry and electrochemical quartz crystal microbalance (EQCM).
The gold EQCM electrodes were first washed with ethanol, acetone and bi-distilled water and after that electrochemically cycled in 0.1 mol dm−3 HClO4 in potential range from − 0.3 to 1.3 V (vs. SCE) at 100 mV s−1 until a stable CV scan was achieved. An aqueous/ethanol (1:5 v/v) dispersion of pristine GO sheets (0.7 mg mL−1) was prepared by sonication for 15 min. A 500 µL of obtained dispersion was spin coated on an Au EQCM electrode that was allowed to dry at room temperature and open air to form an Au/GO electrode. Mass of the GO applied on the electrode was calculated from the quartz oscillation frequency change according to the Sauerbrey equation. The currents obtained for all GO electrodes investigated in this work are expressed as specific currents, Is/A g−1, in regard to GO mass.
The incubation of o-PDA on the GO was carried out by immersion of Au/GO electrodes in water/methanol (9:1 v/v) (GO-PoPDA-w) or 1 mol dm−3 H2SO4 solution (GO-PoPDA-s) both containing 0.1 mol dm−3 of o-PDA monomer at room temperature (25 °C). After 24 h of incubation period, electrodes were vigorously rinsed with water and methanol and immersed for 2 h in methanol to dissolve loosely bounded monomer or possibly existing low molecular mass products. After incubation, electrodes were tested by cyclic voltammetry from − 0.2 to 0.6 V during 2 cycles and from − 0.2 to 0.8 V during 100 cycles in 1 mol dm−3 H2SO4.
Measurements
The electrochemical deposition of polymer layers either on Au (PoPDA) or Au/GO (GO-PoPDA-ec1) electrode was performed by cyclic voltammetry from 0.1 mol dm−3 o-PDA in 1 mol dm−3 H2SO4 aqueous solutions at 20 °C during 100 consecutive cycles in potential range from − 0.2 to 0.8 V at ν = 50 mV s−1. Also, an experiment with Au/GO, (GO-PoPDA-ec2) electrode was performed during 200 consecutive cycles in potential range from − 0.2 to 0.3 V at ν = 50 mV s−1. The usual three-electrode setup was used with EQCM Au disc (A = 1.22 cm2) as a working electrode, saturated calomel electrode (SCE) as reference and Pt sheet as a counter electrode. All potentials in this paper are reported versus saturated calomel electrode (SCE), and experiments were carried out using potentiostat (EG&G Princeton Applied Research, model 263A).
For the EQCM measurements, the frequency of the quartz crystal coated with gold was monitored by a Stanford Research System QCM 200 quartz crystal microbalance connected to the potentiostat. The fundamental frequency was 5 MHz, and the integral sensitivity was 4.85 × 10−7 Hz cm2 g−1. The area of the working electrode was 1.22 cm2 , and piezoelectrically active area was 0.427 cm2.
FTIR-ATR spectra of prepared electrodes were recorded using a Fourier Transform-Infrared Attenuated Total Reflection PerkinElmer UATR Two spectrometer in the range 650–4000 cm−1.
The UV–Vis spectra were recorded on AvaSpec-ULS2048L using UV source Model D 1000 CE, Analytical Instrument Systems Inc., USA. The samples for UV–Vis were prepared by coating of GO on a quartz slides. In order to obtain layers of GO-PoPDA-w and GO-PoPDA-s, GO/quartz layers were treated in a same way as reported for EQCM Au disc electrode.
SEM microphotographs of samples prepared on EQCM Au disc electrodes were taken by JEOL JSM 7000F at accelerating voltage of 5 kV.
Results and discussion
Mechanism of the anodic oxidation of o-PDA at metallic electrodes is well established and documented in the number of papers [75,76,77,78,79]. As shown in a set of cyclic voltammograms given in Fig. 1a, o-PDA oxidation at gold electrode commences at potentials of about 0.5 V resulting in the sharp current rise. The oxidation potential of o-PDA is lower than the oxidation potential of aniline due to the strong positive resonance effect second amine group exerts on the aromatic ring. The oxidation current slowly decreases with the number of cycles forming one well-defined irreversible current peak at 0.612 V. The current peak height continues to decrease with the number of cycles indicating the inhibiting properties of deposited PoPDA. The rate of PoPDA formation is slower than the rate of analogous polyaniline formation probably because an autocatalytic growth is much stronger in the case of polyaniline. PoPDA redox transformations take place in the potential range from − 0.2 to 0.2 V and can be ascribed to either phenazine-like or polyaniline-like structures of the deposited polymer [4, 75, 79]. The coexistence of the two structures is also possible since at least two distinct current waves can be discerned in both cathodic and anodic potential excursions, although the two peaks are better separated and defined in cathodic regions.
When the anodic formation of PoPDA was attempted at GO-covered gold electrode, quite a different behaviour was observed on cyclic voltammograms with several distinct features (Fig. 1b). Although a negligible o-PDA oxidation current was observed at high anodic potentials, the formation of the PoPDA layer follows almost linear relationship with the number of cycles. In addition to the redox transformations of PoPDA which take place in the same potential range as those of the polymer layer deposited on the gold electrode, the most striking feature of the recorded cyclic voltammograms is that polymer redox transformations are not limited only to the potentials between − 0.2 and 0.2 V but almost constant oxidation/reduction current is observed throughout the whole investigated potential range. This means that the PoPDA redox sites are energetically distributed giving rise to the considerable pseudocapacitance of this material. Also, one close to reversible current peak pair appeared at potentials of about 0.7 V and the other at potential 0.45 V. The peaks remained after the electrode was transferred to the monomer-free solution indicating that they arise from the oxidation/reduction of the species firmly attached to the electrode. Since the potentials of the current peaks are much higher than those of the polymer itself, most probably they are the result of dimeric or oligomeric species of o-PDA.
The lack of o-PDA monomer oxidation current at high anodic potentials at Au/GO electrode indicates that the formation of PoPDA layer at or within GO might be a spontaneous reaction. Taking into account various redox states of oxygen functional groups at the surface of GO, the heterogeneous redox reaction between GO and o-PDA might occur. As a consequence, the formation of rGO and various forms of PoPDA might be expected. To test this hypothesis, the cyclic voltammetry experiment from Fig. 1b was carried out to the final potential of 0.3 V only and the results are presented in Fig. 2. The significant current increase was registered with the number of cycles especially in the potential range of PoPDA redox transformation below 0 V indicating spontaneous formation of the polymer on GO surface without initial monomer oxidation.
Additional tests were performed by immersing freshly prepared GO electrodes in the monomer solution and incubated overnight as described in the experimental section. Figure 3 shows first recorded cyclic voltammograms of two incubated electrodes, one incubated in the monomer solution of sulphuric acid (GO-PoPDA-s) and another in the water/methanol solution (GO-PoPDA-w) in comparison with the background current at GO base electrode. Obtained current profiles on cyclic voltammograms demonstrate PoPDA formation which means the layer of PoPDA was formed by chemical interaction of o-PDA and GO. Therefore, apart from PoPDA, reduced form of graphene (rGO) is also present at the surface of electrode. The current increase within the potential range of investigation partially should be assigned also to capacitive currents of rGO.
It looks like that PoPDA formation is favoured in neutral medium since much higher currents were registered (Fig. 3). Also, it seems that the nature of deposited polymer differs. On repetitive cycling involving anodic potentials higher than monomer oxidation potentials, the polymer formed from neutral solutions gradually dissolves or decomposes and the oxidation/reduction currents especially those below 0 V decrease (Fig. 4b), while the polymer obtained from sulphuric acid solution is not only stable at prolonged cycling but the current increases indicating additional polymer formation (Fig. 4a) although the measurements were conducted in monomer-free solution. The influence of the incubation medium on the quantity of deposited polymer might be caused by the pH dependence of the o-PDA oxidation potential due to the involvement of protons in the redox reaction. Chemical interaction between GO and o-PDA and the spontaneous PoPDA formation is thermodynamically favoured in neutral medium, and the reaction proceeds to the completion more readily than in acidic solution where a significant amount of unreacted monomer or dimeric species remained entrapped in the film and available for further electrochemical oxidation.
To confirm the existence of the spontaneous redox reaction between o-PDA and GO, FTIR spectra of the incubated electrodes were taken and compared to the spectra of the same electrodes after their cycling through the 100 consecutive cyclic voltammetry cycles.
The FTIR spectra of GO, GO-PoPDA-s and GO-PoPDA-w are shown in Fig. 5. The influence of pH on oxidation of o-PDA leads to formation of different polymer structures consisting of phenazine units or linear polymers containing free amine groups [80].
FTIR spectrum corresponding to GO shows broad absorption band in the 3600–2500 cm−1 region assigned to –OH stretching mode of mostly water incorporated within GO structure, but also to alkoxy groups present within the graphene structure. Characteristic absorption band for GO at 1734 cm−1 is assigned to carbonyl group originating from carboxyl, aldehydes or ketones. The band at 1631 cm−1 is assigned to asymmetric C=C stretching and –OH bending modes of water physisorbed on the GO [81]. Additional band ascribed to –OH deformation was registered at 1410 cm−1. The absorption bands at 1259 cm−1 and 1047 cm−1 are commonly assigned to epoxy and alkoxy C-O stretching vibrations, respectively. After modification of GO with o-PDA, some of the GO bands decreased indicating removal of different oxygen groups [82], while another bands appeared in spectrum after modification with o-PDA. The strongest peak observed in GO spectrum at 1631 cm−1 disappeared almost completely after incubation process and shifted towards lower wave numbers, to the value of 1620 cm−1. This signifies higher symmetry of C=C bond in incubated samples indicating that during incubation process, oxygen groups responsible for asymmetric vibrations were removed [83]. Probably, process is connected with sp2 restoration on epoxy functional groups by NH2-group of aromatic amine which is in accordance with the literature [37]. Very weak intensity registered for bands at 1532, 1565, 750 and 840 cm−1 suggests the existence of low fraction of phenazine-like structure or linear-polyaniline-like structure in the film [80]. The intense band at 1532 cm−1 and shoulder at 1565 cm−1 confirm the presence of benzenoid or quinoid structures [80, 84]. The bands at 750 and 840 cm−1 are characteristic for out of plane C–H deformation, and it shows different coupling of the aromatic ring suggesting the presence of linear and branched structure. 1,4-coupling (polyaniline-like structure) results in 840 cm−1 band, and 1,3-coupling (phenazine structure) results in 750 cm−1 band. By comparing these two bands, it is evident that 750 cm−1 band was more pronounced in as-prepared GO-PoPDA-w sample in comparison with GO-PoPDA-w recorded after 100 cycles. It suggests that larger amount of the phenazine structures is present within as-prepared GO-PoPDA-w sample which is in correlation with electrochemical measurements. The intensity of these two bands was unchanged for GO-PoPDA-s before and after 100 cycles indicating stable structure with no significant change in spectrum. This is supported by cyclic voltammograms that show similar shape before and after 100 cycles. The band at 1042 cm−1 corresponds to GO structure, and therefore, by comparing the intensity of 750/840 cm−1 bands and 1042 cm−1 band, it is possible to conclude about the amount of conducting polymer within GO structure. The obtained ratio indicates that the higher amount of conducting polymer is present within the as-prepared GO-PoPDA-w compared to GO-PoPDA-w after 100 cycles. Contrary to this result, the as-prepared GO-PoPDA-s contained smaller quantity of conductive polymer. The similar observation was evident from cyclic voltammograms (Fig. 4).
The morphology of the obtained layers was investigated with SEM, and obtained micrographs are shown in Fig. 6. The surface of incubated GO-PoPDA-w is covered by a large number of uniform needles (Fig. 6b). Needle formations in the case of o-PDA have already been observed by other authors [85,86,87]. They were ascribed to low molecular mass products containing 2–7 phenazine monomer units [86]. Kar et al. [87] have also obtained low molecular mass products in the medium of higher pH values, while polymer formation ensued at pHs 1–2. After electrode cycling (Fig. 6c), the change in needle-like structures is evident. On the other hand, GO-PoPDA-s layer does not show any distinct feature different from GO (Fig. 6d, e). Since current response in the cyclic voltammograms indicates the presence of conducting polymer, it follows that polymer formation takes place between the GO layers and not on the outside surface of GO. This phenomenon could be related to higher solubility of ortho-phenylenediamine monomers or oligomers in acid medium.
UV–Vis spectroscopy was used to probe the electronic transitions in the investigated layers. The spectra of GO, GO-PoPDA-w and GO-PoPDA-s electrodes are shown in Fig. 7. Two characteristic absorption peaks were obtained for GO, the absorption maximum at 250 nm and characteristic shoulder around 300 nm. Short wavelength absorption corresponds to π–π* transition in delocalized graphene structure, while 300 nm wavelength absorption corresponds to n–π* transition characteristic for oxygen functionalities, and it proves the presence of oxygen within the graphene oxide structure. After modification of GO surface, the absorption maximum attributed to π–π* transition is red-shifted from 250 to 270 nm indicating lower energy transition that is characteristic of reduced GO. It is also evident that for modified GO the characteristic shoulder at 300 nm has disappeared due to the decreased number of oxygen functionalities. For modified layers, additional absorption maximum was observed at 450 nm due to excitonic transition in quinoid-imine for GO-PoPDA-w and at 500 nm for GO-PoPDA-s due to polaronic transitions of sulphuric acid doped polymer.
Mass changes of PoPDA film at gold electrode taken during oxidation/reduction reaction are given in Fig. 8a, simultaneously with the corresponding cyclic voltammogram. The obtained mass changes were in accordance with those of PoPDA films already described in the literature [78, 79]. As demonstrated by Inzelt et al. [78], at the beginning of potential excursion, mass increase takes place which is followed by a continuous mass decrease during the rest of anodic cycle. In the range of potentials where mass increase was observed, probably some negative counter-ion influx takes place, while at later stages deprotonation of the film follows with concomitant solvent expulsion. In the reverse scan, a significant hysteresis was observed which can be explained by kinetically hindered swelling/deswelling together with the accompanying structural changes [78]. Indeed, although the overall mass change is negligible at the potentials higher than 0.3 V where no oxidation current appears, the amu values calculated from mass-charge plot (Fig. 8b) reveal that the film continues to lose weight (− 68.3). The amu values calculated from the linear segments of the mass-charge curves are given at the corresponding potential ranges at cyclic voltammograms (Fig. 8a).
Continuous mass decrease throughout the whole anodic potential excursion as well as mass gain in the reverse cathodic cycle was registered for the PoPDA formed on the GO support and investigated in sulphuric acid electrolyte (Fig. 9a). In Fig. 9b, corresponding mass-charge plot was displayed revealing the hysteresis similarly to the hysteresis of PoPDA film formed at gold electrode (Fig. 8a). By transferring the electrode to neutral Na2SO4 electrolyte, electroactivity of the film was preserved but the mass change hysteresis disappeared (Fig. 10a, b). Obviously, higher degree of reversibility was obtained in neutral medium.
The facilitated hydration of the GO-PoPDA-s film in neutral solutions is even more obvious by monitoring mass changes during several consecutive cyclic voltammetry cycles in 0.5 M Na2SO4 (Fig. 11). With each cycle, overall mass of the film increases but the increase is much more pronounced in neutral media. The hydration process might be responsible for better reversibility of redox reaction.
Conclusions
It is possible to prepare graphene oxide/poly(o-phenylenediamine) composite material with increased pseudocapacitive properties over the wide potential range compared to pure poly(o-phenylenediamine). The composite can be formed either by electrochemical oxidation of o-phenylenediamine on graphene oxide-covered electrode or by spontaneous heterogeneous redox reaction between graphene oxide and monomer resulting in the reduced graphene oxide and poly(o-phenylenediamine). The response of the composite material is stable, and the experiments demonstrated the great potentials for its utilization in supercapacitors.
References
Jokar E, Shahrokhian S, Zad AI (2014) Electrochemical functionalization of graphene nanosheets with catechol derivatives as an effective method for preparation of highly performance supercapacitors. Electrochim Acta 147:136–142. https://doi.org/10.1016/j.electacta.2014.09.102
Mao L, Zhang K, On Chan HS, Wu J (2012) Surfactant-stabilized graphene/polyaniline nanofiber composites for high performance supercapacitor electrode. J Mater Chem 22:80–85. https://doi.org/10.1039/C1JM12869H
Zhang H, Feng J, Fei T et al (2014) SnO2 nanoparticles-reduced graphene oxide nanocomposites for NO2 sensing at low operating temperature. Sens Actuators B Chem 190:472–478. https://doi.org/10.1016/j.snb.2013.08.067
Nguyen VH, Tran TH, Shim JJ (2014) Glassy carbon electrode modified with a graphene oxide/poly(o-phenylenediamine) composite for the chemical detection of hydrogen peroxide. Mater Sci Eng, C 44:144–150. https://doi.org/10.1016/j.msec.2014.08.028
Sultana I, Rahman MM, Wang J et al (2012) All-polymer battery system based on polypyrrole (PPy)/para (toluene sulfonic acid) (pTS) and polypyrrole (PPy)/indigo carmine (IC) free standing films. Electrochim Acta 83:209–215. https://doi.org/10.1016/j.electacta.2012.08.043
Li S, Zhu F, Meng F et al (2013) Separation of graphene oxide by density gradient centrifugation and study on their morphology-dependent electrochemical properties. J Electroanal Chem 703:135–145. https://doi.org/10.1016/j.jelechem.2013.05.020
Das SR, Srinivasan S, Stromberg L et al (2017) Superhydrophobic inkjet printed flexible graphene circuits via direct-pulsed laser writing. Nanoscale 9:19058–19065. https://doi.org/10.1039/C7NR06213C
Han TH, Kim H, Kwon SJ, Lee TW (2017) Graphene-based flexible electronic devices. Mater Sci Eng R Rep 118:1–43. https://doi.org/10.1016/j.mser.2017.05.001
Bonaccorso F, Sun Z, Hasan T, Ferrari AC (2010) Graphene photonics and optoelectronics. Nat Photon 4:611–622. https://doi.org/10.1038/nphoton.2010.186
Lee C, Wei X, Kysar JW, Hone J (2008) Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science (80-) 321:385–388. https://doi.org/10.1126/science.1157996
Papageorgiou DG, Kinloch IA, Young RJ (2017) Mechanical properties of graphene and graphene-based nanocomposites. Prog Mater Sci 90:75–127. https://doi.org/10.1016/j.pmatsci.2017.07.004
Peres NMR, Guinea F, Castro Neto AH (2006) Electronic properties of disordered two-dimensional carbon. Phys Rev B Condens Matter Mater Phys 73:125411. https://doi.org/10.1103/PhysRevB.73.125411
Neto AHC, Guinea F, Peres NMR et al (2007) The electronic properties of graphene. Rev Mod Phys 81:109–162. https://doi.org/10.1103/RevModPhys.81.109
Balandin AA, Ghosh S, Bao W et al (2008) Superior thermal conductivity of single-layer graphene. Nano Lett 8:902–907. https://doi.org/10.1021/nl0731872
Kim TY, Park C-H, Marzari N (2016) The electronic thermal conductivity of graphene. Nano Lett 16:2439–2443. https://doi.org/10.1021/acs.nanolett.5b05288
Becerril HA, Mao J, Liu Z et al (2008) Evaluation of solution-processed reduced graphene oxide films as transparent conductors. ACS Nano 2:463–470. https://doi.org/10.1021/nn700375n
Wang X, Zhi L, Müllen K (2008) Transparent, conductive graphene electrodes for dye-sensitized solar cells. Nano Lett 8:323–327. https://doi.org/10.1021/nl072838r
Tsai HL, Hsieh C-T, Li J, Gandomi YA (2018) Enabling high rate charge and discharge capability, low internal resistance, and excellent cycleability for Li-ion batteries utilizing graphene additives. Electrochim Acta 273:200–207. https://doi.org/10.1016/j.electacta.2018.03.154
Xia J, Chen F, Li J, Tao N (2009) Measurement of the quantum capacitance of graphene. Nat Nanotechnol 4:505–509. https://doi.org/10.1038/nnano.2009.177
Stoller MD, Park S, Yanwu Z et al (2008) Graphene-based ultracapacitors. Nano Lett 8:3498–3502. https://doi.org/10.1021/nl802558y
Kannappan S, Kaliyappan K, Manian RK et al (2013) Graphene based supercapacitors with improved specific capacitance and fast charging time at high current density. arXiv:1311.1548
Huang H, Chen W, Chen S, Wee ATS (2008) Bottom-up growth of epitaxial graphene on 6H-SiC(0001). ACS Nano 2:2513–2518. https://doi.org/10.1021/nn800711v
Xu X, Zhang Z, Dong J et al (2017) Ultrafast epitaxial growth of metre-sized single-crystal graphene on industrial Cu foil. Sci Bull 62:1074–1080. https://doi.org/10.1016/j.scib.2017.07.005
Chen X, Zhang L, Chen S (2015) Large area CVD growth of graphene. Synth Met 210:95–108. https://doi.org/10.1016/j.synthmet.2015.07.005
Poh HL, Šaněk F, Ambrosi A et al (2012) Graphenes prepared by Staudenmaier, Hofmann and Hummers methods with consequent thermal exfoliation exhibit very different electrochemical properties. Nanoscale 4:3515. https://doi.org/10.1039/c2nr30490b
Ambrosi A, Chua CK, Bonanni A, Pumera M (2012) Lithium aluminum hydride as reducing agent for chemically reduced graphene oxides. Chem Mater 24:2292–2298. https://doi.org/10.1021/cm300382b
Marcano DC, Kosynkin DV, Berlin JM et al (2010) Improved synthesis of graphene oxide. ACS Nano 4:4806–4814. https://doi.org/10.1021/nn1006368
Gao X, Jang J, Nagase S (2010) Hydrazine and thermal reduction of graphene oxide: reaction mechanisms, product structures, and reaction design. J Phys Chem C 114:832–842. https://doi.org/10.1021/jp909284g
Xu Y, Sheng K, Li C, Shi G (2010) Self-assembled graphene hydrogel. ACS Nano 4:4324–4330
Wei M, Qiao L, Zhang H et al (2017) Engineering reduced graphene oxides with enhanced electrochemical properties through multiple-step reductions. Electrochim Acta 258:735–743. https://doi.org/10.1016/j.electacta.2017.11.120
Lindfors T, Österholm A, Kauppila J, Pesonen M (2013) Electrochemical reduction of graphene oxide in electrically conducting poly(3,4-ethylenedioxythiophene) composite films. Electrochim Acta 110:428–436. https://doi.org/10.1016/j.electacta.2013.03.070
Shao Y, Wang J, Engelhard M et al (2010) Facile and controllable electrochemical reduction of graphene oxide and its applications. J Mater Chem 20:743–748. https://doi.org/10.1039/B917975E
Ambrosi A, Bonanni A, Sofer Z et al (2011) Electrochemistry at chemically modified graphenes. Chem A Eur J 17:10763–10770. https://doi.org/10.1002/chem.201101117
Wang J, Manga KK, Bao Q, Loh KP (2011) High-yield synthesis of few-layer graphene flakes through electrochemical expansion of graphite in propylene carbonate electrolyte. J Am Chem Soc 133:8888–8891. https://doi.org/10.1021/ja203725d
Zhang M, Gao B, Vanegas DC et al (2014) Simple approach for large-scale production of reduced graphene oxide films. Chem Eng J 243:340–346. https://doi.org/10.1016/j.cej.2014.01.019
Huang NM, Lim HN, Chia CH et al (2011) Simple room-temperature preparation of high-yield large-area graphene oxide. Int J Nanomed 6:3443–3448. https://doi.org/10.2147/IJN.S26812
Pei S, Cheng HM (2012) The reduction of graphene oxide. Carbon N Y 50:3210–3228. https://doi.org/10.1016/j.carbon.2011.11.010
Xu C, Yuan RS, Wang X (2014) Selective reduction of graphene oxide. Xinxing Tan Cailiao/New Carbon Mater 29:61–66. https://doi.org/10.1016/S1872-5805(14)60126-8
Solís-Fernández P, Rozada R, Paredes JI et al (2012) Chemical and microscopic analysis of graphene prepared by different reduction degrees of graphene oxide. J Alloys Compd 536:S532–S537. https://doi.org/10.1016/j.jallcom.2012.01.102
Cheng M, Yang R, Zhang L et al (2012) Restoration of graphene from graphene oxide by defect repair. Carbon N Y 50:2581–2587. https://doi.org/10.1016/j.carbon.2012.02.016
Araujo PT, Terrones M, Dresselhaus MS (2012) Defects and impurities in graphene-like materials. Mater Today 15:98–109
Chua CK, Ambrosi A, Sofer Z et al (2014) Back cover: chemical preparation of graphene materials results in extensive unintentional doping with heteroatoms and metals (Chem Eur J 48/2014). Chem A Eur J 20:16008. https://doi.org/10.1002/chem.201490201
Kuila T, Bose S, Mishra AK et al (2012) Chemical functionalization of graphene and its applications. Prog Mater Sci 57:1061–1105. https://doi.org/10.1016/j.pmatsci.2012.03.002
Georgakilas V, Otyepka M, Bourlinos AB et al (2012) Functionalization of graphene: covalent and non-covalent approaches, derivatives and applications. Chem Rev 112:6156–6214. https://doi.org/10.1021/cr3000412
Ioniţă M, Vlăsceanu GM, Watzlawek AA et al (2017) Graphene and functionalized graphene: extraordinary prospects for nanobiocomposite materials. Compos Part B Eng 121:34–57. https://doi.org/10.1016/j.compositesb.2017.03.031
Gong X, Liu G, Li Y et al (2016) Functionalized-graphene composites: fabrication and applications in sustainable energy and environment. Chem Mater 28:8082–8118. https://doi.org/10.1021/acs.chemmater.6b01447
Scrosati B (1989) Conducting polymers and their applications. Mater Sci Forum 42:207–220. https://doi.org/10.4028/www.scientific.net/MSF.42.207
Wolfart F, Hryniewicz BM, Góes MS et al (2017) Conducting polymers revisited: applications in energy, electrochromism and molecular recognition. J Solid State Electrochem 21:2489–2515. https://doi.org/10.1007/s10008-017-3556-9
Santana ACO, Southgate EF, Mendes JPBG et al (2014) Characterization of an HRP–AOX–polyaniline–graphite composite biosensor. J Electrochem Sci Eng 4:165–175. https://doi.org/10.5599/jese.2014.0057
Popli SA, Patel UD (2015) Electrochemical decolorization of Reactive Black 5 in an undivided cell using Ti and graphite anodes: effect of polypyrrol coating on anodes. J Electrochem Sci Eng 5:145–156. https://doi.org/10.5599/jese.164
Inzelt G (2017) Recent advances in the field of conducting polymers. J Solid State Electrochem 21:1965–1975. https://doi.org/10.1007/s10008-017-3611-6
Inzelt G (2008) Conducting polymers: a new era in electrochemistry, 1st edn. Springer, Berlin
Inzelt G (2017) Conducting polymers: past, present, future. J Electrochem Sci Eng 8:3–37. https://doi.org/10.5599/jese.448
Mandić Z, Roković MK, Pokupčić T (2009) Polyaniline as cathodic material for electrochemical energy sources. The role of morphology. Electrochim Acta 54:2941–2950. https://doi.org/10.1016/j.electacta.2008.11.002
Zhang J, Zhao XS (2012) Conducting polymers directly coated on reduced graphene oxide sheets as high-performance supercapacitor electrodes. J Phys Chem C 116:5420–5426. https://doi.org/10.1021/jp211474e
Wang L, Ye Y, Lu X et al (2013) Hierarchical nanocomposites of polyaniline nanowire arrays on reduced graphene oxide sheets for supercapacitors. Sci Rep 3:3568. https://doi.org/10.1038/srep03568
Chen S, Zhu J, Wu X et al (2010) Graphene oxide–MnO2 nanocomposites for supercapacitors. ACS Nano 4:2822–2830. https://doi.org/10.1021/nn901311t
Siwal S, Ghosh S, Nandi D et al (2017) Synergistic effect of graphene oxide on the methanol oxidation for fuel cell application. Mater Res Express 4:95306. https://doi.org/10.1088/2053-1591/aa8a88
Sopcic S, Kraljic Rokovic M, Mandic Z (2012) Preparation and characterization of RuO2/polyaniline/polymer binder composite electrodes for supercapacitor application. J Electrochem Sci Eng 14:2021–2026. https://doi.org/10.5599/jese.2012.0010
Mahla DK, Bhandari S, Rahaman M, Khastgir D (2013) Morphology and cyclic voltammetry analysis of in situ polymerized polyaniline/graphene composites. J Electrochem Sci Eng 3:157–166. https://doi.org/10.5599/jese.2013.0038
Zou W-J, Mo S-S, Zhou S-L et al (2011) Preparation of mesoporous carbon/polypyrrole composite materials and their supercapacitive properties. J Electrochem Sci Eng 1:67–73. https://doi.org/10.5599/jese.2011.0001
Pisarevskaya EY, Ehrenburg MR, Ovsyannikova EV et al (2017) Studies of chemical stage of synthesis of electroactive composite based on poly-o-phenylenediamine and graphene oxide. Russ J Electrochem 53:70–77. https://doi.org/10.1134/S1023193517010104
Pisarevskaya EY, Efimov ON, Ehrenburg MR, Andreev VN (2015) Preparation of electrode material with developed surface and pronounced electroactivity by modification of glassy carbon by graphene oxide and poly-o-phenylenediamine. Prot Met. Phys Chem Surf 51:980–984. https://doi.org/10.1134/S2070205115060180
Imran SM, Kim Y, Shao GN et al (2014) Enhancement of electroconductivity of polyaniline/graphene oxide nanocomposites through in situ emulsion polymerization. J Mater Sci 49:1328–1335. https://doi.org/10.1007/s10853-013-7816-5
Konwer S, Guha AK, Dolui SK (2013) Graphene oxide-filled conducting polyaniline composites as methanol-sensing materials. J Mater Sci 48:1729–1739. https://doi.org/10.1007/s10853-012-6931-z
Xu LQ, Liu YL, Neoh KG et al (2011) Reduction of graphene oxide by aniline with its concomitant oxidative polymerization. Macromol Rapid Commun 32:684–688. https://doi.org/10.1002/marc.201000765
Vacchi IA, Spinato C, Raya J et al (2016) Chemical reactivity of graphene oxide towards amines elucidated by solid-state NMR. Nanoscale 8:13714–13721. https://doi.org/10.1039/C6NR03846H
Yang W, Zhou H, Huang Z et al (2017) In situ growth of single-stranded like poly (o-phenylenediamine) onto graphene for high performance supercapacitors. Electrochim Acta 245:41–50. https://doi.org/10.1016/j.electacta.2017.05.088
Su C, Acik M, Takai K et al (2012) Probing the catalytic activity of porous graphene oxide and the origin of this behaviour. Nat Commun 3:1298. https://doi.org/10.1038/ncomms2315
Verma S, Mungse HP, Kumar N et al (2011) Graphene oxide: an efficient and reusable carbocatalyst for aza-Michael addition of amines to activated alkenes. Chem Commun 47:12673. https://doi.org/10.1039/c1cc15230k
Lu Y, Zhang F, Zhang T et al (2013) Synthesis and supercapacitor performance studies of N-doped graphene materials using o-phenylenediamine as the double-N precursor. Carbon N Y 63:508–516. https://doi.org/10.1016/j.carbon.2013.07.026
Mu S (2011) The electrocatalytic oxidative polymerization of o-phenylenediamine by reduced graphene oxide and properties of poly(o-phenylenediamine). Electrochim Acta 56:3764–3772. https://doi.org/10.1016/j.electacta.2011.02.061
Liu X, Zhu H, Yang X (2014) An electrochemical sensor for dopamine based on poly(o-phenylenediamine) functionalized with electrochemically reduced graphene oxide. RSC Adv 4:3743–3749. https://doi.org/10.1039/C3RA45234D
Sačer D, Čapeta D, Šrut Rakić I et al (2016) Tailoring polypyrrole supercapacitive properties by intercalation of graphene oxide within the layer. Electrochim Acta 193:311–320. https://doi.org/10.1016/j.electacta.2016.02.055
Duić L, Kraljić Roković M, Mandić Z (2010) Composite layers consisting of polyaniline and poly(o-phenylenediamine): electrochemical deposition, electrochromic and electrocatalytic properties. Polym Sci Ser B 52:431–437. https://doi.org/10.1134/S1560090410070067
Yano J (1995) Electrochemical and structural studies on soluble and conducting polymer from o-phenylenediamine. J Polym Sci, Part A: Polym Chem 33:2435–2441. https://doi.org/10.1002/pola.1995.080331416
Ujvári M, Láng G, Inzelt G (2000) The problem of the low-frequency dispersion in the case of polymer film electrodes—an experimental impedance study on Au|poly (o-phenylenediamine) electrodes. Electrochem Commun 2:497–502. https://doi.org/10.1016/S1388-2481(00)00067-9
Martinusz K, Czirdk E, Inzelt G (1994) Studies of the formation and redox transformation of poly (o-phenylenediamine) films using a quartz crystal microbalance. J Electroanal Chem 379:437–444
Gharaibeh SA, El Sawy EN, Molero H, Birss VI (2013) Electrochemical and Mass Change Study of the Growth of Poly-(o-Phenylenediamine) Films on Au Substrates. J Electrochem Soc 160:H344–H354. https://doi.org/10.1149/2.098306jes
Palys B, Bokun A, Rogalski J (2007) Poly-o-phenylenediamine as redox mediator for laccase. Electrochim Acta 52:7075–7082. https://doi.org/10.1016/j.electacta.2007.05.029
Dimiev AM, Alemany LB, Tour JM (2013) Graphene oxide. Origin of acidity, its instability in water, and a new dynamic structural model. ACS Nano 7:576–588. https://doi.org/10.1021/nn3047378
Fan X, Peng W, Li Y et al (2008) Deoxygenation of exfoliated graphite oxide under alkaline conditions: a green route to graphene preparation. Adv Mater 20:4490–4493. https://doi.org/10.1002/adma.200801306
Su X, Wang G, Li W et al (2013) A simple method for preparing graphene nano-sheets at low temperature. Adv Powder Technol 24:317–323. https://doi.org/10.1016/j.apt.2012.08.003
Zhang YS, Xu WH, Yao WT, Yu SH (2009) Oxidation-reduction reaction driven approach for hydrothermal synthesis of polyaniline hollow spheres with controllable size and shell thickness. J Phys Chem C 113:8588–8594. https://doi.org/10.1021/jp810491u
Jiang H, Sun X, Huang M et al (2006) Rapid self-assembly of oligo(o-phenylenediamine) into one-dimensional structures through a facile reprecipitation route. Langmuir 22:3358–3361. https://doi.org/10.1021/la053091s
Sestrem RH, Ferreira DC, Landers R et al (2010) Synthesis and spectroscopic characterization of polymer and oligomers of ortho-phenylenediamine. Eur Polym J 46:484–493. https://doi.org/10.1016/J.EURPOLYMJ.2009.12.007
Samanta S, Roy P, Kar P (2015) Influence of pH of the Reaction Medium on the Structure and Property of Conducting Poly(o-Phenylenediamine). Mater Today Proc 2:1301–1308. https://doi.org/10.1016/J.MATPR.2015.07.046
Acknowledgements
The support of Croatian Science Foundation under the Project ESUP-CAP (IP-11-2013-8825) is greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sačer, D., Spajić, I., Kraljić Roković, M. et al. New insights into chemical and electrochemical functionalization of graphene oxide electrodes by o-phenylenediamine and their potential applications. J Mater Sci 53, 15285–15297 (2018). https://doi.org/10.1007/s10853-018-2693-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-018-2693-6