Abstract
The effect of oxygen on phase stability and β–α″ martensitic transformation in Ti–Nb alloys has been studied using first principles calculations. Three stable atomic configurations of Ti–Nb (Ti-12.5, 16.6, and 25 at.% Nb) systems, which can transform from β-phase to α″-phase without changing the local atomic position of Nb atoms, were first identified using the cluster expansion method. Phonon calculations indicated that these structures were stable. Next, the martensitic transformation behavior of Ti–Nb–O system was studied using these structures. We observed a significant lattice distortion around oxygen atoms occupying octahedral interstitial sites that resembles a bcc type of stacking. Our results conclusively revealed that while the oxygen interstitials can oppose the atomic shuffle required for martensitic transformation, they can also cooperatively stabilize the β-phase even at 1 at.% oxygen concentrations by inducing local elastic shear strains. Interestingly, the canceling of these fields can stabilize the β-phase by suppressing the β to α″ transformation which decreases the martensitic start temperature (Ms). Our study revealed that the reduction in Ms is higher at lower Nb concentration. The stabilization of β-phase increases with oxygen concentration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ti alloys have been widely studied due to their high specific strength, exceptional corrosion resistance, biocompatibility, and shape memory properties [1]. However, concerns over toxicity of Ni in well-known NiTi shape memory alloys have prompted the development of a Ni-free β Ti–Nb alloys as replacements [2]. The superelasticity and shape memory effects of Ti–Nb alloys originate from the reversible martensitic transformation between the parent body-centered cubic β and the martensitic orthorhombic α″ phases [3, 4]. The strain recovery during β–α″ martensitic transformation largely depends on the β-phase stability and the Nb concentration. By decreasing Nb content, the recovery strain can reach as high as ~ 10%, which is higher than what is observed for conventional NiTi alloys [5, 6]. However, low Nb content destabilizes the β-phase, increases the martensitic start temperature (Ms) [7], and promotes nucleation of the hexagonal closed-packed (hcp) α-phase in the microstructure. Therefore, achieving higher β-phase stability at low Nb concentration is necessary to attain larger recovery strain and low modulus. So, the challenge is how to stabilize the β-phase with low Nb content.
Alloying elements such as Zr, Ta, V, Sn, Al, O, and N are known to stabilize β-phase, increase the recovery strain, and enhance superelastic behavior of Ti–Nb alloys [8,9,10,11]. Interstitial oxygen and nitrogen stabilize the β-phase significantly by decreasing the martensitic start temperature Ms and increasing the slip resistance. Oxygen is introduced during melting processes, and its concentration can reach up to 0.5 at.% even when vacuum furnaces are used due to the large chemical affinity with Ti, and the significant solubility of oxygen in both body-centered cubic (bcc) and hexagonal closed-packed (hcp) structures of Ti [12]. Kim et al. [13] reported a 160 K in Ms per 1 at.% of oxygen in Ti–22Nb alloy. Nii et al. [14] highlighted the suppression of martensitic transformation with increasing oxygen content and attributed it to the formation of nanosized martensite domains. Tahara et al. [15, 16] investigated the effect of oxygen in Ti–23 at.% Nb–1 at.% O and Ti–20Nb-(0, 0.3, 0.5, 0.7, and 1) at.% O alloys and found that the oxygen distorts the surrounding atoms and forms nanosized lattice modulations, which prevented the long-range α″ formation. In addition to its effect on α” martensitic transformation, oxygen plays an important role in the retarding ω phase formation in gum metals, Ti-based alloys with high strength and high ductility [17]. It is generally accepted that oxygen suppresses ω phase formation by increasing the energy barrier for {111} planes collapse required for β–ω transformation [18]. The utilization of oxygen in microstructure modification can offer an economical substitute for costly alloying of Ti alloys for specific requirements. However, the effect of oxygen in Ti alloys continues remaining purely understood and further research and studies are necessary.
Our current work systematically investigates the effect of interstitial oxygen concentration on the phase stability and the β–α″ martensitic transformation in Ti–Nb alloys. Previous first principles calculations have studied the lattice constants and phase stability in β-type Ti–Nb alloys, Sun et al. [19]. Ojha et al. [20] calculated the transformation strain and slip resistance of ternary Ti–Nb (Zr, Ta) alloys, while Minami et al. [21] investigated the same in Ti–Nb–(Al, Sn, Zr, Ta) alloys. These calculations, of the effect of interstitial oxygen on phase stabilities of Ti alloys, largely focused on the effect of a single oxygen atom in a supercell. They ignored the interaction between oxygen atoms, which may counteract the effect of each other via their induced strain fields [22, 23]. The previous experimental work by Nii et al. [14] and Tahara et al. [15] inspired our investigations on the role of one and two oxygen atoms within supercells, and we observed the induced strain fields can stabilize the β-phase. Earlier, Niu and Geng [24] used first principles to investigate the effect of oxygen on β–α″ transformation in Ti–25 at.% Nb alloys. They observed that the presence of 1 at.% of oxygen, uniformly distributed inside the lattice, was unlikely to induce large enough local strain fields to trigger β–α″ transformation and create any nanodomains. They concluded that a high oxygen concentration is necessary to produce α″ phase.
In this work, we use cluster expansion (CE) coupled with a first principles energy calculator to study the role of oxygen in the β–α″ martensitic transformation. We identify three stable atomic configurations of the Ti–Nb system at different stoichiometry that can transform from β-phase to α″ phases without changing the local atomic positions of Nb atoms. Our results conclusively show that oxygen atoms can cooperatively stabilize the β-phase even at 1 at.% oxygen concentrations by inducing local elastic shear strains. Interestingly, the canceling of these fields can suppress the β to α″ transformation. In fact, oxygen has a greater effect stabilizing β-phase by decreasing the martensitic start temperature (Ms) at lower Nb concentration. In addition, oxygen atoms’ ability to stabilize β-phase increases with oxygen concentration. These results collectively indicate that controlling oxygen content can help tune the properties and phase transformation pathways in Ti–Nb alloys. This is evident from the enhanced stability of β-phase at low Nb content without significant impact on the Ms temperature. In addition to above new insights, the stable Ti–Nb structures identified in our CE calculations can be used as a template to study other ternary Ti–Nb–X alloys to find new Ti alloys with desired properties.
Simulation methodology
The density functional theory (DFT) calculations are performed with the Vienna Ab Initio Simulation Package (VASP) [25, 26] as integrated in the MedeA® software environment [27]. In this approach, the Kohn–Sham equations of DFT are solved with projector-augmented-wave (PAW) potentials and wave functions [25] as implemented in VASP. Electron–electron exchange and correlation effects are described by the generalized gradient approximation (GGA) with the functional form proposed by Perdew–Burke–Ernzerhof (PBE) [26]. A plane-wave kinetic energy cutoff of 500 eV was obtained from careful convergence calculations. 10−5 eV was used for the convergence accuracy for the self-consistent electronic minimization. Monkhorst–Pack k-point meshes with a spacing of 15 × 15 × 15 Å−1 were used in conjunction with Methfessel–Paxton integration scheme using a smearing width of 0.2 eV. These settings yield energy accuracy of 1 meV/atom for different structures. Note that the Ti–Nb–O ternary alloy is non-magnetic. Therefore, our calculations are non-spin-polarized.
One major challenge of ab initio calculations is the choice of the optimal random configurations of the alloys. The UNCLE code, as implemented in Medea, was used to systematically search for the ground-state structures in binary Ti–Nb alloy in a given lattice [27, 28]. In cluster expansion (CE) the Hamiltonian of an alloy, where σ defines the distribution of atoms on a given lattice, is constructed via an Ising-like ansatz. The energy of a structure ECE(σ) is constructed in terms of a sum over points, pairs, and triples and multi-body interactions. These so-called clusters or figures are denoted by F. The CE energy of a structure σ has the form
Here, the symmetry degeneracy \( D_{F} \) and the correlation functions \( \prod_{F} \left( \sigma \right) \) are known for the parent crystal lattice. \( J_{F} \) represents the effective cluster interaction energies (ECIs), which are independent of atomic configuration σ and are obtained by fitting to a set of calculated DFT energies of structures defining the training set. The quality of the obtained \( J_{F} \) is evaluated using the “leave-one out” cross-validation score (CVS). The ground-state curve is obtained in a self-consistent way by adding structures whose energy is predicted by cluster expansion to be lower than the energies of the structure already in its training set. Thereby the most stable structures, which constitute the convex hull of the composition dependent phase diagram, can be found from the converged CE functional [29].
Cluster expansions were performed for both bcc β and the orthorhombic α″ phases for a wide concentration range in Ti–Nb compositions. The DFT total energies were calculated for 152 different training set structures of β-phase and 202 of the α″-phase to determine the cluster interactions. The following three optimization steps were done for these structures—only the unit cell volumes were relaxed in the first and the second steps, while internal positions of the atoms were relaxed in the final step. Stable structures obtained from the CE calculations were used to build supercells with 98, 130 and 194 atoms of Ti–Nb–O alloys for β and α″ phases with varying concentrations of Nb and interstitial oxygen. The supercell dimensions were also varied to understand how the lattice parameters influence energetics.
To understand the effect of oxygen interstitials on the martensitic transformation behavior in Ti–Nb alloys, the solid-state nudged elastic band (SSNEB) method [30] was used to study transformation pathways change with the introduction of oxygen. Five images were linearly interpolated between the initial and final states along the transformation pathway, and the relaxation was done until the force on each image was smaller than 0.05 eV Å−1.
Results and discussion
Phase stability: DFT and CE calculations
Cluster expansion was used to identify thermodynamically stable alloy configurations at T = 0 K, which have low energy values along the contour of the convex hull in the energy–composition plot. The calculated ground-state energy of Ti–Nb system for β and α″ phases is shown in Fig. 1. Our first goal was to find a stable structure that can transform from β-phase to α″-phase without changing the configuration of Ti and Nb. We found that the ground-state structure of α″-phase with 12.5 at.% Nb (1a structure in Fig. 1b) has a chemical configuration similar to the ground-state structure marked 1b in Fig. 1a of β-phase. For 16.6 at.% Nb, the ground-state structure marked 2a in Fig. 1b of the α″-phase has comparable chemical configuration to β-phase ground-state structure 2b in Fig. 1a. These structures, along with their symmetries, are shown schematically in Table 1.
The convex hull plot of Ti–Nb binary obtained using cluster expansion method for: a β-phase. b α″-phase. The gray CE predictions entries represent the structure space within which the ground-state structures are identified, while the red CE entries represent the CE training set structures for which DFT energies are calculated
The ground-state structure for α″-phase with 25 at.% Nb (3a in Fig. 1b) corresponds to the stable structure located on the convex hull of β-phase ground-state diagram with the same atomic configuration. Incidentally, this L60 structure is one of the stable β-phase structures that were predicted by Lazar et al. [31] in their comprehensive study. They discovered that strong Nb–Nb interactions are required for the structural stability of Ti3Nb alloys, which have a total energy difference of only 1.6 meV/atom with respect to the ground-state structure lying on the convex hull.
Next, we investigated the dynamical stability of these low-temperature α″ ground-state structures using phonon calculations via the direct-force method implemented in MedeA [27]. The phonon spectra shown in Fig. 2 reveal that all the low-temperature α″ structures predicted by CE possess real eigenmodes, indicating their dynamical stability. It should be noted that the small, negative frequencies of one of the acoustic branches close to Gamma point in (Fig. 2b) are a numerical artifact. Similar calculations within harmonic approximation showed that β-phase, and its related structures have imaginary phonon modes indicating their instability [31,32,33]. However, the β-phase is known to be stabilized by anharmonic phonon interactions at higher temperatures [34]. The self-consistent phonon theory implemented in the SCAILD method [35] can be used to verify the dynamical stability of β-phase structures at higher temperatures but is beyond the scope of this work due to computational limitations.
The interstitial site occupancy preference for oxygen in the ternary Ti–Nb–O alloy for both β and α″ phases was determined by placing the interstitial atoms in the common octahedral and tetrahedral sites followed by a full atomic relaxation of the supercells. The tetrahedral site for oxygen in β-phase and α″-phase is not stable, and the oxygen relaxes to a nearby stable octahedral site. Similar site preference was observed in previous study in Ti–Nb alloy using an embedded-atom method potential [36]. Unlike the α″-phase, the α-phase has metastable hexahedral and crowdion sites in addition to the stable octahedral site [37] although both these structures have the same hcp stacking. We believe that this difference is likely due to the inherited lattice parameters from β-phase, which destabilizes the local atomic environment when oxygen occupies interstitial sites that are smaller than an octahedral site. Figure 3 shows the formation energy of oxygen interstitial in the various interstitial sites of Im\( \bar{3} \)m β and the orthorhombic (Cmcm) α″-phases. An oxygen in the octahedral site is surrounded by 6 Ti and Nb atoms, with the first nearest neighbors RNN distance of 2.09 and 2.1 Å for β-phase and α″-phase, respectively. The interstitial oxygen distorts the surrounding lattice more than a substitutional atom, and the resulting lattice strain field affects the phase transformation by stabilizing or destabilizing a specific phase [38].
The equilibrium lattice constants for β and α″ phases of binary Ti–Nb alloys with 12.5, 16.6, and 25 at.% of Nb are listed in Table 2. While the ‘a’ lattice constant increases, the ‘b’ and ‘c’ constants decrease with increasing Nb content, in agreement with previous experiments and other first principles calculations [7, 19,20,21]. Table 3 presents the lattice constants of ternary Ti–Nb–O alloys with 12.5, 16.6, and 25 at.% for Nb and with 1 at.%, and 1.53 and 2 at.% of oxygen. These parameters differ slightly from those for the binary Ti–Nb alloys. Therefore, no significant change in recovery strain ϵr is expected from the β–α″ transformation. However, the main role of oxygen involves stabilizing the β-phase and reducing Ms as discussed in the next section. We wish to point out that unlike binary Ti–Nb alloys, the β-phase ternary Ti–Nb–O alloys are unstable and transform to the α″-phase when both the supercell shape and atomic positions are relaxed because of the local stresses induced by the interstitial oxygen. Therefore, a lower energy tolerance for optimization followed by a volume relaxation was employed to obtain the total energies and lattice constants of their metastable high-temperature β-phase.
The formation energy, which quantifies the relative stabilities of β and α″ phases in Ti–Nb alloys, is defined as:
here N is the total number of atoms in the Ti–Nb–O supercell, Etotal is the total energy of a β or α″-supercells, and E(Ti), E(Nb), and E(O), respectively, represents the energy per atom of Ti, Nb, and O in their stable structure. Also, to get energetics values as close as possible to a real random solution, we averaged the total energies of the three different configurations for each composition. The energetics of β–α″ phase stability was estimated using the formation energy difference given by:
The α″-phase becomes less stable at larger values of the formation energy difference ∆E″–βf.
The ∆E″–βf values for Ti–Nb and Ti–Nb–O alloys are shown in Fig. 4. For the binary Ti–Nb alloys, the α″-phase becomes less stable with respect to the β-phase at increasing Nb content, in agreement with the previous work [4, 7, 18]. For the Ti–Nb–O alloys, ∆E″–βf increases with increasing oxygen content between 0 and 2 at.%, which indicates a decrease in Ms. However, Fig. 4b shows that the slope of (∆Ef/at.% O) is higher for Ti–12.5 at.% Nb than at the other two Nb concentrations. Thus, oxygen appears to have a greater influence on increasing the ∆E″–βf, and thereby stabilizing the β-phase. Figure 4a, b shows that ∆E″–βf for Ti–Nb alloys increases by 2.8 meV/at.% Nb, which corresponds to a ∆T of ~ 34 K for each atomic percent increase in Nb. The experimental estimate ∆T of 40 K for each atomic percent of Nb is in reasonable agreement with our results [7, 39]. On the other hand, the increase in ∆E″–βf for Ti–Nb–O alloys is 6.5 meV/at.% O for the alloys containing 25 and 16.6 at.% Nb and 12.5 meV/at.% O for 12.5 at.% Nb alloys, which corresponds to a ∆T of ~ 78 K/at.% O and ~ 145 K/at.% O, respectively. The ∆T value for 25 and 16.6 at.% Nb significantly differs from the experimental value of 160 K [40]. This might be due to the fact that oxygen effect is more sensitive to temperature than Nb and merits further study. Also, a rigorous study that accounts for the finite temperature effect would likely provide better insights on the larger the ∆E″–βf values and the increased stability of β-phase at higher temperatures. On the other hand, the ∆T value for 12.5 at.% Nb is much higher than the other Nb concentrations, so we expect to see more significant decrease in Ms with increasing oxygen content in the alloy with low Nb concentration. It should be noted the most of the experimental studies investigating the effect of oxygen content on Ms in Ti–Nb–O alloys were restricted to 22 and 23 at.% Nb and had ∆T value of 160 K [13, 41]. Therefore, the effect of oxygen should yield a different behavior at smaller Nb concentrations due to decreasing stability of the β-phase.
Mechanism and energetics of β → α″ martensitic transformation
Figure 5 schematically illustrates the β–α″ martensitic transformation mechanism. The lattice constants of the α″ (Cmcm) orthorhombic phase lie between the β (bcc) and α′ (hcp) phases, and its stacking sequence along the c-axis resembles hcp structures. Thus, the β–α″ transformation may be viewed as a special case of the β–α′ transformation. However, the transformation strain needed to form the α″-phase from the β-phase differs from that to form α′ and depends on the Nb content [38, 39, 42]. The martensitic transformation mechanism in Ti alloys involves two processes: (a) shuffling displacement of adjacent {110}β planes along <110>β directions. This displacement increases from 0.20 to 0.32 Å with increasing Nb content [43, 44]. (b) lattice distortion of {110}β that involves stretching along <110> and compression along <001>. The shape memory effect and superelasticity originate from the lattice distortion [15].
In the energy-minimized Ti–Nb–O system, each oxygen atom occupies an octahedral site and is surrounded by six lattice atoms. Two of the lattice atoms are the first nearest neighbors along <001>, and the other four are the next nearest neighbors along <110>. The interstitial oxygen atoms induce local strain fields along a <001> direction, and three types of octahedral sites are possible depending on the strain field direction. The local strain fields are relaxed by the shuffling of adjacent {110} planes along <110> directions during the first step of β–α″ martensitic transformation.
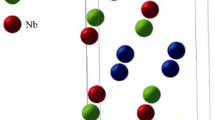
Figure 6 shows the energy-minimized β and α″ structures in Ti–16 at.% Nb and Ti–16 at.% Nb–1 at.% O alloys. In the ternary system, the β–α″ transforms to the α″-phase upon relaxation. This is likely due to strain fields induced by oxygen as inferred by the absence of this transformation in the binary Ti–16.6 at.% Nb system (Fig. 6a). Figure 7 shows the six shuffling modes, based on appropriate combinations of shuffling planes and directions, which trigger the β–α″ martensitic transformation as observed in transmission electron microscopy (TEM) by Tahara et al. [15]. The strain fields around oxygen at each octahedral interstitial site can relax via four of the six shuffling modes as shown in Fig. 7. For example, if we consider oxygen atom at site-1, the elastic strain energy induced by the oxygen atom can be relaxed by the shuffling modes 1, 2, 3, and 4, which involve the opposite shuffling of adjacent {110} planes along <001> directions. At site-2, the oxygen-induced strain fields can relax via shuffling modes 1, 2, 5, and 6.
The energy-minimized β and α” structures in binary Ti–16.6Nb (a, b) and ternary Ti–16.6Nb–1O systems (c, d), respectively. It is evident that the β-phase in Ti–16.6Nb–1O relaxed to α″-phase when oxygen atom is present at a stable octahedral site. The blue, green, and red colors correspond to Ti, Nb, and O atoms, respectively
The six shuffling modes that can trigger the β–α″ transformation and relax the stain fields induced by oxygen atoms are shown [15]. For each site, four modes of shuffling can relax the oxygen strain field. The arrows indicate <100> shuffling directions of the {110} planes for each mode
In the absence of an applied stress, the oxygen atoms are randomly distributed in β-phase between the three sites as shown in Fig. 7. The relaxation of the local strain fields induced by the oxygen atoms creates nanosized lattice modulations (domains), which appear as a diffuse streak with higher intensity halfway along <110>β directions the selected area diffraction patterns (SADP) obtained using TEM. The intensity of these spots increases at lower temperatures due to the increased stability of α″-phase over the β-phase [41]. However, the random distribution of these nanosized domains suppresses the martensitic transformation due to the opposing effect of the preferred shuffling modes for each domain. The TEM study also reveals that oxygen interstitials suppress the martensitic transformation in Ti–Nb alloys and stabilize the β-phase although it is impossible to track the oxygen atom in such experiments. On the other hand, atomistic simulations of the Ti–Nb alloys allow us to systematically investigate the nanodomain formation by quantifying strain fields associated with oxygen interstitials.
To understand the role of oxygen interstitials on the martensitic transformation in Ti–Nb alloys, the solid-state nudged elastic band (SSNEB) method has been used to map the minimum energy pathways (MEP) as a function of oxygen concentration. For each Nb concentration in the ternary Ti–Nb–O alloys, three oxygen concentrations were investigated. Three cases were studied for each oxygen concentration, and oxygen atoms occupy two octahedral sites. In the first case, two oxygen atoms occupy the same octahedral sites and experience the same mode to relax oxygen strain fields induced during martensitic transformation. For example, this could involve two favorable sites wherein oxygen atoms occupy site-1 with mode-3 as shown in Fig. 7. In the second case, the two oxygen interstitials occupy the same octahedral sites but exhibit different modes such that their strain fields oppose each other. For example, this could involve one oxygen atom at site-1 with mode-3 and the other at site-1 with mode-4. In the third case, both octahedral sites have same modes but both oppose the shuffling displacement of the martensitic transformation. The case 1 is shown schematically in Fig. 8.
Figure 9a shows the MEPs for binary Ti–Nb alloys as a function of Nb concentration. Note that these paths are calculated at T = 0 K. No energy barrier is observed, and the pathways reflect a preference for α″-phase at low temperatures. However, the transformation energy increases with increasing Nb concentration. This indicates that the α″ transformation is increasingly suppressed, in agreement with the previous studies showing a decrease in the Ms with increasing Nb content driven β stabilization in Ti–Nb alloys [7, 18, 37].
For the ternary Ti–Nb–O systems, Fig. 9b–d shows the MEPs for different Nb concentrations and oxygen site occupancies. For clarity, this figure only shows the change in MEPs for systems with 2 at.% oxygen concentration. We observed that for the case 1 of oxygen occupancy, no change is seen in the relative stability of β or α″-phases. However, for the other two cases, an increase in the relative stability of β-phase and a decrease in the stability of α″-phase are observed for increasing oxygen content. The third case also shows the highest increase in the relative stability of β-phase as the oxygen content increases from 1 to 2 at.%. This is likely due to the opposing effect between the strain fields around the unfavorable octahedral sites and the (0\( \bar{1} \)1)[011] shuffling required for martensitic transformation.
We next examine the differences in the local environment around the interstitial oxygen atom in the three cases studied above. Figure 10 shows the ternary Ti–25 at.% Nb–2 at.% O system after relaxation for the three cases of oxygen occupancy. Case 1 shows a negligible change in the displacements of first nearest neighbors of Ti and Nb atoms around the oxygen atom (see Fig. 10a). On the contrary, cases 2 and 3 reveal significant lattice distortions around oxygen atoms. Figure 10b, c shows octahedral sites where atom shuffling opposes the relaxation strain fields around oxygen atoms and reveals a bcc type of stacking. This can be visualized as the local strain fields of oxygen retarding the shuffle required to initiate the martensitic transformation. However, a full shuffle displacement occurs near the oxygen atom where the strain fields are aligned favorably. These lattice distortions correlate well with the increasing relative stability of β-phase at increasing oxygen content in case 2 and case 3 studied above. Again, note that these calculations are done at T = 0 K. These lattice distortions will likely suppress the martensitic transformation at higher temperatures where the β-phase is increasingly stable. Experimental studies in Ti–23 at.% Nb–1 at.% O showed that intensity of the lattice modulations in the TEM SADP was pronounced at temperatures below room temperature [41]. Our observations agree well with those observations of the suppression of martensitic transformation at low temperatures.
To study how the separation distance between two oxygen atoms affects the energy of Ti–Nb–O supercell in Fig. 8, we calculated the formation energy of the α″-phase at different separation distances. We have two scenarios—one with two oxygen atoms undergoing different shuffling modes and the second with oxygen atoms undergoing the same shuffling mode but with their strain fields opposing the shuffling directions of the martensitic transformation. Figure 11 shows the formation energy of the ternary Ti–Nb–O system as a function of the separation distance between two oxygen atoms for these two conditions. We found that the formation energy in Ti–Nb–O ternary system varies depending on the type octahedral sites occupied by oxygen atoms and does not depend on their separation, which supports our earlier findings about the energy dependency on oxygen site occupancy in Ti–Nb–O alloys.
Summary
By coupling CE method with a first principle energy calculator, we identified three stable atomic Ti–Nb configurations that can transform from β-phase to α″ phases without changing the local atomic position of Nb atoms. The solid-state nudged elastic band (SSNEB) method has been used to map MEPs for Ti–Nb and Ti–Nb–O systems. The transformation energy increases with increasing Nb concentration in Ti–Nb system. For Ti–Nb–O system three cases were studied for each oxygen concentration with interstitial oxygen occupying two octahedral sites. A progressive increase in the stability of β-phase and a decrease in the stability of α″-phase are observed with increasing oxygen content. The highest increase in the relative stability of β-phase as the oxygen content increases from 1 to 2 at.% is observed in third case where two oxygen atoms occupy the same type of octahedral sites but both oppose the shuffling displacement of the martensitic transformation. This is likely due to the counter effect between the strain fields around the unfavorable octahedral sites and the shuffling mode (0\( \bar{1} \)1)[011] required for martensitic transformation. The increase in the relative stability of β-phase with increasing oxygen content is translated into a decrease in Ms. The highest decrease in Ms was observed in Ti–12.5 at.% Nb alloy which agrees with Yuan et al. work [45] where they could achieve a low Ms with low Nb concentration of 11 at.% by adding oxygen. Also, we observed a significant lattice distortion resembling a bcc type of stacking around oxygen atoms occupying octahedral sites that oppose the shuffling directions of martensitic transformation. However, these distortions are absent if the oxygen atoms occupy the octahedral sites that do not oppose the shuffling directions of the transformation. This change in the local environment around the interstitial oxygen atoms confirms the ability of oxygen to increase the stability of β-phase and to change the martensitic transformation behavior in Ti–Nb alloys. In addition, we found that the formation energy varies depending on the type of the octahedral sites occupied by oxygen atoms and does not depend on the separation distance between oxygen atoms, which supports our findings about the energy dependency on oxygen site occupancy in Ti–Nb–O alloys.
Our results confirm the stabilization effect of oxygen on β-phase in Ti–Nb alloys. The highest stabilization effect is expected at lower Nb concentration. Therefore, a higher decrease in Ms temperature can be obtained by adjusting Nb and oxygen content in Ti–Nb alloys. Current theoretical work suggests adding oxygen to Ti–Nb alloy with Nb concentration of 12.5 at.% will yield higher decrease in Ms.
References
Hao YL, Li SJ, Sun SY, Zheng CY, Yang R (2007) Elastic deformation behaviour of Ti–24Nb–4Zr–7.9Sn for biomedical applications. Acta Biomater 3(2):277–286
Shabalovskaya S (1994) Shape memory and superelastic technologies. In: First International Conference, p 209
Takahashi E, Sakurai T, Watanabe S, Masahashi N, Hanada S (2002) Effect of heat treatment and Sn content on superelasticity in biocompatible TiNbSn alloys. Mater Trans 43(12):2978–2983
Bönisch M, Calin M, Waitz T, Panigrahi A, Zehetbauer M, Gebert A, Skrotzki W, Eckert J (2013) Thermal stability and phase transformations of martensitic Ti–Nb alloys. Sci Technol Adv Mater 14(5):55004
Hao YL, Li SJ, Prima F, Yang R (2012) Controlling reversible martensitic transformation in titanium alloys with high strength and low elastic modulus. Scr Mater 67:487–490
All EBV, With I (2017) Stabilizing the body centered cubic crystal in titanium alloys by a nano-scale concentration modulation. J Alloys Compd 700:155–158
Kim HY, Ikehara Y, Kim JI, Hosoda H, Miyazaki S (2006) Martensitic transformation, shape memory effect and superelasticity of Ti–Nb binary alloys. Acta Mater 54(9):2419–2429
Kim JI, Kim HY, Inamura T, Hosoda H, Miyazaki S (2005) Shape memory characteristics of Ti–22Nb–(2–8) Zr(at.%) biomedical alloys. Mater Sci Eng A 403(1–2):334–339
Hao YL, Li SJ, Sun SY, Yang R (2006) Effect of Zr and Sn on Young’s modulus and superelasticity of Ti–Nb-based alloys. Mater Sci Eng A 441(1–2):112–118
Kim HY, Sasaki T, Okutsu K, Kim JI, Inamura T, Hosoda H, Miyazaki S (2006) Texture and shape memory behavior of Ti–22Nb–6Ta alloy. Acta Mater 54(2):423–433
Masumoto K, Horiuchi Y, Inamura T, Hosoda H, Wakashima K, Kim HY, Miyazaki S (2006) Effects of Si addition on superelastic properties of Ti–Nb–Al biomedical shape memory alloys. Mater Sci Eng A 438–440:835–838
Nayak SK, Hung CJ, Sharma V, Alpay SP, Dongare AM, Brindley WJ, Hebert RJ (2018) Insight into point defects and impurities in titanium from first principles. npj Comput Mater 4(1):11
Il Kim J, Kim HY, Hosoda H, Miyazaki S (2005) Shape memory behavior of Ti–22Nb–(0.5–2.0) O (at%) biomedical alloys. Mater Trans 46(4):852–857
Nii Y, Arima TH, Kim HY, Miyazaki S (2010) Effect of randomness on ferroelastic transitions: disorder-induced hysteresis loop rounding in Ti–Nb–O martensitic alloy. Phys Rev B Condens Matter Mater Phys 82(21):1–7
Tahara M, Kim HY, Inamura T, Hosoda H, Miyazaki S (2011) Lattice modulation and superelasticity in oxygen-added β-Ti alloys. Acta Mater 59(16):6208–6218
Tahara M, Inamura T, Kim HY, Miyazaki S, Hosoda H (2015) Role of oxygen atoms in α″ martensite of Ti-20 at.% Nb alloy. Scr Mater 112:15–18
Saito T (2003) Multifunctional alloys obtained via a dislocation-free plastic deformation mechanism. Science 300(5618):464–467
Tane M, Nakano T, Kuramoto S, Niinomi M, Takesue N, Nakajima H (2013) ω Transformation in cold-worked Ti–Nb–Ta–Zr–O alloys with low body-centered cubic phase stability and its correlation with their elastic properties. Acta Mater 61(1):139–150
Sun J, Yao Q, Xing H, Guo WY (2007) Elastic properties of β, α″ and ω metastable phases in Ti–Nb alloy from first-principles. J Phys Condens Matter 19(48):486215
Ojha A, Sehitoglu H (2016) Slip resistance of Ti-based high-temperature shape memory alloys. Shape Mem Superelasticity 2(1):50–61
Minami D (2016) Effect of alloying element X on transformation strains and phase stabilities between alpha double prime and beta Ti–Nb–X (X = Al, Sn, Zr, Ta) ternary alloys. Mater Trans 57(3):263–268
Niu JG, Ping DH, Ohno T, Geng WT (2014) Suppression effect of oxygen on the β to ω transformation in a β-type Ti alloy: insights from first-principles. Model Simul Mater Sci Eng 22(1):15007
Hennig RG, Trinkle DR, Bouchet J, Srinivasan SG, Albers RC, Wilkins JW (2005) Impurities block the alpha to omega martensitic transformation in titanium. Nat Mater 4(2):129–133
Niu JG, Geng WT (2014) Oxygen-induced lattice distortion in β–Ti3Nb and its suppression effect on β to α’’ transformation. Acta Mater 81:194–203
Blöchl PE (1994) Projector augmented-wave method. Phys Rev B 50(24):17953–17979
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77(18):3865–3868
Materials Design (2012) Medea version 2.10 (Angel Fire, NM Mater Des)
Lerch D, Wieckhorst O, Hart GLW, Forcade RW, Müller S (2009) UNCLE: a code for constructing cluster expansions for arbitrary lattices with minimal user-input. Model Simul Mater Sci Eng 17(5):55003
van de Walle A (2008) A complete representation of structure-property relationships in crystals. Nat Mater 7(6):455–458
Sheppard D, Xiao P, Chemelewski W, Johnson DD, Henkelman G (2012) A generalized solid-state nudged elastic band method. J Chem Phys 136(7):074103
Lazar P, Jahnátek M, Hafner J, Nagasako N, Asahi R, Blaas-Schenner C, Stöhr M, Podloucky R (2011) Temperature-induced martensitic phase transitions in gum-metal approximants: first-principles investigations for Ti3Nb. Phys Rev B Condens Matter Mater Phys 84(5):1–17
Togo A, Tanaka I (2015) First principles phonon calculations in materials science. Scr Mater 108:1–5
Trinkle DR, Jones MD, Hennig RG, Rudin SP, Albers RC, Wilkins JW (2006) Empirical tight-binding model for titanium phase transformations. Phys Rev B Condens Matter Mater Phys 73(9):1–9
Souvatzis P, Eriksson O, Katsnelson MI, Rudin SP (2008) Entropy driven stabilization of energetically unstable crystal structures explained from first principles theory. Phys Rev Lett 100(9):1–4
Souvatzis P, Arapan S, Eriksson O, Katsnelson M (2011) Temperature driven α to β phase-transformation in Ti, Zr and Hf from first principles theory combined with lattice dynamics. Condmat 66006(1):4
Yu L, Yin F, Ping D (2007) Natural mechanism of the broadened Snoek relaxation profile in ternary body-centered-cubic alloys. Phys Rev B Condens Matter Mater Phys 75(17):1–12
Wu HH, Wisesa P, Trinkle DR (2013) Oxygen diffusion in HCP metals. Phys Rev B 14307:1–15
Nowick AS, Berry BS (1972) Anelastic relaxation in crystaline solids. Academic, New York
Kim HY, Ohmatsu Y, Il Kim J, Hosoda H, Miyazaki S (2004) Mechanical properties and shape memory behavior of Ti–Mo–Ga alloys. Mater Trans 45(4):1090–1095
Miyazaki S, Kim HY, Hosoda H (2006) Development and characterization of Ni-free Ti-base shape memory and superelastic alloys. Mater Sci Eng A 438–440:18–24
Tahara M, Kanaya T, Kim HY, Inamura T, Hosoda H, Miyazaki S (2014) Heating-induced martensitic transformation and time-dependent shape memory behavior of Ti–Nb–O alloy. Acta Mater 80:317–326
Duerig TW, Williams JC (1984) Overview: microstructure and properties of beta titanium alloys. In: Proceedings of the symposium on beta titanium alloys in the 80’s, TMS, Atlanta, GA, pp 16–67
Ojha A, Sehitoglu H (2016) Critical stresses for twinning, slip, and transformation in Ti-based shape memory alloys. Shape Mem Superelasticity 2:180–195
Bonisch M, Calin M, Giebeler L, Helth A, Gebert A, Skrotzki W, Eckert J (2014) Composition-dependent magnitude of atomic shuffles in Ti–Nb martensites. J Appl Crystallogr 47(4):1374–1379
Yuan B, Yang B, Gao Y, Lai M, Chen XH, Zhu M (2016) Achieving ultra-high superelasticity and cyclic stability of biomedical Ti–11Nb–4O (at.%) alloys by controlling Nb and oxygen content. Mater Des 92:978–982
Acknowledgements
This research was supported by the NSF DEMREF program (Grant Number 1435611). The computations were done on Talon3 supercomputer at the University of North Texas and TACC Stampede2 at Texas Advanced Computing Center through XSEDE program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salloom, R., Reith, D., Banerjee, R. et al. First principles calculations on the effect of interstitial oxygen on phase stability and β–α″ martensitic transformation in Ti–Nb alloys. J Mater Sci 53, 11473–11487 (2018). https://doi.org/10.1007/s10853-018-2381-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-018-2381-6