Abstract
Motivated by experimental observation of B36 borophene as a molecular model of bulk boron, here, we report the impact of nitrogen substitution on the electronic and chemical properties of neutral and anionic B36 clusters. Three N-doped configurations resulted from nitrogen substitution at different positions with respect to the central hexagonal hole of B36 are considered. The effect of N-doping on the structure, curvature, and π bonding pattern of the B36 is analyzed. High level of N-dopant enhances the curvature, and the cluster adopts a buckled form. We also study the binding strength between alkali metals with the pristine and N-doped substrates. Moreover, the adsorption properties of metallized substrates toward H2 molecule are explored, highlighting the role of dopant. Depending on the type of metal and the nitrogen content, the H2 adsorption energies vary in the range between − 0.11 and − 0.21 eV which fall into the range for the practical applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Superexcellent properties of boron nanostructures have been led these wonder materials in the center of attention [1,2,3,4,5]. Boron nanostructures have the same merit as carbon nanomaterials such as lightweight, extreme hardness, and porous [6, 7]. Recently, a new two-dimensional boron sheet, borophene, has been synthesized on the Ag(111) substrates [8]. The exploration of borophene offers a broad perspective in medicine, material research, catalysis, and photonic devices [9]. Furthermore, the quasi-planar structure, low atomic weight, and high stability of borophene make it an attractive potential choice for the energy storage and conversion systems [10,11,12,13]. Over the past decade, combined experimental and theoretical studies have led to a systematic understanding of the structural and bonding properties of small boron clusters [14, 15]. Recently, a quasi-planar boron cluster, B36, with a central hexagonal vacancy and sixfold symmetry has been synthesized by Piazza and coworkers [16]. This B36 cluster represents a record size for freestanding quasi-planar atomic clusters and is an embryo for the formation of extended two-dimensional boron sheets [6, 7, 17]. The unique structure of B36 with a natural hole is capable of lots of applications. For example, Rastgu et al. [18] investigated the reactivity and electronic sensitivity of B36 borophene toward four nucleobases of adenine, guanine, thymine, and cytosine. Their results suggest a new method for determination of DNA sequencing by use of B36. In a recent study, the potential application of B36 as a sensor for detecting toxic gases such as formaldehyde has been demonstrated [19]. Increasing research interests in B36 have encouraged scientists to explore further beneficial methods for energy generation prospects [20,21,22,23,24,25]. It is known that metallization of nanostructures is an efficient and industrial way to develop forefront of energy-related materials. Although alkali metals [26, 27], alkaline earth metals [28, 29], and transition metals [30, 31] are extensively investigated in this case, lighter metal adatoms can help to achieve higher gravimetric density [32]. On the other side, there are many literature studies indicating that B doping on carbon nanostructure can enhance storage capacity in metal ion batteries, decreases diffusion barrier, and raises metal reversible storage [33, 34]. Therefore, it is expected that pure boron or boron-rich materials achieve great performance as an electrode in rechargeable ion batteries. The adsorption and diffusion of commercial metal ions on freestanding borophene have received great research interest in recent years [35]. Experimental and theoretical studies indicate that borophene can serve as an ideal electrode material with high electrochemical performance for both Li-ion and Na-ion batteries [22, 24].
The advantage of borophene over carbon nanostructures and the experimental observation of B36 give us an impetus to examine it as a possible hydrogen storage material. Liu et al. [6] showed that Li adsorbed on neutral and anionic B36 can serve as reversible hydrogen storage media. Nonetheless, for efficient and economic H2 operation (adsorption/desorption) the optimal binding energy between hydrogen molecule and the host should be 0.1–0.3 eV/H2 [36]. To cover downside of the weak interaction between H2 molecules and the host, chemical modifications and decoration with foreign metal are successful techniques [37]. Accordingly, in the current contribution, we study the effect of local nitrogen doping on the structure, electronic, and adsorption properties of B36 and its anion. Our main concerns are as follows: (1) How does the local position of dopant affect the structure and the curvature of the B36 cluster? (2) How do the electronic structures of pristine and doped B36 change after decoration with light alkali metals (Li, Na, K). Does the binding strength between metal and the B36 clusters depend on the concentration and location of nitrogen dopant? (3) How do the B/N content and the metal affect the adsorption behaviors of metallized N-doped B36 toward H2 molecule?
Computational methods
All structures were fully relaxed by density functional theory (DFT) calculations at PBE0/6-31G* level. The PBE0 was lately benchmarked as a reliable DFT method for the boron system [17, 38, 39]. Further, Romanescu et al. [40] performed an UV–IR double-resonance experiment to obtain absorption spectra of boron clusters. They also calculated IR spectra of the three low-lying isomers of B11, B16, and B17 in the wavelength range of 650–1550 cm−1 using different DFT methods. The best agreement between experimental IR spectrum and calculated IR was observed for PBE0 method. Accordingly, all structural optimization and electronic surfaces analyses in the present study were accomplished by PBE0 as implemented in GAUSSIAN 09 package [41].
In order to have criteria for structural stability of the clusters, the formation energy is calculated as follows:
where \( E_{\text{cluster}} \) is the total energy of the cluster, \( E_{\text{N}} \) is the energy of a nitrogen atom in \( {\text{N}}_{2} \) molecule, \( E_{\text{B}} \) is the energy of an isolated boron atom, and \( n_{\text{N}} \), \( n_{\text{B}} \), and \( n_{\text{tot}} \) represent the number of nitrogen, boron, and total atoms in the cluster, respectively.
The binding strength between metal (M = Li, Na, and K) and boron cluster is obtained from the expression, \( E_{\text{b}} = E_{\text{M/cluster }} - E_{\text{M}} - E_{\text{cluster}} \), in terms of the energy of an isolated metal \( E_{\text{M}} \), and the energy of boron cluster with and without metal adatom (\( E_{\text{M/cluster}} \) and \( E_{\text{cluster}} \)). Also to evaluate adsorption behavior of metallized substrates toward single hydrogen molecule, the adsorption energy is calculated by:
\( E_{{{\text{H}}_{2} - {\text{M}}/{\text{cluster}}}} \) denotes the total energy of a hydrogen molecule adsorbed on metallized substrate and \( E_{{{\text{H}}_{2} }} \) is the energy of an isolated hydrogen molecule. Apart from energetic criteria, for all systems under our consideration, including pristine, metallized, and hydrogen adsorbing systems, the harmonic vibrational frequencies were calculated to affirm that the obtained structures are true minima. Bonding analyses were performed using the adaptive natural density partitioning (AdNDP) approach [42]. The AdNDP patterns, the deformation maps of electron density, and the topological analysis of the electron localization function (ELF) were performed with MultiWFN program [43, 44].
Results and discussion
Nitrogen doping
Structure and energetics of N-doped borophene
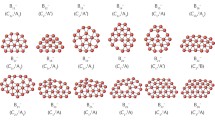
Both B36 and its anion are quasi-planar with the shape of a bowl having a central hexagonal vacancy [16]. B36 encircles three different sizes of rings as shown in Fig. 1. Inner, middle, and outer rings each contain 6, 12, and 18 boron atoms, respectively. The N-doped analogous of the B36 cluster can be designed by replacing B–B unit by B–N in each ring. We consider three doped clusters by introducing three, six, or nine nitrogen atoms into the inner, middle, and outer rings of B36 resulting into formation of B33N3, B30N6, and B27N9 clusters (Fig. 1). All the atomic coordinates of doped clusters were fully relaxed during the geometry optimization without symmetry constraints. To screen the perturbation effect of N-doping on the structure and curvature of B36, we consider three structural parameters including the height of bowl, the length of the cluster, and the diameter of a sphere fitted inside of cluster cavity. The detailed structural information is given in Table S1 in Supplementary Material. As shown in Fig. 1, doping at the inner ring has little effect on the cluster geometry and B33N3 retains its bowl shape. The bowl height becomes (1.56 Å) which is larger than that of B36 (1.17 Å), but the cluster length remains almost unchanged. On the contrary, B36 undergoes great structural deformation upon N-doping at the middle and outer rings. In B30N6, substitution six boron atoms at the middle ring with six nitrogens result in a cone-like structure with the height of 3.65 Å. Also in B27N9 the bowl transfigures to a disordered geometry having the height and the length of 4.01 Å and 7.33 Å, respectively. The bond length between B and N is 1.28–1.50 Å which is smaller than the distance between two B atoms (1.58–1.74 Å). Consequently, the cluster undergoes compression upon N-doping. In B33N3, the core cavity becomes slightly smaller than that of all-boron B36. Moreover, B33N3 has six additional pentagonal vacancies around the central pore. In the case of B30N6, some boron atoms move above the nominal ring and some move below that caused buckling. Deformation around the inner and middle rings shrinks the central cavity and produces several larger vacancies at the bottom of the cone. In B27N9, the nitrogen dopants have farther distance from the central vacancy and the compression occurs mostly at the cluster length. Thus, B27N9 has the largest central cavity that may be helpful for its application as an adsorbent. It should also be noted that the structures of N-doped B36 − clusters are similar to those of neutral B36 with small geometry change (see Supplementary Material for detailed structural parameters).
In addition to the structure, the thermodynamic and kinetic stability of the doped systems is also crucial for application purpose. The feasibility of N-substitutions in the borophene is assessed through the formation energy using Eq. (1). The negative values of formation energies indicate that the N-doped borophenes are energetically stable although their surfaces are no longer quasi-planar and prefer to buckle. The variation of formation energies is presented in Fig. 2. For both neutral and anion, the formation energy decreases by increasing the impurity concentration, indicating a decrease in the thermodynamic stability of the N-doped clusters as compared to the all-boron structure. On the other hand, a standard way of evaluating the stability of such molecular systems is to study the energy separation between the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO). The higher HOMO–LUMO gap (E g) is an indicator of a more stable system with less chemical reactivity. The HOMO–LUMO gaps of B36 and B36 − are 1.22 and 1.18 eV. Interestingly, we see an opposite trend for the variation of E g in the neutral and anionic clusters (Fig. 3). The N-doping at the inner and middle rings of the neutral B36 lowers the HOMO–LUMO gap, while doping at the outer ring raises the E g. In the case of anionic clusters, the trend is quietly reversed. However, it is worthy to note that in all N-doped B36 − clusters, the gaps are greater than that of the pure undoped cluster.
To identify the effect of N-doping on the electronic structure of boron clusters, we have plotted total density of states (TDOS) for considered systems (Fig. 4). Comparing the TDOS of the original B36 cluster (solid line) with N-doped clusters (dashed line) reveals the appearance of new peaks in the vicinity of Fermi energy which in turn implies the strong hybridization between nitrogen and boron atoms. The N-doping at the inner and middle rings shifts the Fermi level to lower energies and induces the p-type behavior. Moreover, we also present the spin-polarized DOS plots of anionic clusters in Fig. S1 in Supplementary Material. The asymmetric behaviors of up and down spins near the Fermi level result in a nonzero magnetization in B36 − and its N-doped structures.
Chemical bonding analysis: adaptive natural density partitioning
To understand more details on the bonding properties of considered doped systems, we performed AdNDP analysis. AdNDP has been introduced as a bridge between canonical molecular orbitals (CMOs) and natural bond orbitals (NBOs). CMOs are highly delocalized; meanwhile, NBOs are appreciably localized. AdNDP is an extension of NBO analysis featuring both localized and delocalized bonding without invoking the concept of resonance. In AdNDP analysis, density matrix has been localized by n-center two electron orbitals (nc-2e) with n spanning the interval from one to the total number of atoms in the system. However, there is no unique rule on how to search orbitals and pick out candidate orbitals as AdNDP orbitals. Actually, some molecules may have more than one reasonable AdNDP patterns. In this context, different AdNDP patterns have already been proposed for B36 [16, 45]. Herein, we just focus on the AdNDP results for the π bonding frameworks. To be specific, comparisons of the π orbitals between the inner rings of B36 and B33N3 (6c-2e), the middle rings of B36 and B30N6 (12c-2e), and the outer rings of B36 and B27N9 (18c-2e) are made (Figs. 5, 6, 7). Earlier studies indicate that B36 and B36 − are all-boron analogs of coronene (C24H12) featuring concentric dual π aromaticity [45, 46]. Accordingly, we also performed AdNDP analysis for coronene and its doped derivatives (C21N3H12 and C15N9H6) resulted from nitrogen substitution at the inner and outer ring, respectively. The AdNDP patterns of π bonding in the inner rings of B36 and B33N3, as well as C24H12 and C21N3H12, are presented in Fig. 5a–d. Interestingly, similar bonding patterns are observed not only for B36 and C24H12 but also for the doped structures B33N3 and C21N3H12, demonstrating the close analogy between the π bonds in the B33N3 and C21N3H12. In Figs. 6 and 7, we proposed two new optimal π orbitals in middle and outer rings with acceptable occupation numbers (ON). In a previous study, Chen et al. [45] demonstrated a one-to-one correspondence between 2c-2e, 4c-2e, and 6c-2e π bonds of B36 with those of C24H12. Here, such correspondence has also been observed for 6c-2e and 18c-2e π orbitals of borophene and coronene. More interestingly, our AdNDP analysis for the doped clusters indicates the same analogy between π bonding patterns of N-doped coronene and N-doped borophene.
Electron density deformation upon N-doping
To achieve a deep insight into the redistribution of electron density upon N-doping, the electron density deformation is adapted. Deformation densities can be generated to visualize the difference between the total electron density of the molecule and the superimposed densities of the constituent non-interacting atoms. Graphical representation of the deformation density maps is shown in Fig. 8. The blue and red lines represent the positive and negative density differences which arise from charge density accumulation and depletion, respectively. Evidently, in B36 regions of positive deformation density indicate the formation of covalent bonds between boron atoms of an individual ring and boron atoms belonging to two neighboring rings (Fig. 8a). N-doping at the inner ring does not lead to significant change into the distribution of electron density around the middle and outer rings (Fig. 8b). Nonetheless, the map shows that extra electrons of the N-dopant stay locally on nitrogen and weaken the bonds in the inner ring. In the nitrogen-rich structures, the symmetric bonding pattern is lost (Fig. 8c, d) and the depletion of electron density leads to an anisotropic distribution of electron density over the whole B30N6 and B27N9 clusters. Thus, although B36 itself is a highly stable cluster, however, it cannot bear introducing the dopant.
Metallization
To understand the effect of N-doping and charge state of the substrate on the alkali metal binding properties, we first calculate the binding energy between Li atom and B36 cluster. Different initial adsorption sites of Li on B36 are considered, including above the convex and concave sides of the central hexagonal cavity, triangle hollow site, and above the B–B bonds of the inner, middle, and outer rings. After structural relaxation, we found that the Li atom preferably is adsorbed above the central hexagonal vacancy at a distance of 1.60 Å with the maximum binding energy of E b = 1.88 eV. The binding of Li to B36 − is about 0.2 eV stronger. We further examined whether the Li binding energy changes on the N-doped clusters. When compared to the case of pristine B36, the binding energy is significantly enhanced by ~ 1.2–2.15 eV. As shown in Fig. 9, the maximum enhancement is observed in the case of Li adsorption on B33N3. A similar trend has also been obtained for Li adsorption on anionic B36 −. This finding suggests that different values of binding energies can be achieved by changing the charge state and introducing nitrogen into the B36 cluster. Moreover, the calculated binding energies are high enough to hinder clustering of alkali metals [47].
We also considered similar configuration for the adsorption of Na and K on the neutral and anionic B36 as well as their N-doped analogous. The trends in the binding energies are supported by the adsorption distance and the charge transfer between metal and substrates. The collected data in Table S2 show that the size of metal adatom and the dimension of the cavity on the boron cluster are two influential factors that determine the adsorption distance. In fact, the optimal distance (dM-substrate) is determined by the repulsive forces between metal and the cluster. Hence, the largest distance belongs to the adsorption of K atom on B30N6 (having the smallest cavity size). Similar to the bonding mechanism of alkali metals on carbon surfaces [48], the adsorption of alkali metals on considered boron clusters is along with the electron transfer from metal to the cluster. The calculated natural charges of the metal center (QM) are summarized in Table S2. As might be anticipated, the magnitudes of charge transfer between alkali metals and B33N3 cluster are remarkably higher than those of other complexes.
This binding mechanism has also been confirmed by conducting the ELF analysis. The ELF theory is an important tool to study electronic structure and chemical bond analysis. Generally, a high value of ELF at a certain point is an indicator of the localized electrons therein. ELF is a dimensionless quantity that varies between 0 and 1. ELF = 0 corresponds to a completely delocalized situation, and ELF = 1 shows perfect localization; Fig. 10 presents the two-dimensional color-filled maps of Li adsorption on neutral B36 and three N-doped clusters. The left panel shows the ELF maps of bare clusters. In B36, the high value of ELF (red color) in the outer ring indicates that the electrons are more likely confined in that region. Introducing nitrogen impurity reduces the degree of localization. In the case of B27N9, the map dominantly shows regions with ELF less than 0.4 implying delocalized features. In the adsorption systems (right panel) the quite low ELF, the blue area between Li and boron atoms of inner ring suggest weak interaction with electrostatic nature. It is interesting to note that the higher stability of Li/B33N3, as showed above by the binding energy, can also be demonstrated by the ELF map of the interacting system. The ELF in Fig. 10b indicates the large degree of localization between boron atoms of the outer ring which is extended toward the middle ring in some region. In addition, the weak interaction between Li and boron atoms of the inner ring is also observed.
H2 adsorption
We next study the adsorption of single hydrogen molecule on pristine and metallized substrates. Several initial configurations of the H2 molecule including parallel and perpendicular to the hexagonal and triangle hollow sites as well as above the metal are examined. Upon relaxation, the hydrogen molecule prefers to locate above the central hexagonal hollow site in pristine and to tilt toward the metal in metallized substrates. The optimized structures of the hydrogen adsorption on the bare and metallized substrates are shown in Fig. 11, and the corresponding adsorption energies are presented in Fig. 12. The H2 adsorption energy for pristine B36 is − 0.119 eV, and the adsorption distance is 3.50 Å. In the case of metallized B36, the hydrogen molecule prefers to tilt toward alkali metal atoms so that one of the two H atoms becomes relatively closer to the metal atom. A summary of geometries related to the adsorption of H2 on alkali metal decorated neutral and anionic B36 is given in Tables S3 and S4 in Supplementary Material. As shown in Fig. 12, metallization of B36 enhances the H2 adsorption energies by 28–82%. The increase in the binding strength is in parallel with the decrease in the adsorption distance between metal and H2. A similar trend has also been observed for the anionic B36 cluster.
Previous studies have demonstrated that N-doping decreases the adsorption energies of H2 in carbon nanostructures [49, 50]. It is of our interest to know the effect of N-doping on the hydrogen adsorption behavior of B36. Accordingly, we have systematically studied the adsorption behaviors of hydrogen molecules on N-doped B36. For the sake of comparison, we considered similar adsorption configuration for N-doped clusters and their alkali metal coated forms. Inspection of Fig. 12 reveals that for B33N3, the H2 adsorption energy slightly increases as compared to B36. At higher dopant concentration, a decrease in adsorption energy is observed. This result is in line with earlier reports for hydrogen adsorption of N-doped carbon materials. To obtain further understanding of H2 adsorption mechanism, we have compared PDOS of an isolated hydrogen molecule with those of adsorbed H2 on B33N3 and Li/B33N3 (Fig. 13). Isolated hydrogen molecule has two symmetric peaks at − 8 and 8 eV. After adsorption on B33N3, the PDOS of H2 remains almost intact and a weak hybridization between H2 and PDOS of nitrogen atoms is observed far below the Fermi level at − 9 to − 8 eV. In the case of Li/B33N3, the PDOS clearly reveals the charge transfer mechanism due to polarization interaction between H2 and Li. The great contribution of metal upon the H2 adsorption is reflected by the obvious orbital interaction above the Fermi level at the region between 7 and 9 eV.
Conclusions
In conclusion, we have investigated the effect of nitrogen doping on the electronic structure and hydrogen adsorption properties of metallized B36 and B36 − clusters. Three N-doped configurations resulted from N-doping at the inner, middle, and outer rings of B36 were considered. The structural features such as cluster curvature and size of the central hollow site have been systematically analyzed. Chemical bonding analysis of the doped clusters indicates the same analogy between concentric π bonding patterns of N-doped coronene and N-doped borophene. On the other hand, analysis of deformation electron density reveals that in the nitrogen-rich structures (B30N6 and B27N9 clusters), depletion of electron density results in the anisotropic distribution of electron density and cluster buckling.
We have also studied the adsorption of alkali metals on pristine and N-doped neutral and anionic B36 clusters. The binding energies of alkali metals depend on the curvature and nitrogen content of the substrate. Maximum binding strength is observed in the case of metallized B33N3. Further, we have reported the effect of N-doping on the hydrogen adsorption behavior of B36. We showed that in B33N3, the H2 adsorption energy slightly increases as compared to B36, but at the higher level of dopant a decrease in adsorption energy is observed. However, depending on the type of metal and the nitrogen content, the H2 adsorption energies vary in the range between − 0.11 and − 0.21 eV which lie in desirable range for reversible desorption near room temperature. We hope that our theoretical results will provide a useful guide for designing and synthesis of efficient hydrogen storage media.
References
Kiran B, Bulusu H, Zhai HJ, Yoo S, Zeng XC, Wang LS (2005) Planar-to-tubular structural transition in boron clusters: B20 as the embryo of single-walled boron nanotubes. Proc Natl Acad Sci USA 102:961–964
Huang W, Sergeeva AP, Zhai HJ, Averkiev BB, Wang LS, Boldyrev AIA (2010) Concentric planar doubly π-aromatic B19 − cluster. Nat Chem 2:202–206
Popov IA, Piazza ZA, Li WL, Wang LS, Boldyrev AI (2013) A combined photoelectron spectroscopy and ab initio study of the quasi-planar B24(-) cluster. J Chem Phys 139:144307-1–144307-8
Oger E, Crawford NRM, Kelting R, Weis P, Kappes MM, Ahlrichs R (2007) Boron cluster cations: transition from planar to cylindrical structures. Angew Chem Int Ed 46:8503–8506
Aihara JI, Kanno H, Ishida T (2005) Aromaticity of planar boron clusters confirmed. J Am Chem Soc 127:13324–13330
Liu CH, Wang X, Ye XJ, Yan X, Zeng Z (2014) Curvature and ionization-induced reversible hydrogen storage in metalized hexagonal B36. J Chem Phys 141:194306-1–194306-8
Mukherjee S, Thilagar P (2016) Borophene: a new paradigm! Curr Sci 111:1302–1304
Mannix AJ, Zhou XF, Kiraly B, Wood J, Alducin DD, Myers BD, Liu X, Fisher BL, Santiago U, Guest JR et al (2015) Synthesis of borophenes: anisotropic, two-dimensional boron polymorphs. Science 350:1513–1516
Sachdev H (2015) Disclosing boron’s thinnest side. Science 350:1468–1469
Tang H, Beigi S (2007) Novel precursors for boron nanotubes: the competition of two-center and three-center bonding in boron sheets. Phys Rev Lett 99:115501-1–115501-4
Yang X, Ding Y, Ni J (2008) Ab initio prediction of stable boron sheets and boron nanotubes: structure, stability, and electronic properties. Phys Rev B 77:041402-1–041402-4
Balendhran S, Walia S, Nili H, Sriam S, Bhaskaran M (2015) Elemental analogues of graphene: silicene, germanene, stanene, and phosphorene. Small 11:640–652
Sachdev H, Muller F, Hufner S (2010) BN analogues of graphene: on the formation mechanism of boronitrene layers—solids with extreme structural anisotropy. Diamond Relat Mater 19:1027–1033
Sergeeva PA, Popov IA, Piazza ZA, Li WL, Romanescu C, Wang L, Boldyrev AI (2014) Understanding boron through size-selected clusters: structure, chemical bonding, and fluxionality. Acc Chem Res 47:1349–1358
Li S, Zhang Z, Long Z, Sun G, Qin S (2016) Comparative study on the spectral properties of boron clusters Bn0/−1(n = 38–40). Sci Rep 6:25020-1–25020-9
Piazza ZA, Hu HS, Li WL, Zhao YF, Li J, Wang LS (2014) Planar hexagonal B36 as a potential basis for extended single-atom layer boron sheets. Nat Commun 5:3113-1–3113-6
Li WL, Chen Q, Tian WJ, Bai H, Zhao YF, Hu HS, Zhai HJ, Li D, Wang LS (2014) The B35 cluster with a double-hexagonal vacancy: a new and more flexible structural motif for borophene. J Am Chem Soc 136:12257–12260
Rastgou A, Soleymanabadi H, Bodaghi A (2017) DNA sequencing by borophene nanosheet via an electronic response: a theoretical study. Microelectron Eng 169:9–15
Kootenaei A, Ansari G (2016) B36. borophene as an electronic sensor for formaldehyde: quantum chemical analysis. Phys Lett A 380:2664–2668
Jiang HR, Lu Z, Wu MC, Ciucci F, Zhao TS (2016) Borophene: a promising anode material offering high specific capacity and high rate capability for lithium-ion batteries. Nano Energy 23:97–104
Zhang X, Hu J, Cheng Y, Yang H, Yau Y, Yang SA (2016) Borophene as an extremely high capacity electrode material for Li-ion and Na-ion batteries. Nanoscale 8:15340–15347
Shi L, Zhao T, Xu A, Xu J (2016) Ab initio prediction of borophene as an extraordinary anode material exhibiting ultrafast directional sodium diffusion for sodium-based batteries. Sci Bull 61:1138–1144
Zhao Y, Zeng S, Ni J (2016) Phonon-mediated superconductivity in borophenes. App Phys Lett 108:242601-1–242601-4
Zhang Y, Wu ZF, Gao PF, Zhang S, Wen Y (2016) Could borophene be used as a promising anode material for high-performance li ion battery? ACS Appl Mater Interfaces 8:2175–22181
Meng F, Chen X, Sun S, He J (2017) Electronic and magnetic properties of pristine and hydrogenated borophene nanoribbons. Phys E 91:106–112
Ataca C, Akturk E, Ciraci S, Ustunel H (2008) High-capacity hydrogen storage by metallized graphene. Appl Phys Lett 93:043123–043125
Kim D, Lee S, Hwang Y, Yun K, Chung Y (2014) hydrogen storage in li dispersed graphene with stone–wales defects: a first-principles study. Int J Hydrog Energy 39:13189–13194
Yoon M, Yang S, Hicke C, Wang E, Geohegan D, Zhang Z (2008) Calcium as the superior coating metal in functionalization of carbon fullerenes for high-capacity hydrogen storage. Phys Rev Lett 100:206806–206809
Chen C, Zhang J, Zhang B, Duan H (2013) Hydrogen adsorption of mg-doped graphene oxide: a first-principles study. J Phys Chem C 117:4337–4344
Cabria I, Lopez MJ, Fraile S, Alonso J (2012) Adsorption and dissociation of molecular hydrogen on palladium clusters supported on graphene. J Phys Chem C 116:21179–21189
Guo Y, Lan X, Cao J, Xu B, Xia Y, Yin J (2013) A comparative study of the reversible hydrogen storage behavior in several metal decorated graphyne. Int J Hydrog Energy 38:3987–3993
Zhang H, Zhao M, Bu H, He X, Zhang M, Zhao L, Luo Y (2012) Ultra-high hydrogen storage capacity of li-decorated graphyne: a first-principles prediction. J Appl Phys 112:084305-1–084305-5
Lu RF, Rao D, Meng Z, Zhang X, Xu G, Liu Y, Kan E, Xiao C, Deng K (2013) Boron-substituted graphyne as a versatile material with high storage capacities of Li and H2: a multiscale theoretical study. Phys Chem Chem Phys 15:16120–16126
Wan W, Wang H (2015) First-principles investigation of adsorption and diffusion of ions on pristine, defective and B-doped graphene. Materials 8:6163–6178
Rao D, Zhang L, Meng Z, Zhang X, Wang Y, Qiao G, Shen X, Liu J, Lu F (2017) Ultrahigh energy storage and ultrafast ion diffusion in borophene-based anodes for rechargeable metal ion batteries. J Mater Chem A 5:2328–2338
Zhou J, Wang Q, Sun Q, Jena P, Chen XS (2010) Electric field enhanced hydrogen storage on polarizable materials substrates. Proc Natl Acad Sci USA 107:2801–2806
Hussain T, Islam MS, Rao GS, Panigrahi P, Gupta D, Ahuja R (2015) Hydrogen storage properties of light metal adatoms (Li, Na) decorated fluorographene monolayer. Nanotechnology 26:275401-1–275401-6
Li FY, Jin P, Jiang DE, Wang L, Zhang SB, Zhaoand JJ, Chen ZF (2012) B80 and B101-103 clusters: remarkable stability of the core-shell structures established by validated density functionals. J Chem Phys 136:74302-1–074302-8
Bai H, Chen Q, Miao C, Qmu YW, Wu YB, Lu GH, Zhai HJ, Li SD (2013) Ribbon aromaticity in double-chain planar BnH22 − and Li2BnH2 nanoribbon clusters up to n = 22: lithiated boron dihydride analogues of polyenes. Phys Chem Chem Phys 15:18872–18880
Romanescu C, Harding DJ, Fielicke A, Wang LS (2012) Probing the structures of neutral boron clusters using infrared/vacuum ultraviolet two color ionization: B11, B16, and B17. J Chem Phys 137:014317-1–014317-6
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, et al (2013) Gaussian 09, revision D.01: Gaussian, Inc: Wallingford CT
Zubarev DY, Boldyrev AI (2008) A developing paradigms of chemical bonding: adaptive natural density partitioning. Phys Chem Chem Phys 10:5207–5217
Lu T Multiwfn, version 3.3.9. http://multiwfn.codeplex.com/
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comp Chem 33:580–592
Chen Q, Wei GF, Tian WJ, Bai H, Liu ZP, Zhai HJ, Li SD (2014) Quasi-planar aromatic B36 and B36 − clusters: all-boron analogues of coronene. Phys Chem Chem Phys 16:18282–18287
Wang LS, Zhai HJ, Li SD, Li J, Boldy AI (2016) From planar boron clusters to borophene and borospherene. Proc of SPIE 10174:1017402-1–1017402-9
Legrian F, Manzhos S (2015) Aluminum doping improves the energetics of lithium, sodium, and magnesium storage in silicon. J Power Sources 274:65–70
Mohajeri A, Shahsavar A (2017) Tailoring the optoelectronic properties of graphyne and graphdiyne: nitrogen/sulfur dual doping versus oxygen containing functional groups. J Mater Sci 52:5366–5379
Zhou Z, Gao X, Yan J, Song D (2006) Doping effects of B and N on hydrogen adsorption in single-walled carbon nanotubes through density functional calculations. Carbon 44:939–947
Correa DJ, Florez F, Mora-Ramosb ME (2016) Ab initio study of hydrogen chemisorption in nitrogen-doped carbon nanotubes. Phys Chem Chem Phys 18:25663–25670
Acknowledgements
This work is supported by the Shiraz University Research Council.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest regarding the publication of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10853_2017_1900_MOESM1_ESM.docx
The structural properties of base and doped borophene as well as hydrogen adsorption properties for metallized substrates are given in supplementary material. (DOCX 336 kb)
Rights and permissions
About this article
Cite this article
Shahsavar, A., Mohajeri, A. Impact of position and number of nitrogen atom substitution on the curvature and hydrogen adsorption properties of metallized borophene. J Mater Sci 53, 4540–4553 (2018). https://doi.org/10.1007/s10853-017-1900-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1900-1