Abstract
Dendrimers have been used to control the pore size and morphology of porous materials during their synthesis. Various characterization techniques have also been used to validate the formation of mesoporosity. Materials such as cetyltrimethylammonium bromide (CTAB) and other co-polymers are commonly used as templates for the synthesis of mesoporous materials. However, advantages of using dendrimers as templates for the synthesis of mesoporous materials include: (1) ease of control of the final pore size (depending on the dendrimer employed); (2) ease of removal of the dendrimer template by a simple extraction method or calcination process, which does not strongly interact with the inorganic species; (3) the monodispersed structure of the dendrimer leads to the formation of monodispersed pores with a narrow size distribution; and (4) the synthetic process require room (or relatively low) temperatures as opposed to elevated temperatures used for other surfactants. This mini-review is therefore focussed on the use of dendrimers as templating or pore-directing agents for the synthesis of micro- and mesoporous materials. The catalytic application of the mesoporous materials as heterogeneous supports is also discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dendrimers are well-defined, monodispersed, tree-like, three-dimensional macromolecular structures characterized by a high density of peripheral groups [1]. They are composed of three main distinguishing architectural components: (a) an interior core; (b) interior layers (generations), which are made up of repeating branching units attached to the initiator core; and (c) the exterior (periphery) attached to the outermost interior generation (Fig. 1) [2, 3].
Dendrimers can be synthesized by either divergent or convergent methods. In the divergent approach, dendrimers are produced through an iterative sequence of reaction steps, where additional iterations lead to higher generations. The first example of a divergent approach for the synthesis of a well-defined, branched structure was reported by Vögtle et al. in 1978 [4], who called it “cascade synthesis”. In 1985, Tomalia et al. reported a modification of this procedure to synthesize independent, divergent and macromolecular “true dendrimers” in the form of poly(amidoamide) (PAMAM) dendrimers [5]. In the same year, Newkome et al. [6] reported the preparation of “arborols” (a synonym for dendrimers). In contrast to the divergent method, the convergent method involves the construction of the dendrimer from the surface and inwards towards the core using a two-stage process. The two or more dendritic segments or dendrons synthesized in the first stage are simply joined together in the second stage to create the final and complete dendrimer product. The first convergent synthetic approach for accessing dendritic macromolecules was reported in 1990 by Hawker and Fréchet [7].
Dendrimers have successfully been used as templates for the synthesis of metal nanoparticles, which are often referred to as dendrimer-encapsulated nanoparticles (DENs) [3, 8, 9]. Researchers have also employed dendrimers as templating or pore-directing agents in the synthesis of porous materials [10, 11]. A summary of different types of dendrimers that have been used for the synthesis of porous silica materials is outlined in Table 1.
Traditionally, porous silicas, aluminosilicates [22, 23] and other oxides [24] were prepared in the presence of micelles as structure directing agents. Mesoporous materials are defined as those materials that contain a pore with a diameter of between 2 and 50 nm, while microporous materials have a pore diameter of less than 2 nm. Highly ordered mesoporous silica materials were first discovered by scientists at the Mobil Corporation in 1992 [23]. The first reported so-called Mobil Crystalline Material abbreviated as MCM-41 was micrometre-sized and possessed hexagonally ordered mesopores [22]. These particles had a variable morphology with a very small amount of hexagonally shaped nanoparticles. Due to the large surface area (700–1500 m2/g), narrow pore-size distribution (ranging from 2 to 10 nm), high chemical and thermal stability and the ease of silica functionalization, these materials were considered ideal as supports for adsorption, catalysis, chemical separations and biotechnology devices. However, one of the biggest challenges encountered during the synthesis of these porous materials was the ability to control pore structure and pore size. Template chemistry aims to address this challenge by enabling the synthesis of tailored micro- [25] and mesoporous [23] silica structures through the use of surfactant micellar structures and long-chain alkyl-amine templates [26]. Since the discovery of these materials, research efforts have been initiated to achieve control over other physicochemical properties of mesoporous silicas, especially surface area, pore size and morphology. Through such research efforts, other families of mesoporous materials such as Santa Barbra Amorphous (SBA) [24], Michigan State University (MSU) [27], and Folded Sheet Mesoporous (FSM) [28] were discovered. Since their discovery, mesoporous materials have found application in areas such as catalysis [29, 30] and hydrogen storage [31]. To the best of our knowledge, a review of the use of dendrimers as templating or pore-directing agent for the synthesis of micro- and mesoporous material has, at the time of preparation of this mini-review, not appeared in the literature. To this end, this review focused on the use of dendrimers as templates or pore-directing agents for accessing micro- and mesoporous materials.
Dendrimers as templating agent for the synthesis of micro- and mesoporous silica materials
Synthesis of disordered/ordered mesoporous silica materials
As mentioned previously, dendrimers have been employed as template for the synthesis of colloidal metal nanoparticles [3]. However, lately, dendrimers have also been successfully used as templates for the control of pore size and morphology during the synthesis of porous silica materials [11, 14]. For example, Chujo et al. [14] prepared porous silica using the acid-catalysed sol–gel reaction of tetramethoxysilane (TMOS) in the presence of PAMAM dendrimers (generations 1–5) containing amino and ester end-groups. The same research group has explored other polymers (e.g. polyoxazoline, which were not successful in controlling the pore size [32]. Although the problem of phase separation of the mixture was observed when PAMAM dendrimers) containing primary amino end-groups were used, this was not the case with ester-terminated dendrimers. The resulted polymer hybrids were subjected by pyrolysis at 600 °C to remove organic segments (i.e. the dendrimers). The pore size of the silica gels was found to correlate well with the diameter of the dendrimers template. In this regard, the radius of the generation 3.5 dendrimer of 12.9 Å is similar to that calculated by Tomalia [5, 33]. The resulting porous silica materials had a surface area ranging from 200 to 610 m2/g, making these materials ideal for use as catalyst supports. Similarly, Larsen et al. [11] reported the use of amine-terminated PAMAM dendrimers as templates for the formation of amorphous silica. Two possible pathways for the formation of these materials were cited: (1) depending on the dendrimer size, production of mesopores and micropores with single molecules instead of micelles and (2) spheroidal pores imprinted on the inorganic solids are the expected pore shapes similar to the dendrimer used. When dissolved in an aqueous-organic solution containing silicon sol–gel precursors, these amine-terminated dendrimers were expected to behave in a manner similar to the alkyl-amine templates used by the Mobil researchers for the synthesis of mesoporous silica (MCM-41) and silica-alumina materials. However, given key differences between the dendrimer and alkyl-amine template chemistry, internal hydrocarbon chains should be packed more densely in alkyl-amine templates when compared with dendrimers since the former has greater flexibility relating to micellare diameter. The production of a sol–gel material both from the meso- and microporous structures has also been achieved without the removal of the carbosilane dendrimer template [20]. This motivated Larsen et al. to produce dendrimer-free mesoporous cavities in xerogels using dendrimers as templates. In this study, the sample was prepared by mixing generation 4 PAMAM dendrimers with n-butanol, tetraethyl orthosilicate (TEOS) and HCI, to produce a TEOS/dendrimer molar ratio of approximately 374:1. This mixture was left in a container for 3 days at 343 K to form a white, paste-like product which was dried for a further 3 h at 373 K. The resulting fine powder was placed in a quartz U-tube for a further 1.5 h drying under a nitrogen flow at 803 K. Lastly, the sample was calcined under air flow for 1.5 h at 833 K to remove the dendrimer template. During the temperature programmed drying cycle, alkenes, carbon monoxide, alcohols and water evolved, but this evolution ceased once the 1.5 h temperature plateau of 803 K was reached. Water and carbon monoxide evolved exclusively during the temperature programmed oxidation steps until the final temperature of 833 K was reached, resulting in a carbon/hydrogen-free material. Other methods for template removal (e.g. solvent extraction with Cl2CH2, methanol and ethanol) proved ineffective. Characterization of the synthesized material was performed using XRD spectroscopy and other methods and techniques. The X-ray diffraction patterns for the calcined and uncalcined samples showed low 2θ reflections (see Fig. 2). However, the intensity was significantly less pronounced in the case of the uncalcined sample.

Image reproduced from Ref. [11] with permission from copyright (2000) American Chemical Society
The XRD pattern obtained for the calcined and uncalcined samples.
The 2θ values for the calcined and uncalcined materials were found to be 2.7 and 2.5°, respectively. This was consistent with the notion that contraction of the X-ray coherent distance in the gel structure occurred during calcination and removal of the template. The calculated radius for the calcined sample was roughly 32 Å. The experimental radius of the PAMAM generation 4 dendrimer was determined to be 40 Å, and this led to the conclusion that successful imprinting of a mesoporous cavity was achieved.
The nitrogen adsorption–desorption isotherm for the calcined sample, which was measured at 77 K, was found to be characteristic of microporous solids. Evidence for the existence of the microporosity was derived from the strong adsorption at low P/Po, and the correlation of the data with the Dubinin–Radushkevich equation. In order to determine the nature of the microporosity, the Kr adsorption uptake of this sample at 195 K was measured in a temperature range closer to that of conventional applications and was thereafter modelled with a modified Horvath–Kawazoe (HK) algorithm [34, 35]. The spherical cavity model of Cheng and Yang [35] was adopted, given the quasi-spherical nature of the dendrimer template. Two zeolites, 5A and HY, were used to calibrate this method (see Fig. 3a). The surface area of the calcined sample was found to be 623 m2/g and the material showed an unusual pore-size distribution (see Fig. 3b). For comparison purpose, the HK curve for a blank material prepared without dendrimer template is also shown in Fig. 3b. The blank materials showed a broad pore-size distribution curve. As expected for microporous (disordered) silica materials, a maximum around 15–16 Å was recorded [20].

Image reproduced from Ref. [11] with permission from copyright (2000) American Chemical Society
Horvath–Kawazoe plots of the zeolite reference materials. Dashed lines are first derivatives (a). Horvath–Kawazoe plots of the sample material (star-1) and the SiO2 blank (b). Derivatives are shown as solid (SiO2) and dashed (star-1) lines.
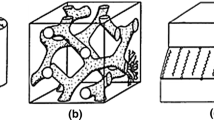
Upon heating the sample, contraction was observed due to an inward collapse of the low-density dendrimer structure (see Fig. 4). This collapse is attributed to the fact that the low-density structure of a dendrimer (unlike an alkyl-amine) cannot withstand the high pressure created by gel densification during heating. The amorphous nature of this material makes it an interesting and unique class, which, however, does not compete with zeolites in terms of production of well-defined micro-cavities. The value of the N2 uptake for this material is approximately equal to the one reported for zeolite 5A [36].

Image reproduced from Ref. [11] with permission from copyright (2000) American Chemical Society
A proposed mechanism of pore formation: short-range ordering.
Although the size distribution of the cavities was not well defined, the X-ray domains are expected to become large enough for the elucidation of the geometric arrangements of the cavities (via the analysis of higher-order reflections) when the synthetic procedure is improved. From all characterization techniques, it was evident that PAMAM dendrimer templates can be successfully used for the synthesis of microporous materials. However, these materials possessed poor structural properties that led to the collapse of pores at high temperatures. Therefore, these materials are not ideal for use as catalysts or as catalyst support materials [11]. In an attempt to overcome these problems, Larsen et al. [19] used polypropylenimine tetrahexacontaamine (DAB-Am-64) dendrimers as templates for the production of mesoporous silicas, which are referred to as NU-1. In this study, the XRD 2θ values of 3.25° and 3.6° were obtained for the uncalcined and calcined samples. Both of these values were found to be higher than those obtained using PAMAM dendrimers as a template. A BET-specific surface area of 637 m2/g was obtained for the calcined sample, making these silica materials more suitable for catalyst support application. This can be attributed to the removal of the dendrimer template. In a related study, Larsen group reported the use of poly(propylene) imine (DAB-Am-32 and DAB-Am-64) dendrimers as templates for the synthesis of silicas using the sol–gel method [16]. The materials synthesized using DAB-Am-32 and DAB-Am-64 were labelled NU-2 and NU-1, respectively. In this method, no acid was added to enable an investigation of whether a similar material could be reproduced without using HCl or any other acid. The pyrolysis and oxidation behaviour of the synthesized materials was also studied. The absence of higher-order reflection from the XRD patterns for both materials (NU-1 and NU-2), both before and after calcination indicated the non-formation of three-dimensional arrangements with uniform cavities. Both NU-1 and NU-2 materials showed a broad signal appearing at 2θ ~ 22°, which is typical of amorphous materials. No significant difference relating to the XRD data of the NU-1 synthesized with and without acid was observed [16]. The BET-specific surface areas for NU-1 and NU-2 materials were found to be 767.4 and 585.1 m2/g, respectively, as opposed to a low surface area of 241.0 m2/g measured for the material prepared in the absence of dendrimers. The average pore-size diameter (PSD) of NU-1 and NU-2 were found to be 27 and 17 Å, respectively. The PSD for NU-1 was approximately 2 Å above the value predicted using the XRD data, and the PSD curve was found to fall well above the norm (about 16 Å) for the microporous silicas [37]. Based on the broader PSD observed for NU-2, it was concluded that the DAB-Am-32 dendrimer is the less effective template for the synthesis of stable mesoporous materials under these reaction conditions (without use of acid).
The synthesis of porous and non-porous xerogels, using functionalized dendrimers and arborols respectively, has also been reported [38]. However, the nature of the porosity of these xerogels was not clearly explained and still remains a point of interest. Tilley et al. [20] used carbosilane dendrimers as building blocks for the synthesis of mesoporous dendrimer-based silica xerogels. In this study, the second- and third-generation triethoxysilyl-terminated dendrimers were hydrolysed via the sol–gel method to form micro- and mesoporous hybrid dendrimer–silica xerogels. The dendrimers were constructed according to a reported synthetic methodology [39], which involves the hydrosilation of terminal alkene groups with HSiCl3, followed by Grignard functionalization of vinyl or allyl groups to the corresponding trichlorosilyl functionalities. However, a formation of impurities is often observed as a result of redistribution at silicon [40]. The redistribution of by-products, which can potentially complicate the reaction, was avoided by direct hydrosilation of the resulting vinyl- or allyl with triethoxysilane. As shown in Fig. 5, the reaction led to a high yield of G2′-(OEt)36 or G3′ -(OEt)108. Hydrolyses of the G2′-(OEt)36 and G3′-(OEt)108 in THF (2.5 M in OEt groups) with small quantities of 1 N HCl led to the formation of homogeneous sol solutions. These sol solutions were allowed to gel for over 48 h in polyethylene bottles, to produce clear monoliths. As shown in Fig. 5, the monolith was removed from the polyethylene bottles and the solvent processed to give the xerogels X-G2′ and X-G3′, respectively.

Image reproduced from Ref. [20] with permission from copyright (1999) American Chemical Society
Synthesis of an alkylsilane-based xerogel.
Infrared spectroscopy, XRD, and N2 adsorption–desorption techniques were used to characterize the synthesized X-G2′ and X-G3′ materials. Infrared spectroscopic data seem to indicate that both these materials contain partially condensed polysilsesquioxane moieties and the amorphous nature of both materials was confirmed by XRD data. Nitrogen adsorption–desorption isotherms were used to determine the surface area, pore volume, and pore radius of the sample material. The average pore radii of G-X2′ and G-X3′ were calculated to be 13.1 and 13.5 Å, respectively. As shown in Table 2, an increase in total surface area and pore volume was observed with an increase in dendrimer generation.
Three different amine-terminated dendrimer molecules were used as templates to produce both microporous and mesoporous silica materials via a neutral templating route based on hydrogen bonding interactions between neutral amines and the inorganic precursors (see Fig. 6) [15]. The three dendrimers were [tris-(2-aminoethyl)amine] 1, a structurally related low molecular weight polyamidoamine-type dendrimer 2 and a low molecular weight dendrimer derived from pentaerythritol 3. The preparation and composition of these silica materials were similar to those reported by Tanev and Pinnavaria [41, 42]. While the reaction time for the material synthesized using templating molecule 1 was 18 h, both 2 and 3 were produced within 36 h. As evidenced by thermogravimetric analysis data, these materials displayed some weight loss. The final silica materials were obtained after calcination at 540 °C. It was determined from the characterization data that while the materials prepared using 1 and 3 were microporous, they differed significantly with respect to surface areas. Although the materials prepared using 2 showed a mesoporous character, the observed pore architecture that is not well-defined was most likely as a result of a combination of micropores and mesopores.

Image reproduced from Ref. [15] with permission from Elsevier
Dendrimer templating molecules during the synthesis of amorphous silica.
Xu et al. reported the use dendrimers as “green” template for the synthesis of mesoporous silica materials [21]. Amphiphilic dendritic polyglycerol were employed as template and was simply removed by water extraction instead of calcination process as preferred by other authors. The surface area of the silica material was found to be higher for calcined samples when compared with water extracted samples. For instance, the surface area for water extracted samples was 623.5 m2/g while a surface area of 749.5 m2/g was obtained for the calcined sample. The higher surface area of the silica material was attributed to condensation of hydroxyl groups taking place on the surface of silica and/or small changes that may have occurred to the pore framework during calcination. However, surprisingly, no significant difference was observed in the respective average pore sizes of the water extracted and calcined silica material samples. In another related study, Dai et al. [12] reported on the synthesis of mesoporous silica materials using different generations of amphiphilic polyamidoamine dendrimers as “green” template. The dendrimer template was also removed using the water extraction method. Higher surface area for the calcined samples was observed in relation to those subjected to water extraction method for the removal of the dendrimer template. Similarly, no change in average pore sizes was recorded for the calcined and water extracted samples. While dendrimer-templated silica materials reported by other authors did not have well-ordered structures, Tanglumlert et al. [43] managed to synthesize well-ordered mesoporous silica materials using PAMAM dendrimers. For the synthesis of these well-ordered mesoporous silica materials, the silatrane was used as a silica precursor under mild acidic condition using the sol–gel process. The formation of amorphous silica was circumvented by decreasing the dendrimer concentration.
Recently, mesoporous dendrimer-like mesoporous silica nanohybrids (see Fig. 7) were fabricated for biomedical application [44]. The synthesis of these porous dendrimer-like materials was inspired by previous reports on the use of dendrimers as drug carriers [45]. Qiao et al. [44, 46, 47] have published some interesting work on the synthesis of dendrimer-like mesoporous silica nanohybrid materials for biomedical application. In this work, CTAB was used as a templating agent (surfactant) and was subsequently removed by extraction method. Advantage of these dendrimer-like mesoporous silica materials (when compared with conversional mesoporous silica materials) include: (1) the formed centre-radial pores are more accessible by various size molecules, (2) the uniform pore-size distribution enable the use of these materials as nanocontainers for small drug molecules and (3) the existence of multi-scale pores allow the diffusion through the porous matrices of the guest molecules of different sizes. As a result, dendrimer-like mesoporous silica materials are perceived to possess enhanced performance in different applications such as catalysis, bioseparation and biomedicine [47, 48]. Interestingly, since these mesoporous silica materials have dendrimer-like structures, they can potentially be used as hard templates for the synthesis of other mesoporous oxide materials such as Co3O4, Fe2O3 and MnO2 as previously reported by other authors [49]. The synthesis and characterization and potential applications of dendrimer-like mesoporous silica materials in biomedicine, catalysis and energy were extensively reviewed by Qiao et al. [48].

Image reproduced from Ref. [48] with permission from John Wiley and Sons
Schematic illustration of dendritic silica particles with centre-radial pore channels, whose sizes gradually increase from a to c.
Dendrimers as templating agent for the synthesis of mesoporous silica nanospheres/nanocages
Other than in the control of the pore size and morphology of mesoporous silicas, Knecht and Wright [17, 18] have demonstrated the use of these templates for controlling the size of the formed nanospheres. For example, silica nanospheres were produced by adding monosilic acid to either PPI or PAMAM dendrimers with a known amine concentration in phosphate-buffered solution at a neutral pH [18]. Analysis of the reaction products revealed a sigmoidal and linear correlation between amine concentrations and silica production of PPI and PAMAM dendrimers, respectively. The concentration of the phosphate in solution was identified as a key factor for controlling the size of the product, with the activity and particle size increasing with an increase in phosphate concentration [50, 51]. In a related study, Knecht et al. [17] used PPI and PAMAM dendrimers for the selective precipitation of 30–300 nm silica nanospheres using a defined concentration of phosphate buffer and main group metal salts. Although the silica production was not affected by phosphate-buffered solutions, SEM showed a linear dependence of silica particle size on the phosphate buffer concentration lower than 20 mM. Depending on the generation of the dendrimer template used, the silica spheres with a constant average diameter of between 250 and 350 nm were produced at phosphate buffer concentration higher than 20 mM. We have recently reported a related encapsulation of Au and Ag nanoparticles within silica nanospheres using generation 4 of amine-terminated PAMAM dendrimers [13]. It was observed that the phosphate buffer concentration has an effect on the size of the silica nanosphere (see Fig. 8). The silica nanospheres were found to have characteristics of mesoporous materials after the removal of the dendrimer template by a 500 °C calcination method. Surface area and average pore size of 698.3 m2/g and 3.4 nm were obtained, respectively.

Image reproduced from Ref. [13] with permission from Elsevier
TEM images of G4-PAMAM-NH2 templated silica nanosphere (G4-PAMAM-NH2-SiO2) prepared in different buffer concentrations: a 20 mM, b 40 mM, c 60 mM and d 80 mM.
Imae et al. also reported on the synthesis of sphere-like porous silica materials [52]. Using amine-terminated PAMAM dendrimers as a structure directing agent, the synthesis was carried out at elevated temperatures (70–140 °C). Depending on the pH, concentration of the dendrimer template and reaction temperature, the ageing time was varied from 2 to 6 days. The average size of the sphere-like porous particles was found to be 30–300 nm, depending on the reaction conditions employed. Lee et al. [10] synthesized small silica nanocages (~ 10 nm) using generation 4 in-house immolative dendrimers. A notable advantage about this method is option of being able to functionalize the interior of the nanocages, which makes them suitable for the encapsulation of other nano-sized materials.
In an attempt to extend this chemistry, Wright et al. [53] were able to produce multicomponent silica nanospheres using PAMAM dendrimers as templates for both Au0 nanoparticles and silica condensation. The resulting nanocomposite materials yielded randomly distributed gold nanoparticles encapsulated within the mesoporous silica nanospheres with a diameter of 80 nm (see Fig. 9).
The synthesis of silica in the presence of pre-formed Pt DENs was reported by Chandler et al. [54]. Although the porosity of the silica support that was formed was not investigated, it can be safely assumed that the mesopore structures were formed upon dendrimer removal by calcination. The catalytic activity of the synthesized composite material was evaluated for the hydrogenation of toluene.
Dendrimers as templating agents for the synthesis of micro- and mesoporous titania materials
Titanium dioxide is one of the most important inorganic oxides because of its versatility in terms of its properties and applications. It is used in the paint industry, as clothes colourants and sunscreen lotions [55,56,57,58,59]. Wright et al. [60] adopted a previously reported procedure for the synthesis of dendrimer-templated silica nanospheres by using different generations (G0, G2, G4, G5 and G6) of amine-terminated PAMAM and PPI dendrimers to synthesize titania nanospheres. As in the case of silica [17], the size of the titania nanospheres was found to increase when the synthesis was carried out in phosphate-buffered solution as opposed to water. Average sizes of 310–470 nm were recorded for different generations of the PAMAM dendrimer-templated TiO2 nanospheres. Different phase transitions were observed during annealing to remove the dendrimer template. For instance, the crystalline anatase phase was observed upon heating at 600 °C. Another phase transition from anatase to rutile was observed when the annealing temperature was increased to 900 °C. In another interesting study, Brahmi et al. [61] synthesized mesoporous anatase nanocrystals at low temperature from generation 4 phosphorus dendrimers. The norm has always been that titania transforms from amorphous phase to anatase during annealing at higher temperatures (400–500 °C). These mesoporous titanias were found to possess a relatively high surface area relative to other reported mesoporous titania materials synthesized using different surfactants. The BET surface area ranging from 158 to 240 m2/g was recorded for these phosphorus-dendrimer-templated mesoporous titania materials. As demonstrated by Bouchara et al. [62], oxo-based mesoporous hybrid titania materials produced from generation 5 or 7 dendrimers and carboxyl (COO−) peripheral groups were found to possess a BET-specific surface area of 37 m2/g and average pore size of 9–30 nm. The average pore size of 9 nm was attributed to the size a specific dendrimer molecule, while the 30 nm pore size was due to the agglomeration of dendrimer templates. The existence of mesopore structure was assumed based on TEM measurements. The nitrogen adsorption–desorption isotherms of the calcined materials were found to be of type II, which is characteristic of non-porous materials. Such an observation was attributed to the incomplete removal of the dendrimer template during calcination at 325 °C. The dual function of the amine-terminated PAMAM dendrimers to template both the nanoparticles and pores of the support during the synthesis was also demonstrated by Crooks et al. [63]. To this end, the sol–gel synthesis of metal nanoparticles supported onto titania was achieved. In this instance, the interior of the dendrimer templated the nanoparticles, while the exterior templated the titania pores within the sol–gel matrix. While the synthesized titania supported DENs composite was found to be amorphous prior to calcination, it was evident from powder X-ray diffraction data that it was partially converted to the crystalline anatase phase following calcination at 500 °C. With a BET surface of 34.0 m2/g prior to calcination and a type III isotherm, the synthesized composite material typifies a macroporous material. After calcination, the BET surface area increased to 50.2 m2/g and a type IV isotherm was observed, which is characteristic of mesoporous materials. The post-calcination increase in the surface area of the composite material was attributed to the mesoporosity introduced by the dendrimer template. Crooks’ research group has also achieved the synthesis of titanium-supported Pd/Au bimetallic nanoparticles using the same method [64].
Dendrimers as templating agent for the synthesis of micro- and mesoporous alumina materials
Similar to silica, alumina has important industrial applications such use as adsorbents and catalyst support in chemical reactions. However, one of the limitations of alumina catalysts is the deactivation emanating from coke formation and pore plugging that negatively affects the diffusion of reactants and products [65]. Therefore, the synthesis of alumina with highly specific surface areas, and narrow, tunable pore-size distributions is of great industrial importance. Very few reports on the use of dendrimers as pore-directing agents for the synthesis of mesoporous alumina appear in the literature. For instance, Zhang and Wang [66] used different half-generation (Generation 2.5, 3.5 and 4.5) PAMAM dendrimer templates to control pore size during the synthesis of mesoporous alumina materials. These mesoporous alumina granules were synthesized using a new Yoldas sol–gel process combined with an oil-drop granulation process [67]. The oil-drop method for the fabrication of mesoporous alumina was first proposed by Wang and Lin [68]. Aluminium isopropoxide was used as a precursor for the synthesis of alumina granules. The materials prepared in the presence of generation 2.5, 3.5 and 4.5 PAMAM dendrimers were denoted as SC10, SA10 and SB10, respectively. TEM images revealed a sponge-like, three-dimensional and randomly connected mesoporous network. The core structure and pore channel for the uncalcined samples (SA10 and SB10) appeared relatively narrower than that of the calcined samples. However, the mesoporous aluminium oxide was found to be different from the MCM-41 due to the lack of long-range order in the pore structure. The prepared mesoporous alumina displayed a Type IV nitrogen adsorption–adsorption isotherm and type H1 hysteresis loops, which are both characteristics of mesoporous materials. The BET surface area was found to decrease with an increase in the dendrimer generation, while the opposite was observed for the average pore size (see Table 3). The generation 4 amine-terminated PAMAM dendrimers was used as a single molecular templating agent for the synthesis of mesoporous aluminophosphate materials [69]. The amorphous pre-calcination nature of these materials was attributed to the presence of the dendrimer template in the hybrid materials. The materials were found to possess mesopore structures after calcination, and the average pore diameter of 5-8 nm was found.
Dendrimer-templated porous zeolite formation
Zeolites are another class of well-known nanoporous materials, used largely in industrial processes as catalysts and adsorbent materials. They have also been recently applied in agricultural, environmental and biological technologies [70, 71]. Zeolites are obtained by hydrothermal treatment of aluminosilicate materials in the presence of templates or surfactants which act as structure directing agents and are responsible for the final porosity [36, 72, 73]. Lombardo et al. [74] demonstrated the use of carboxyl-terminated PAMAM dendrimers as templates for the formation of porous zeolites. In this study, the zeolite LTA was formed by a simple addition of the aluminosilicate to an aqueous solution of the generation 3.5 PAMAM dendrimer. The dendrimer used in this case possessed a charged surface that acts as the main driving force influencing the crystallite aggregation and provides the long-range assembly conditions for the zeolite growth. The formation of porous, stable, and monodispersed spherical aggregates, with an average radius of 3500 Å, was detected following characterization of the material with XRD, SAXS, and SEM techniques. The research group of Lombardo et al. [75] have also synthesized SAPO-34 zeolite using generation 4 amine-terminated PAMAM dendrimers as templating agents.
Dendrimers as templates for the fabrication of other inorganic mesoporous materials
Non-silicious inorganic dendrimer-templated mesoporous materials have also been reported. For example, amine-terminated PAMAM dendrimers (generation 4) were used as templates for the synthesis of mesoporous ZnWO4 materials [76]. These as-formed materials were characterized using various techniques and used as catalysts in the degradation of rhodamine B (RhB) and malachite green (MG) dyes. Pramanik and Imae [77] prepared PAMAM dendrimers templated mesoporous hydroxyapatite (HAp) using the hydrothermal method. The precursor for HAp was prepared by mixing aqueous solutions of calcium nitrate and diammonium hydrogen phosphate. For comparative purposes, the same materials were also prepared using cetyltrimethylammonium bromide (CTAB) and both porogens gave comparable physicochemical properties. For instance, a BET surface area of 62.58 and 56.46 m2/g were obtained for micelle and dendrimer porogens, respectively. Additionally, the pore volume for the micelle templated materials was found to be 0.19 cm3/g, while 0.18 cm3/g was achieved for the dendrimer-templated materials.
The oxidation of an alkene is an essential step in the production of numerous fine chemicals and pharmaceuticals [78,79,80,81,82,83]; hence, the need for effective homogeneous and heterogeneous catalysts for the oxidation of alkenes. The use of generation 4 and 5 poly(propylene)imine (DAB-Am), dendrimers as templating agents for the sol–gel synthesis of mesoporous titanosilicate and vanadosilicate oxidation catalysts has been reported [84]. Unlike other studies, where the synthesis and characterization of dendrimer-templated mesoporous materials were the only objectives of the studies, Bruce et al. [84] went further by evaluating the catalytic activity in the oxidation of cyclohexene by tert-butylhydroperoxide (TBHP). Doping of these mesoporous catalysts with vanadium and titanium led to the incorporation of the transition metals into the final dendrimer-templated mesoporous silicate materials (see Fig. 10). The synthetic protocol was adopted from the work of Larsen et al. [11, 85].
Atomic absorption spectroscopy (AAS) and combustion analyses, which were used for elemental analysis of the calcined metal-doped samples, revealed that the expected titanium and vanadium loadings were slightly less than was expected from the sol–gel synthetic method. In order to determine whether the metals were randomly dispersed throughout the particles or formed a separate oxide phase, energy dispersion X-ray analysis (EDX) was performed. In both titanium and vanadium-doped samples, the metal atoms were found to be randomly dispersed throughout the solid structures. The temperature required to initiate the decomposition of the dendrimer was found to be 108 °C lower than the temperature reported for the decomposition of transition metal-free silicate by Larsen et al. [85]. The respective average particle sizes of CU-D32 and CU-D64 samples were approximately 0.15 and 0.20 µm. The PXD patterns showed that CU-D32-Ti and CU-D64-Ti materials had a d-spacing of 21.1 and 23 Å, respectively. The catalytic activity of these materials was evaluated for the epoxidation of cyclohexene (see Fig. 11) [84]. The reaction products showed that all catalysts were very effective in the production of cyclohexene-oxide under mild oxidative conditions.
Catalytic oxidation of cyclohexene by TBHP [84]
Iron phosphate has been reported as one of the best oxidation catalysts [86]. Therefore, the synthesis of mesoporous iron phosphate catalysts is of great importance in the chemical industry. It should, however, be borne in mind that the synthesis of mesoporous iron phosphate materials by simple surfactant template method has proven to be difficult. Despite such challenges, Lu and Imae [87] have managed to use PAMAM dendrimer template to access mesoporous iron phosphate. Mesoporous cerium oxide, another potential oxidation catalyst has also been synthesized in the presence of the carboxyl-terminated PAMAM dendrimers [62]. The materials were prepared using cerium isopropoxide as a precursor and SEM revealed globular morphology of about 700 nm in size.
Overall conclusions
It is clear that dendrimers are capable of acting as templating agents, not only for synthesis of metal nanoparticle, but also for the production of stable micro- and mesoporous materials. When synthesizing the latter, dendrimer templates can be used for controlling both the particle and pore sizes. The concentration of the phosphate buffer used in the preparation of certain types of dendrimers plays an important role in determining the particle size of templated mesoporous silicas. It is also possible to simultaneously produce mesoporous oxide and metal nanospheres supported on the very same oxide material using dendrimers as the template.
Although PAMAM dendrimers are effective templates for the synthesis of micro- and mesoporous oxide materials, materials derived from these dendrimers have in some cases poor structural properties which cause the pores to collapse at high temperatures. As a result, pores that are significantly smaller than those of the PAMAM template itself are formed. Consequently, some of the mesoporous oxide produced in this way may not necessarily be ideal for catalysts support material. The metal-doped mesoporous oxide, which is catalytically active, can also successfully be synthesized using dendrimer templates.
The molar ratio of Si/dendrimer is assumed to play an important role in pore connectivity and resistance to collapsing; it is actually key when tailoring the wall-thickness between voids. Thicker pore walls improve the thermal and hydrothermal stability of the frameworks [42]. The use of higher generation dendritic molecules with an appropriate silica/template ratio can potentially yield well-defined mesoporous structures. It has been found that the total pore volume and size as well as relative fraction of micro- and mesopores can be controlled by changing the reaction period and temperature and Si/surfactant ratio [88, 89].
References
Newkome GR, Moorefield CN, Vögtle F (1996) Dendritic molecules: concepts, synthesis, perspectives. Wiley, Weinheim
Tomalia DA, Baker H, Dewald JR, Halls M, Kallos G, Martin S, Roeck J, Ryder J, Smith P (1986) Dendritic macromolecules: synthesis of starburst dendrimers. Macromolecules 19:2466–2468
Crooks RM, Zhao M, Sun L, Chechik V, Yeung LK (2001) Dendrimer-encapsulated nanoparticles: synthesis, characterization, and application to catalysis. Acc Chem Res 34:181–190
Buhleier EW, Wehner W, Vögtle F (1978) “Cascade”-and “Nonskid-Chain-like” syntheses of molecular cavity topologies. Synthesis 2:155–158
Tomalia DA, Baker H, Dewald JR, Halls M, Kallos G, Martin S, Roeck J, Ryder J, Smith P (1985) A new class of polymers: starburst-dendritic macromolecules. Polym J 17:117–132
Newkome GR, Yao Z-Q, Baker GR, Gupta K (1985) Micelles. Part 1. Cascade molecules: a new approach to micelles. A [27]-Arborol. J Org Chem 50:2003–2004
Hawker CJ, Fréchet JMJ (1990) Preparation of polymers with controlled molecular architecture. A new convergent approach to dendritic macromolecules. J Am Chem Soc 112:7638–7647
Balogh L, Tomalia DA (1998) Polu(Amidoamine) dendrimer-templated nanocomposites. 1. Synthsesis of zerovalent copper nanoclusters. J Am Chem Soc 120:7355–7356
Kim Y-G, Oh S-K, Crooks RM (2004) Preparation and characterization of 1–2 nm dendrimer-encapsulated gold nanoparticles having very narrow size distributions. Chem Mater 16:167–172
Lee J-K, Kung MC, Suh Y-W, Kung HH (2008) Discrete molecular-sized nanocages derived from disintegratable dendrimer template. Chem Mater 20:373–375
Larsen G, Lotero E, Marquez M (2000) Amine dendrimers as templates for amorphous silicas. J Phys Chem B 104:4840–4843
Dai H, Yang J, Ma J, Chen F, Fei Z, Zhong M (2012) A green process for the synthesis of controllable mesoporous silica materials. Microporous Mesoporous Mater 147:281–285
Nemanashi-Maumela M, Nongwe I, Motene RC, Davids BL, Meijboom R (2017) Au and Ag nanoparticles encapsulated within silica nanospheres using dendrimers as dual templating agent and their catalytic activity. Mol Catal 438:184–196
Chujo Y, Matsuki H, Kure S, Saegusa T, Yazawa T (1994) Control of pore size of porous silica by means of pyrolysis of an organic–inorganic polymer hybrid. Chem Commun. https://doi.org/10.1039/C39940000635
Hukkamäki J, Pakkanen TT (2003) Amorphous silica materials prepared by neutral templating route using amine-terminated templates. Microporous Mesoporous Mater 65:189–196
Larsen G, Loreto E, Marquez M (2000) Facile sol–gel synthesis of porous silicas using poly(propylene)imine dendrimers as templates. J Mater Res 15:1842–1848
Knecht MR, Sewell SL, Wright DW (2005) Size control of dendrimer-templated silica. Langmuir 21:2058–2061
Knecht MR, Wright DW (2004) Amine-terminated dendrimers as biomimetic templates for silica nanosphere formation. Langmuir 20:4728–4732
Larsen G, Lotero E, Marquez M (2000) Use of polypropylenimine tetrahexacontaamine (DAB-Am-64) dendrimer as a single-molecule template to produce mesoporous silicas. Chem Mater 12:1513–1515
Kriesel JW, Tilley TD (1999) Dendrimers as building blocks for nanostructured materials: micro- and mesoporosity in dendrimer-based xerogels. Chem Mater 11:1190–1193
Xu Y, Xu S, Emmler T, Roeflos F, Boettcher C, Haag R, Buntkowsky G (2008) A novel green template for the synthesis of mesoporous silica. Chem Eur J 14:3311–3315
Beck JS, Vartuli JC, Roth WJ, Leonowicz ME, Kresge CT, Schmitt KD, Chu CTW, Olson DH, Sheppard EW, McCullen SB, Higgins JB, Schlenker JL (1992) A new family of mesoporous molecular sieves prepared with liquid crystal templates. J Am Chem Soc 114:10834–10843
Kresge CT, Leonowicz ME, Roth WJ, Vartuli JC, Beck JS (1992) Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature (London) 359:710–712
Zhao E, Hernandez O, Pacheco G, Hardcastle S, Fripiat JJ (1998) Thermal behavior and texture of mesoporous zirconia obtained from anionic surfactants. J Mater Chem 8:1635–1640
Müller A, Reuter H, Dillinger S (1995) [Mo154(NO)14O420(OH)28(H2O)70](25 ± 5)−: a water-soluble big wheels with more than 700 atoms and a relative molecular mass of about 24,000. Angew Chem Int Ed Engl 34:2122–2124
Kadib AE, Katir N, Bousmina M, Majoral J-P (2012) Dendrimer–silica hybrid mesoporous materials. New J Chem 36:241–255
Bagshaw SA, Prouzet E, Pinnavaia TJ (1995) Templating of mesoporous molecular sieves by nonionic polyethylene oxide surfactants. Science 269:1242–1244
Inagaki S, Fukushima Y, Kuroda K (1993) Synthesis of highly ordered mesoporous materials from a layered polysilicate. Chem Commun. https://doi.org/10.1039/C39930000680
Kapoor MP, Kuroda H, Yanagi M, Nanbu H, Juneja LR (2009) Catalysis by mesoporous dendrimers. Top Catal 52:634–642
Poyraz AS, Kuo C-H, Kim E, Meng Y, Seraji YS, Suib SL (2014) Tungsten-promoted mesoporous group 4 (Ti, Zr, and Hf) transition-metal oxides for room-temperature solvent-free acetalization and ketalization reactions. Chem Mater 26:2803–2813
Dundar-Tekkaya E, Yürüm Y (2016) Mesoporous MCM-41 material for hydrogen storage: a short review. Int J Hydrogen Storage 41:9789–9795
Chujo Y, Saegusa T (1992) Control of pore size of porous silica by means of pyrolysis of an organic–inorganic polymer hybrid. Adv Polym Sci 100:11–29
Tomalia DA, Naylor AM, Goddard WA (1990) Starburst dendrimers: molecular-level control of size, shape, surface chemistry, topology, and flexibility from atoms to macroscopic matter. Angew Chem Int Ed Engl 29:138–175
Horvath G, Kawazoe KJ (1983) Method for the calculation of effective pore size distribution in molecular sieve carbon. Chem Eng Jpn 16:470–475
Cheng LS, Yang RT (1994) Improved Horvath–Kawazoe equations including spherical pore models for calculating micropore size distribution. Chem Eng Sci 49:2599–2609
Breck DW (1974) Zeolite molecular sieves. Wiley, New York
Brinker CJ, Scherer GW (1985) Sol → gel → glass: I. Gelation and gel structure. J Non-Cryst Solids 70:301–322
Boury B, Corriu RJP, Nuñez R (1998) Hybrid xerogels from dendrimer and arborols. Chem Mater 10:1795–1804
Kriesel J, Konig S, Freitas MA, Marshall AG, Leary JA, Tilley TD (1998) Synthesis of highly charged organometallic dendrimers and their characterization by electrospray mass spectrometry and single-crystal x-ray diffraction. J Am Chem Soc 120:12207–12215
Curtis MD, Epstein PS (1981) Redistribution reaction on silicon catalyzed by transition metal complexes. Adv Organomet Chem 19:213–255
Tanev PT, Pinnavaia TJ (1996) Biomimetic tem-plating of porous lamellar silicas by vesicular surfactant assemblies. Science 271:1267–1269
Tanev PT, Pinnavaia TJ (1995) A neutral templating route to mesoporous molecular sieves. Science 267:865–867
Tanglumlert W, Wongkasemjit S, Imae T (2009) Fabrication of dendrimer porogens-capsulated mesoporous silica via sol–gel process of silatrane precursor. J Nanosci Nanotechnol 9:1844–1850
Du X, Shi B, Liang J, Bi J, Dai S, Qiao SZ (2013) Developing functionalized dendrimer-like silica nanoparticles with hierarchical pores as advanced delivery nanocarriers. Adv Mater 25:5981–5985
Kesharwani P, Jain K, Jain NK (2014) Dendrimer as nanocarrier for drug delivery. Prog Polym Sci 39:268–307
Du X, Shi B, Tang Y, Dai S, Qiao SZ (2014) Label-free dendrimer-like silica nanohybrids for traceable and controlled gene delivery. Biomaterials 35:5580–5590
Du X, Xiong L, Dai S, Kleitz F, Qiao SZ (2014) Intercellular microenvironment-responsive dendrimer-like mesoporous nanohybrids for traceable, effective, and safe gene delivery. Adv Funct Mater 24:7627–7637
Du X, Qiao SZ (2015) Dendritic silica particles with center-radial pore channels: promising platforms for catalysis and biomedical applications. Small 11:392–413
An K, Alayoglu S, Musselwhite N, Plamthottam S, Melaet M, Lindeman AE, Somorjai GA (2013) Enhanced co oxidation rates at the interface of mesoporous oxides and Pt nanoparticles. J Am Chem Soc 135:16689–16696
Sumper M, Lorenz S, Brunner E (2003) Biomemetic control of size in the polyamine-directed formation of silica nanospheres. Angew Chem Int Ed 42:5192–5195
Brunner E, Lutz K, Sumper M (2004) Biomimetic synthesis of silica nanospheres depends on the aggregation and phase separation of polyamines in the aqueous solution. Phys Chem Chem Phys 6:854–857
Mitra A, Bhaumik A, Imae T (2004) Synthesis and characterization of nanoporous silica using dendrimer molecules. J Nanosci Nanotechnol 4:1052–1055
Knecht MR, Wright DW (2004) Dendrimer-mediated formation of multicomponent nanospheres. Chem Mater 16:4890–4895
Beakley LW, Yost SE, Cheng R, Chandler BD (2005) Nanocomposite catalysts: dendrimer encapsulated nanoparticles immobilized in sol–gel silica. Appl Catal A 292:124–129
Weir A, Westerhoff P, Fabricius L, Hristovski K, Goetz NV (2012) Titanium dioxide nanoparticles in food and personal care products. Environ Sci Technol 46:2242–2250
Pfaff G, Reynders P (1999) Angle-dependent optical effects deriving from submicron structures of films and pigments. Chem Rev 99:1963–1982
Phillips LG, Barbano DM (1997) The influence of fat substitutes based on protein and titanium dioxide on the sensory properties of lowfat milks. J Dairy Sci 80:2726–2731
Meacock G, Taylor KDA, Knowles MJ, Himonides A (1997) The improved whitening of minced cod flesh using dispersed titanium dioxide. J Sci Food Agric 73:221–225
Jaroenworaluck A, Sunsaneeyametha W, Kosachan N, Stevens R (2006) Characterization of silica-coated TiO2 and its UV absorption for sunscreen cosmetic applications. Surf Interface Anal 38:473–477
Sewell SL, Rutledge RD, Wright DW (2008) Versatile biomimetic dendrimer templates used in the formation of TiO2 and GeO2. Dalton Trans. https://doi.org/10.1039/b802842g
Brahmi Y, Katir N, Ianchuk M, Collière V, Essassi E-M, Ouali A, Caminade A-M, Bousmina M, Majoral JP, Kadib AE (2013) Low temperature synthesis of ordered mesoporous stable anatase nanocrystals: the phosphorus dendrimer approach. Nanoscale 5:2850–2856
Bouchara A, Rozes L, De DJ, Soler-Illia AA, Sanchez C, Turrin CO, Caminade AM, Majoral JP (2003) Use of functional dendritic macromolecules for the design of metal oxo based hybrid materials. J Sol–Gel Sci Technol 26:629–633
Scott RWJ, Wilson OM, Crooks RM (2004) Titania-supported Au and Pd composites synthesized from dendrimer-encapsulated metal nanoparticle precursors. Chem Mater 16:5682–5688
Scott RWJ, Sivadinarayana C, Wilson OM, Yan Z, Goodman DW, Crooks RM (2005) Titania-supported PdAu bimetallic catalysts prepared from dendrimer-encapsulated nanoparticle precursors. J Am Chem Soc 127:1380–1381
Firouzi A, Kumar D, Bull LM, Besier T, Sieger P, Huo Q, Walker SA, Zasadzinski JA, Glinka C, Nicol J, Margolese D, Stucky GD, Chmelka BF (1995) Cooperative organization of inorganic-surfactant and biomimetic assemblies. Science 267:1138–1143
Zhang B, Wang J (2006) Pore control of mesoporous alumina with half-generation poly-amidoamine dendrimers. Nanoscince 11:114–118
Buelna G, Lin YS (2001) Preparation of spherical alumina and copper oxide coated alumina sorbents by improved sol–gel granulation process. Microporous Mesoporous Mater 42:67–76
Wang ZM, Lin YS (1998) Sol–gel synthesis of pure and copper oxide coated mesoporous alumina granular particles. J Catal 174:43–51
Zhang X, Lin S, Chen X, Chen J, Yang L, Luo M (2007) Preparation of mesoporous aluminophosphate using poly(amido amine) as template. Front Chem China 2:419–421
Bekkum HV, Flanigen EM, Jansen JC (1991) Introduction to zeolite science and practice. Stud Surf Sci Catal. 58:359–390
Chen NY, Degnan TF, Smith CM (1994) Molecular transport and reaction in zeolites, design and application of shape selective catalyst. VCH Publishers, New York
Barrer RM (1982) Hydrothermal chemistry of zeolites. Academic Press, London
Barrer RM (1978) Zeolite and clay minerals as sorbents and molecular sieves. Academic Press, New York
Bonaccorsi L, Lombardo D, Longo A, Proverbio E, Triolo A (2009) Dendrimer template directed self-assembly during zeolite formation. Macromolecules 42:1239–1243
Bonaccorsi L, Calandra P, Amenitsch H, Proverbio E, Lombardo D (2013) Growth of fractal aggregates during template directed SAPO-34 zeolite formation. Microporous Mesoporous Mater 167:3–9
Lin S, Chen J, Weng X, Yang L, Chen X (2009) Fabrication of photocatalysis of mesoporous ZnWO4 with PAMAM as a template. Mater Res Bull 44:1102–1105
Pramanik N, Imae T (2012) Fabrication and characterization of dendrimer-functionalized mesoporous hyroxyapatite. Langmuir 28:14018–14027
Centi G, Misono M (1998) New possibilities and opportunities for basic and applied research on selective oxidation by solid catalysts: an overview. Catal Today 41:287–296
Sheldon RA (1991) Heterogeneous catalytic oxidation and fine chemicals. Stud Surf Sci Catal 59:33–54
Sheldon RA, Dakka J (1994) Heterogeneous catalytic oxidations in the manufacture of fine chemicals. Catal Today 19:215–246
Sheldon RA, Kochi JK (1981) Metal catalyzed oxidations of organic compounds. Academic Press, New York
Arends IWCE, Sheldon RA (2001) Activities and stabilities of heterogeneous catalysts in selective liquid phase oxidations: recent developments. Appl Catal A 212:175–187
Rafelt JS, Clark JH (2000) Recent advances in the partial oxidation of organic molecules using heterogeneous catalysts. Catal Today 57:33–44
Rogers MC, Adisa B, Bruce DA (2004) Synthesis and characterization of dendrimer-templated mesoporous oxidation catalysts. Catal Lett 98:29–36
Larsen G, Lotero E, Marquez M (2000) Facile sol–gel synthesis of porous silicas using poly(propylene)imine dendrimers as templates. J Mater Res 15:1842–1848
Wang Y, Otsuka K (1997) Partial oxidation of ethane by reductively activated oxygen over iron phosphate catalyst. J Catal 171:106–114
Luo X, Imae T (2005) Synthesis of mesoporous iron phosphate using PAMAM denrimer as a single molecular template. Chem Lett 34:1132–1133
Voort PVD, Ravikovitch PI, Jong KPD, Neimark AV, Janssen AH, Benjelloun M, Bavel EV, Cool P, Weckhuysen BM, Vansant EF (2002) Plugged hexagonal templated silica: a unique micro-mesoporous composite material with internal silica nanocapsules. Chem Commun 38:1010–1011
Miyazawa K, Inagaki S (2000) Control of the microporosity within the pore walls of ordered mesoporous silica SBA-15. Chem Commun. https://doi.org/10.1039/B005128O
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Nemanashi, M., Noh, JH. & Meijboom, R. Dendrimers as alternative templates and pore-directing agents for the synthesis of micro- and mesoporous materials. J Mater Sci 53, 12663–12678 (2018). https://doi.org/10.1007/s10853-018-2527-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-018-2527-6